Introduction
Olfactory dysfunction is an underreported symptom that is highly prevalent in the adult general population (up to 25 per cent reported).Reference Murphy, Schubert, Cruickshanks, Klein, Klein and Nondahl1 The incidence of olfactory dysfunction increases dramatically with age and is extremely common in the elderly.Reference Doty, Shaman, Applebaum, Giberson, Siksorski and Rosenberg2 Olfactory dysfunction is often described as hyposmia (reduced sense of smell), and more severe anosmia (complete loss of the sense of smell). This has a significant impact on patients' quality of life and can lead to serious incidents; for example, a missed gas leak.
Disturbances in olfaction can result from pathological processes at any level along the olfactory pathway. Patients presenting with olfactory dysfunction often represent a diagnostic dilemma. Subsequently, many patients are investigated with imaging of the olfactory tract, usually to rule out structural abnormalities.
Recent studies and reviews have suggested that imaging of the olfactory tract may not be necessary in many patients with olfactory dysfunction and normal examination findings.Reference Powell, Reda ElBadawey and Zammit-Maempel3–Reference Busaba5
This pictorial review aimed to illustrate the most common causes of olfactory dysfunction in clinical practice and enable clinicians to request the most appropriate imaging modality. However, this review is not exhaustive; the causes of olfactory dysfunction are numerous. Close collaboration between the ENT surgeon and radiologist is important in deciding upon further investigations.
Normal radiological anatomy of olfaction
Extracranial anatomy
Specialised olfactory epithelium lines the roof of the nasal cavity, on the extracranial surface of the cribriform plate (Figure 1). This 5 cm2 of epithelium contains the olfactory sensory neurons, the true cranial nerves or fila olfactoria, whose unmyelinated processes traverse the cribriform plate synapsing with the ipsilateral olfactory bulb where the second-order neurones reside. There are approximately 20 fila olfactoria in each nostril.
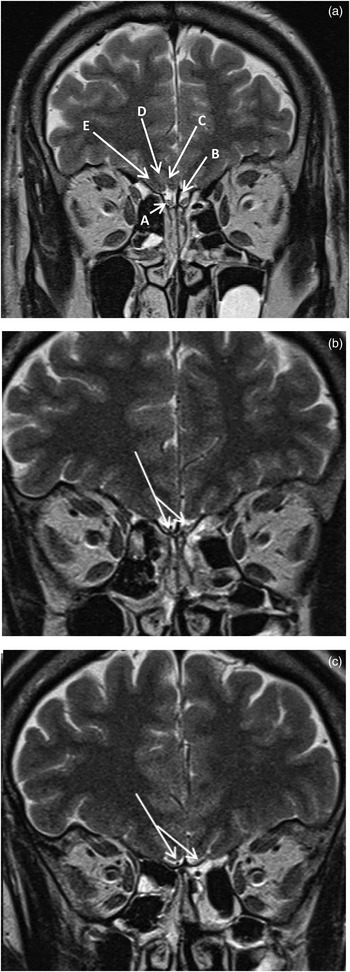
Fig. 1 (a) Coronal T2-weighted magnetic resonance imaging (MRI) scan showing normal olfactory bulbs (‘A’), subfrontal cistern (‘B’), gyrus rectus (‘C’), olfactory sulcus (‘D’) and medial orbital gyrus (‘E’). (b) & (c) Coronal T2-weighted MRI scans showing normal olfactory tracts (arrows).
Intracranial anatomy
Although historically the olfactory bulbs and tracts were referred to as the first cranial nerve, as second-order neurones they are in fact extensions of the brain itself. Coronal magnetic resonance imaging (MRI) in the form of T1-weighted, T2-weighted and fluid-attenuated inversion recovery (‘FLAIR’) sequences depict the olfactory bulbs and tracts. The olfactory bulb is closely apposed to the cribriform plate in the subfrontal cistern (Figure 1). The second-order neurones project posteriorly in the olfactory tracts, which course along the floor of the anterior cranial fossa subjacent to the olfactory sulcus (Figure 1). At the anterior perforated substance, the olfactory tract trifurcates into lateral, medial and intermediate olfactory striae. Most fibres follow the lateral olfