Introduction
The genus Coomansus was proposed by Jairajpuri & Khan (Reference Jairajpuri and Khan1977) based on the type species Coomansus parvus (de Man, 1880) and several species which were transferred from the genus Clarkus Jairajpuri, 1970. The genus Coomansus differs from the genus Clarkus Jairajpuri, 1970 in having a weak longitudinal ridge opposite to dorsal tooth, anteriorly gradually merging into the ventral wall (Jairajpuri, Reference Jairajpuri1970; Ahmad & Jairajpuri, Reference Ahmad and Jairajpuri2010). Loof & Winiszewska-Ślipińska (Reference Loof and Winiszewska-Ślipińska1993) have reviewed this genus Coomansus and transferred nine species of ‘zschokkei-group’ from the genus Iotonchus Cobb, 1916 and the species Clarkus major (Cobb, 1893) Jairajpuri, 1970 to the genus Coomansus. On the other side, the species Coomansus sphagni (Brzeski, 1960) Jairajpuri, 1970 was moved to the genus Iotonchus. Recently, nine species of ‘zschokkei-group’ were transferred from the genus Coomansus to the genus Parkellus Jairajpuri, Tahssen & Choi, 2001 based on the more posterior position of the dorsal tooth apex (Ahmad & Jairajpuri, Reference Ahmad and Jairajpuri2010). The main feature of the genus Coomansus that distinguishes it from the genus Parkellus is the location of the dorsal tooth apex in the anterior half of the buccal cavity (Jairajpuri et al., Reference Jairajpuri, Tahseen and Choi2001). The main diagnostic features of the genus are: (1) buccal cavity barrel-shaped with one dorsal tooth situated in the anterior half of buccal cavity; (2) presence of weak longitudinal ridge on ventral wall; (3) non-tuberculate cardia; (4) didelphic–amphidelphic female reproductive system; and (5) conoid, ventrally curved tail, 2–6 anal body diameter long without spinneret at the tail terminus.
Currently, 24 Coomansus species have been described, with the four most recent species discovered being Coomansus inca Andrássy, 2011 from Peru, Coomansus mapuche Andássy, 2011 from Chile, C. papua Andrássy, 2011 from Papua New Guinea and Coomansus prodontus Shah & Hussain, 2015 from Korea. In Vietnam, two species of the genus Coomansus – C. parvus (de Man, 1880) Jairajpuri & Khan, 1977 and Coomansus venezolanus (Loof, 1964) Jairajpuri, 1970 have been reported (Nguyen, Reference Nguyen2007; Vu, Reference Vu2016).
In the present study, Coomansus batxatensis sp. nov. is described based on morphological, morphometric and molecular data from the Bat Xat Nature Reserve in Lao Cai Province, Vietnam. An updated key to species based on females and a compendium of all the species known are also provided.
Materials and methods
Nematode extraction, preservation and morphological studies
Soil samples were collected from a pristine forest in Lao Cai Province, Vietnam. Nematodes were extracted from soil samples using modified Baermann funnel technique (Southey, Reference Southey1986).
They were heat killed, fixed in 4% formaldehyde (for morphological observations) or in a DESS mixture (Yoder et al., Reference Yoder, De Ley, King, Mundo-Ocampo, Mann, Blaxter, Poiras and De Ley2006) (for molecular analyses), transferred to anhydrous glycerol (Seinhorst, Reference Seinhorst1959, Reference Seinhorst1962) and mounted on glass slides for microscopic observation. After filming and taking pictures, selected specimens were submitted for molecular studies. Measurements were performed with a Nikon DS-Fi3 digital microscope camera (Minato, Tokyo, Japan) on a Nikon Eclipse Ni-U Upright microscope (Minato, Kyoto, Japan) at the Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology (VAST), Vietnam. Observations of morphological diagnostic features and light microscope were taken with a Nikon digital camera mounted on a Nikon Eclipse Ni microscope. Illustrations were drawn using a Nikon Eclipse Ni microscope equipped with a Nikon Y-IDT drawing tube (Minato, Tokyo, Japan). Photographs and illustrations were edited using Adobe Photoshop CC 2018 (www.adobe.com/productions/photoshop.html). Permanent slides are stored at the Department of Nematology, Institute of Ecology and Biological Resources, VAST, Hanoi, Vietnam.
DNA extraction, polymerase chain reaction (PCR) and sequencing
Nematode DNA was extracted from a single individual as described by Holterman et al. (Reference Holterman, Rybarczyk, Evan den Elsen, van Megen, Mooyman, Pena Santiago, Bongers, Bakker and Helder2008) and DNA extracts were stored at −20° until used as PCR template. The D2–D3 expansion segment of 28S ribosomal DNA (rDNA) and 18S were amplified using the forward D2A (5′-ACAAGTACCGTGGGGAAAGTTG-3′) and reverse D3B (5′-TCGG AAGGAACCAGCTACTA-3′) primers (Subotin et al., Reference Subotin, Sturhan, Chizhov, Vovlas and Baldwin2006) and primers 18S (18F: 5′-TCTAGAGCTAATACATGCAC-3′/18R: 5′-TACGGAAACCTTGTTACGAC-3′). All PCR reactions contained 12.5 μl Hot start green PCR Master Mix (2×) (Promega, Madison, Wisconsin, USA), 1 μl of the forward and reverse primer (10 μM each), the 3 μl DNA template and sterile Milli-Q water to 25 μl of the total volume. All PCR reactions were performed in SimpliAmp Thermal cycler (Thermo Fisher Scientific, Waltham, Massachusetts, USA)) as follows: an initial denaturation step at 95°C for 4 min, followed by 40 cycles at 95°C for 30 s, 54°C for 30 s and 72°C for 60 s with a final incubation for 5 min at 72°C. Amplicons were visualised under ultraviolet illumination after Simplisafe gel staining and gel electrophoresis. After sequencing, the obtained C. batxatensis sp. nov. rDNA sequence fragments were deposited in GenBank under the following accession numbers: MW525195–MW525197 (18S rDNA) and MW525198–MW525200 (28S rDNA).
Phylogenetic analyses
For phylogenetic relationships, analyses were based on 18S and 28S rDNA. The newly obtained rDNA sequences were analysed using the BioEdit sequences available in GenBank using the ClustelW alignment tool implemented in the MEGA 7 version 7.0 (Kumar et al., Reference Kumar, Stecher and Tamura2016). The final 18S and 28S rDNA datasets for phylogenetic study included sequences from the current study C. batxatensis sp. nov. and available sequences of Mononchida representatives from GenBank. The prepared multiple alignments of 18S and 28S rDNA generated by the ClustelW algorithm were routinely manually edited in order to eliminate improper phylogenetic signals. The phylogenies were constructed with the program MEGA 7 version 7.0. Maximum likelihood with T92+G substitution model for both 18S and 28S data sets was used.
Results and discussion
Coomansus batxatensis sp. nov.
Material examined
Holotype. Ten paratype females and one paratype male were collected at Sang Ma Sao commune. Another population including 13 females and two males was collected at Y Ty commune. All specimens were preserved in good condition.
Measurements. See table 1.
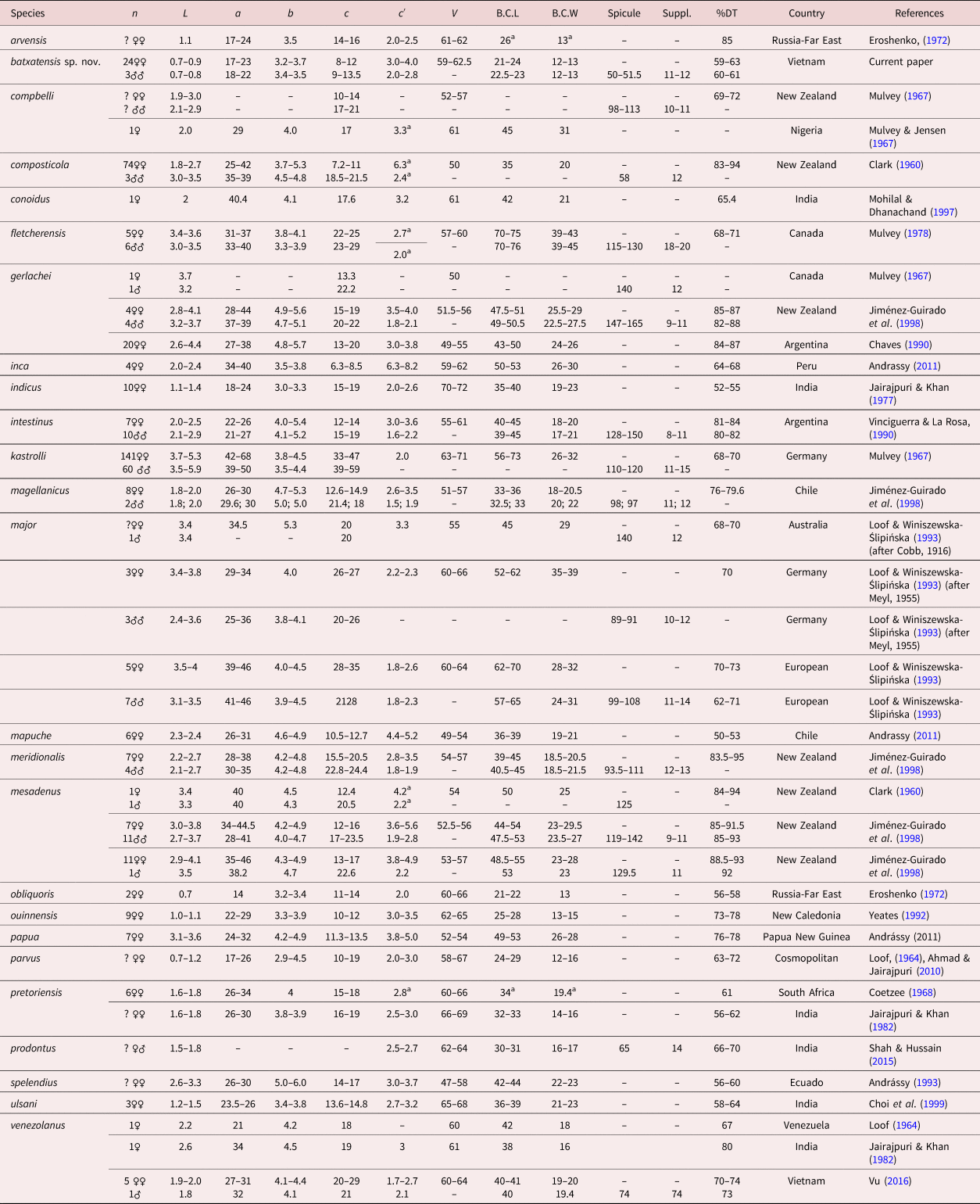
Table 1. Morphometrics of Coomansus batxatensis sp. nov. All measurements are in μm except where indicated.

L = body length, V = distance from vulva to the anterior end of body (mk) × 100/body length (mk), a = body length (mk)/maximum body diameter (mk), b = body length (mk)/pharynx length (mk), c = body length (mk)/tail length (mk), c' = tail length (mk)/anal body diameter (mk), G1 = anterior genital branch length (mk) × 100/body length (mk), G2 = posterior genital branch length (mk) × 100/body length (mk) (mk = micrometer).
Description
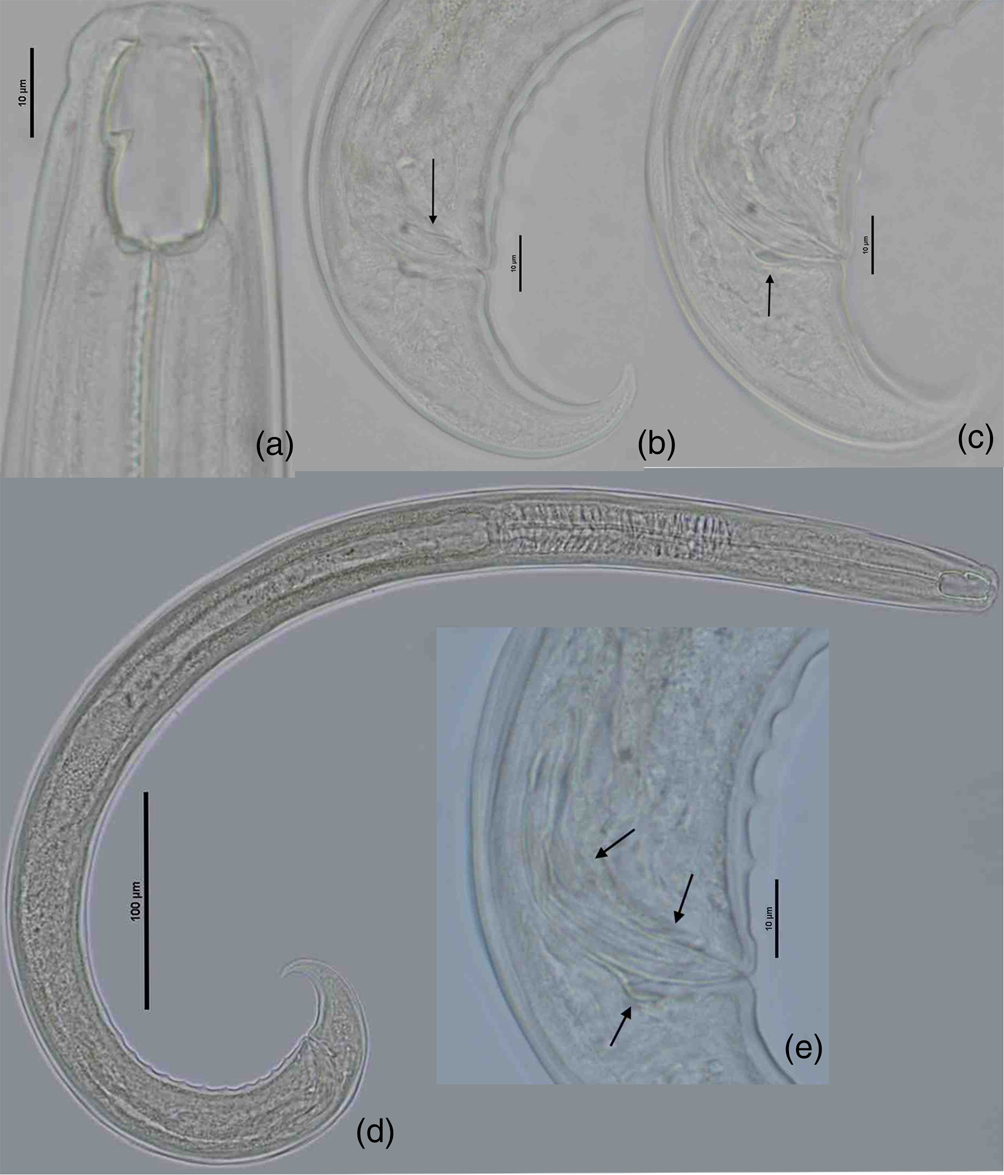
Small nematodes, 0.7–0.9 mm long. Relaxed specimens arcuate, more curved ventrad at posterior end. Body tapering slightly anterior to base of pharynx but more sharply towards posterior end. Maximum body width at the level of vulva. Cuticle smooth, 2.5 (2–3) μm thick.
Lip region rounded, 3.0 (2.7–3.3) times as wide as high, offset by a depression. Lips moderately separated and rounded. Anterior sensilla arranged in two circles: an anterior one of six inner labial papillae, posterior crown with six outer labial papillae and four cephalic papillae slightly protruding beyond the body outline. Amphid fovea funnel-like, its aperture, 3.6 (3.0–4.0) μm wide, located 4.6 (4.0–6.0) μm from the anterior end of buccal cavity or 4.4 (3.5–5.5) μm to the dorsal tooth apex. Buccal cavity barrel to sub-rectangular shape, flattened at base, 1.9 (1.7–2.0) times as long as wide. Dorsal tooth medium-sized, forward directed, its apex located 60 (59–63)% of buccal cavity length from buccal cavity base. About the posterior fifth of buccal cavity embedded in pharyngeal tissues.
Nerve ring encircling the cylindrical and muscular pharynx at about 35 (32–36)% of its length. Secretory–excretory pore (SE-pore) indistinct, situated just posterior to nerve ring or 39 (38–42)% its length from anterior end. Pharyngo–intestinal junction non-tuberculate. Rectum slightly arcuate, 0.8 (0.8–1) times the anal body diameter long. Tail conical, ventrally bent, regularly tapering, with sharp tip. Caudal glands and terminal opening absent.
Female. Genital system didelphic–amphidelphic, with both branches equally developed. Ovaries on alternate sides of the intestine, well developed, with numerous oocytes, overlap the uterus–oviduct junction. Oviduct and sphincter just on the other side of the reflexed ovary. Uterus simple. Vagina length occupying about 1/3 of corresponding body diameter. Pars proximalis vaginae as long as wide, cylindrical, surrounded by constrictor muscles not drawn. Pars refringens vaginae with faint sclerotized but clear and small (2.5 × 1.7 μm), teardrop-shaped pieces. Pars distalis vaginae short. Vulva transverse in ventral view (see figures 1 & 2).

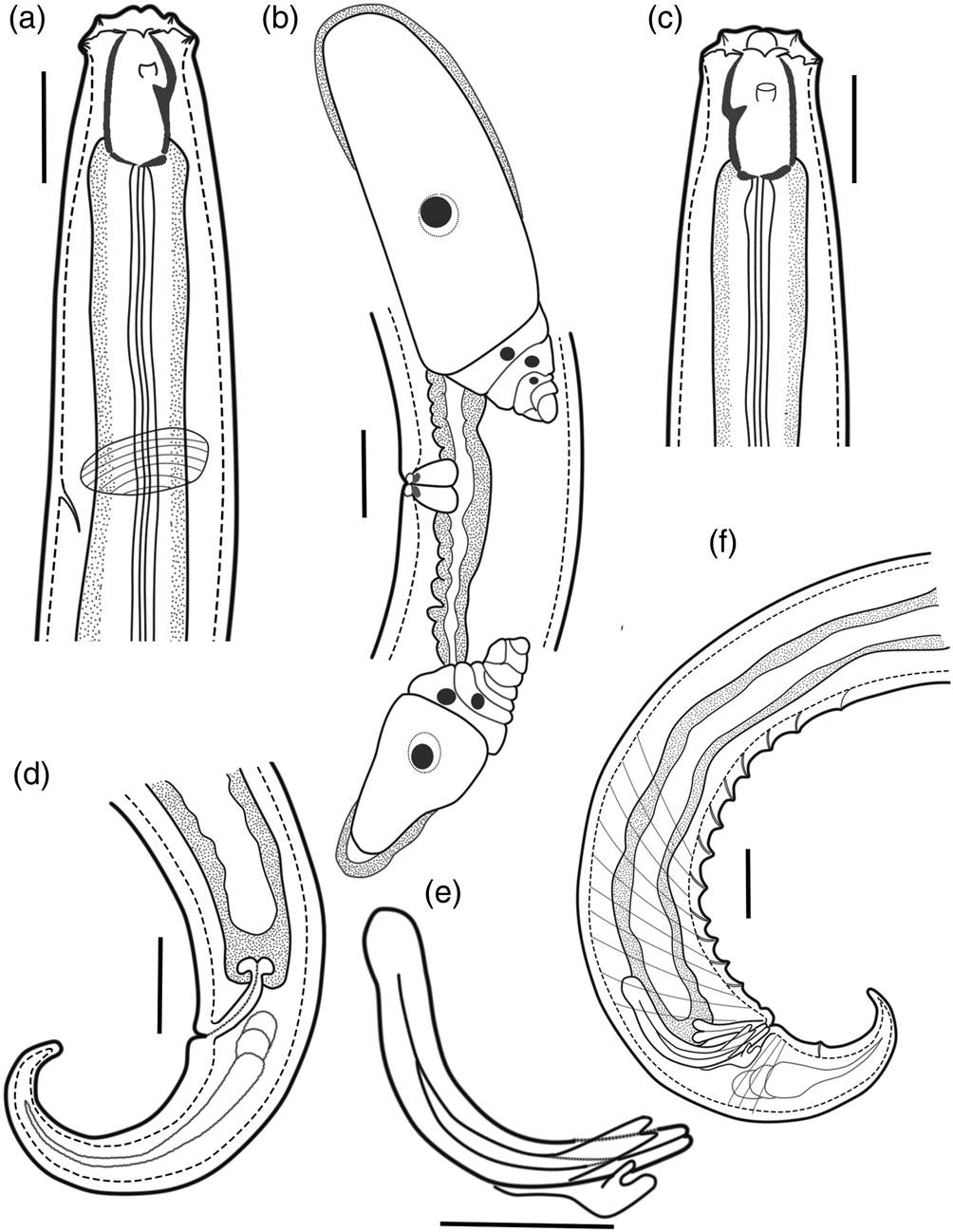
Fig. 1. Holotype female and paratype male of Coomansus batxatensis sp. nov.: (a) female head region; (b) female reproductive system; (c) male head region; (d) female tail; (e) spicule, gubernaculum and accessory piece; (f): male posterior region. Scale bars: 20 μm.

Fig. 2. Holotype female Coomansus batxatensis sp. nov.: (a) holotype female head region; (b) amphidial aperture; (c) reproductive system; (d) vulval region; (e) entire body; (f) tail.
Male. Genital system diorchic, with opposed testes. Spicules are relatively slender, ventrally curved, with bifurcate terminus, 1.7–1.9 times longer than body diameter at cloacal aperture; the head of spicule round-shaped, offset by a shallow depression, conical blade part. Lateral guiding pieces with arched edges and bifurcate terminus, 12–15 μm. This furcation is symmetrical and moderately marked. Gubernaculum is well developed, spatuliform in the terminal, 18–19.5 μm long. Ventromedian supplements 11–12 in number, conical and regularly arranged, occupy 13.6–14.6% of the body length. The anteriormost supplement is situated at 97–114 μm from cloacal aperture. The distance between the last and anteriormost supplement is 85–100 μm. Male tail with two pairs of small subventral papillae (see figures 2 & 3).

Fig. 3. Paratype male Coomansus batxatensis sp. nov.: (a) paratype male head region; (b) posterior region with accessory piece; (c): posterior region with spicule and gubernaculum; (d) entire body; (e) spicule, gubernaculum and accessory piece.
Type habitat and locality. The type population was collected in soil around the roots of forest trees at the Ky Quan San mountain (22°30′22″N, 103°36′52″E, altitude 2150 m) in Sang Ma Sao commune, Bat Xat Nature Reserve, Lao Cai Province, Vietnam. The other population was collected in soil around the roots of forest trees at the Nhieu Co San mountain (22°35′45″N, 103°37′09″E, altitude 2320 m) in Y Ty commune, Bat Xat Nature Reserve, Lao Cai Province, Vietnam.
Type materials. Holotype female and three paratype females from Sang Ma Sao population on slide C. batxatensis sp. nov. no. 1; seven other paratype females and one paratype male from Sang Ma Sao population on slides C. batxatensis sp. nov. nos 2–4. Thirteen females and two males from Y Ty population on slides C. batxatensis sp. nov. nos 5–10. All slides have been deposited in the nematode collection of the Department of Nematology, Institute of Ecology and Biological Resources of VAST, Vietnam.
Etymology. The name of the species refers to its geographical origin from Bat Xat Nature Reserve, Lao Cai Province in Vietnam.
Diagnosis
Coomansus batxatensis sp. nov. is characterized by small adult body size (0.7–0.9 mm), small size of buccal cavity (21–24 × 12–13 μm), rather posterior position of dorsal tooth apex (12.5–15.0) μm from posterior margin of buccal cavity or 59–63% of buccal cavity length, pars refringens vaginae with faint and small (2.5 × 1.7 μm) teardrop-shaped pieces, short pars distalis vaginae, the presence of males with short spicules (50–51.5 μm), rounded head and conical blade.
In general appearance, C. batxatensis sp. nov. is similar to C. parvus (de Man, 1880) Jairajpuri & Khan, 1977, Coomansus arvensis (Eroshenko, Reference Eroshenko1972) Jairajpuri & Khan, 1977, Coomansus ouinnensis Yeates, 1992, Coomansus obliquoris (Eroshenko, Reference Eroshenko1972) Jairajpuri & Khan, 1977, Coomansus indicus Jairajpuri & Khan, 1982 and C. ulsani Choi, Khan & Lee, 1999 based on a small body size less than 1.6 mm (Coetzee, Reference Coetzee1968; Eroshenko, Reference Eroshenko1972; Jairajpuri & Khan, Reference Jairajpuri and Khan1977, Reference Jairajpuri and Khan1982; Popovici, Reference Popovici1990; Yeates, Reference Yeates1992; Choi et al., Reference Choi, Khan and Lee1999; Zullini et al., Reference Zullini, Loof and Bongers2002) but it differs all of them by having presence of males.
Coomansus batxatensis sp. nov. is distinguished from C. parvus by having more posterior dorsal tooth apex (59–63% vs. 62–73%); lower c value (7.6–11.7 vs. 10–19) but higher c′ value (3–4 vs. 2–3). From C. arvensis it differs by having more posterior dorsal tooth apex (59–63% vs. 74–85%); lower c value (7.6–11.7 vs. 14–16) but higher c′ value (3–4 vs. 2–2.5). Coomansus batxatensis sp. nov. is different from C. ouinnensis by having body stouter (a = 17.4–23.3 vs. 22–29), vulva more anterior (V = 59–62.5% vs. 62–64%), more posterior dorsal tooth apex (59–63% vs. 73–78%) and smaller size of buccal cavity (21–24 × 12–13 vs. 25–28 × 13–15) μm.
The new species C. batxatensis sp. nov. is very close to C. obliquoris in having a very short body and in the shape and size of buccal cavity. It is distinguished from C. obliquoris by having a slenderer body (a = 17.4–23.3 vs. 14), lower c value (7.6–11.7 vs. 11–14), more anterior dorsal tooth apex (59–63% vs. 56–58%), weakly sclerotized walls of buccal cavity vs. strongly sclerotized and in the shaped of pieces of pars refringens vaginae (teardrop vs. round). From C. indicus it differs by having shorter body length (0.7–0.9 vs. 1.1–1.4) mm, vulva position more anteriorly (V = 59–63% vs. 70–72%), lower c value (7.6–11.7 vs. 15–19) but higher c′ ratio (3–4 vs. 2–2.6), more anterior position of dorsal tooth apex (59–63% vs. 52–55%) and smaller size of buccal cavity (21–24 × 12–13 vs. 35–40 × 19–23) μm.
The new species C. batxatensis sp. nov. can be distinguished from C. ulsani by having vulva more anterior (V = 59–63% vs. 65–68%), body stouter (a = 17.4–23.3 vs. 23.5–26), lower c value (7.6–11.7 vs. 13.6–14.8) and smaller size of buccal cavity (21–24 × 12–13 vs. 36–39 × 21–22) μm.
Table 2 presents a compendium of Coomansus species morphometrics for comparative purposes.
Table 2. Compendium of species belonging to the genus Coomansus Jairajpuri & Khan, 1977.

Measurements in μm excepted L in mm.
L = body length, a = body length (mk)/maximum body diameter (mk), b = body length (mk)/pharynx length (mk), c = body length (mk)/tail length (mk), c' = tail length (mk)/anal body diameter (mk), V = disance from vulva to the anterior end of body (mk) × 100/body length (mk), B.C.L = buccal cavity length, B.C.W = buccal cavity width, Spicule = length of spicule, Suppl. = ventromedian supplements, DT: dorsal tooth position length from the base of buccal cavity (mk) × 100/buccal cavity length (mk), mk = micrometer.
a Calculated from original illustrations.
Key to species
Currently, 25 species of the genus Coomansus Jairajpuri & Khan, 1977 have been recorded. The following key is based on characteristics of females:
1 Small nematode, L < 1.6 mm 2
• Large nematode, L ≥ 1.6 mm 8
2 V = 70–72 indicus Jairajpuri & Khan, 1977
– V < 70 3
3 Dorsal tooth apex from the base of buccal cavity at >75% 4
– Dorsal tooth apex from the base of buccal cavity at <75% 5
4 Tail 3–3.5 anal body diameter long; buccal cavity = 25–28 × 13–15 μm); ♂: unknown ouinnensis (Yeates, 1992) Andrássy, Reference Andrássy1993
– Tail 2–2.5 anal body diameter long; buccal cavity = 23–24.5 × 12–13 μm; ♂: unknown arvensis (Eroshenko, 1972) Jairajpuri & Khan, 1977
5 Plump body; a = 14; ♂: unknown obliquoris (Eroshenko, 1972) Jairajpuri & Khan, 1977
– Body slenderer; a > 17 6
6 Buccal cavity longer than 30 μm; ♂: unknown ulsani Choi, Khan & Lee, 1999
– Buccal cavity length less than 30 μm 7
7 Tail 3–4 anal body diameter long; small size of buccal cavity: 21–24 × 12–13 μm, c = 7.6–11.7 batxatensis sp. nov.
– Tail 2–3 anal body diameter long; larger size of buccal cavity: 24–29 × 14–16 μm, c = 10–19; ♂: unknown parvus (de Man, 1880) Jairajpuri & Khan, 1977
8 Tail 6–8.5 anal body diameter long 9
– Tail < 6 anal body diameter long 10
9 Larger size of buccal cavity = 50–53 × 26–30 μm; ♂: unknown inca Andrássy, 2011
– Small size of buccal cavity = 32–38 × 20 μm composticola (Clark, 1960c) Jairajpuri & Khan, 1977
10 Dorsal tooth apex from the base of buccal cavity at > 80% 11
– Dorsal tooth apex from the base of buccal cavity at ≤ 80% 14
11 Tail > 4 anal body diameter long mesadeus (Clark, 1960c) Jairajpuri & Khan, 1977
– Tail ≤ 4 anal body diameter long 12
12 a < 28 intestinus (Vinciguerra & La Rosa, 1990) Andrássy, 1993
– Body slenderer; a ≥ 28 13
13 Body length: L > 2.8 mm gerlachei (de Man, 1904) Jairajpuri & Khan, 1977
– Body length: L < 2.8 mm meridionalis Jiménez-Guirado, Wouts & Bell, 1998
14 Dorsal tooth apex from the base of buccal cavity at ≥ 66% 15
– Dorsal tooth apex from the base of buccal cavity at < 66% 22
15 Body length ≤ 3 mm 16
– Body length > 3 mm 19
16 V ≥ 60 17
– V < 60 18
17 Body length : L > 1.8 mm; buccal cavity size = 38–42 × 16– 20 μm venezolanus (Loof, 1964) Jairajpuri & Khan, 1977
– Body length: L ≤ 1.8 mm; smaller size of buccal cavity = 30–31 × 16–17 μm prodontus Shah & Hussain, 2015
18 Larger size of buccal cavity = 45 × 31 μm; dorsal tooth apex from the base of buccal cavity at <75% compbelli (Allgén, 1929) Jairajpuri, 1970a
– Smaller size of buccal cavity = 33–36 × 18–22 μm; dorsal tooth apex from the base of buccal cavity at >75% magellanicus Jiménez-Guirado, Wouts & Bell, 1998
19 Tail length = 3.8–8.0 anal body diameter long; ♂: unknown papua Andrássy, 1993
– Tail length shorter; c' < 3.5 20
20 c' ≤ 2 anal body diameter long kastrolli (Altherr, 1958) Jairajpuri, 1970
– c' > 2 anal body diameter long 21
21 Large buccal cavity size = 70–75 × 39–43 μm fletcherrensis Mulvey, 1978
– Smaller size of buccal cavity = 45–70 × 29–39 μm major (Cobb, 1893) Loof & Winiszewska-Ślipińska, 1993
22 V < 60; body length: L > 2 mm 23
– V < 60; body length: L ≤ 2 mm 24
23 Body length: L ≥ 2.6 mm; c > 14; c' = 3.0–3.7; buccal cavity size =42–44 × 22–23 μm; ♂: unknown splendius Andrássy, 1993
– Body length: L < 2.6 mm; c' = 4.4–5.2; buccal cavity size = 36–39 × 19–21 μm; ♂: unknown mapuche Andrássy, 2011
24 a = 26–34; c' = 2.5–3.0; buccal cavity size = 32–34 × 14–19 μm; ♂: unknown pretoriensis (Coetzee, 1968) Jairajpuri & Khan, 1977
– Body slenderer, a = 40; c' = 3.2; larger size of buccal cavity = 42 × 21 μm; ♂: unknown conoidus Mohilal & Dhanachand, 1997
Phylogenetic analysis
Molecular sequences of three individuals of C. batxatensis sp. nov. were analysed in this study. After sequencing and editing, six sequences were obtained: three 847–919 bp nearly full length of SSU rRNA (small subunit ribosomal ribonucleic acid) (18S), GenBank accession numbers MW525195, MW525196, MW525197 and three 765–798 bp length D2D3 of LSU rRNA (large subunit ribosomal ribonucleic acid) (28S), GenBank accession numbers MW525198, MW525199 and MW525200.
The interspecific nucleotide variation within the acquired 18S rDNA sequences was: C. parvus (accession no. AY284767) vs. C. batxatensis sp. nov. = 0.01. There was no intraspecific variation within the acquired 28S rDNA sequences. The interspecific variation was: C. parvus (accession no. MT705327) vs. C. batxatensis sp. nov. = 0.02.
rDNA phylogenetic relationships among Mononchida
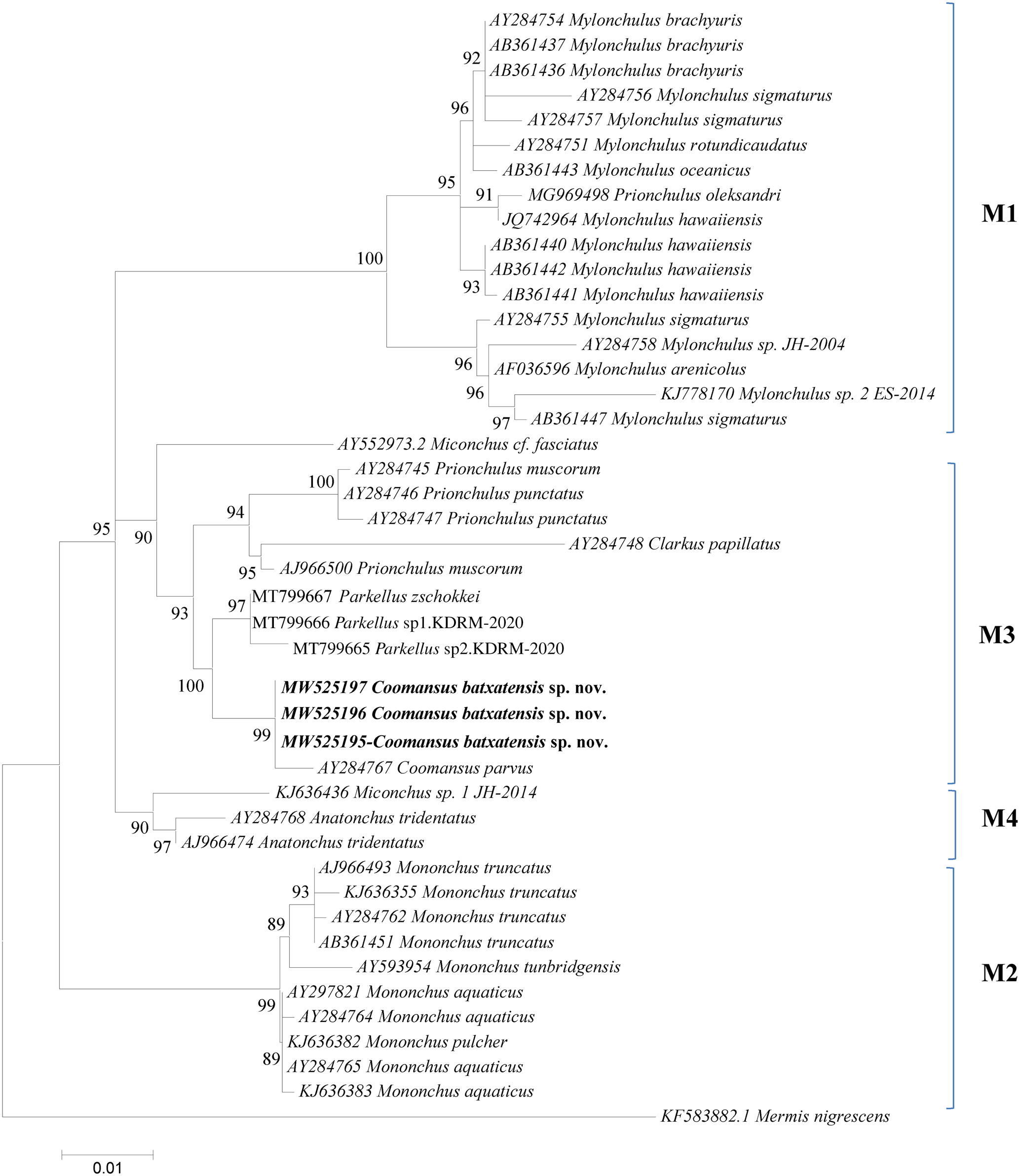
The results derived from the analyses of the 18S and D2–D3 region of 28S sequences are presented in the molecular trees of figs 4 and 5, respectively. In both phylogenetic trees the new species C. batxatensis sp. nov. clustered with the only other species of the genus presented, C. parvus, both species forming a sister group to the genus Parkellus in agreement with Jairajpuri et al. (Reference Jairajpuri, Tahseen and Choi2001) and Ahmad & Jairajpuri (Reference Ahmad and Jairajpuri2010).

Fig. 4. 18S rDNA-based Bayesian phylogeny of the Mononchida. The new Coomansus batxatensis species is indicated in bold. Numbers near nodes indicate posterior probabilities. The scale bar indicates the expected number of substitutions per site.

Fig. 5. D2–D3 region of 28S rDNA-based Bayesian phylogeny of the Mononchida. The new Coomansus batxatensis species is indicated in bold. Numbers near nodes indicate posterior probabilities. The scale bar indicates the expected number of substitutions per site..
The new species C. batxatensis sp. nov. and C. parvus, clustered together in a group, were positioned within the M3 clade (following the nomenclature of Holterman et al., Reference Holterman, Rybarczyk, Evan den Elsen, van Megen, Mooyman, Pena Santiago, Bongers, Bakker and Helder2008; Olia et al., Reference Olia, Ahmad, Araki, Minaka, Oba and Okada2008), encompassing representatives of genera Clarkus, Parkellus and Prionchulus of the Mononchidae family (figs 4 and 5). This positioning was confirmed by phylogenetic analyses based on both the 18S and D2–D3 region of 28S rDNA data.
In conclusion, the validity of C. batxatensis sp. nov. is supported by its morphological and molecular characterization. Based on 18S rDNA and D2–D3 extension region of 28S rDNA sequences, the genus Coomansus appears as a sister group of Parkellus, supporting the validity of the latter genus.
Acknowledgement
The author would like to thank Dr BUI Hong Quang (Institute of Ecology and Biological Resources, VAST) for supporting the collection of these soil specimens.
Financial support
This work was supported by the Vietnam Academy of Science and Technology (grant number VAST04.05/21-22) with the title ‘The role of predatory nematodes Mononchida in the nematode communities at the nature reserves in Lao Cai Province’.
Conflicts of interest
None.
Ethical standards
The author assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of animals.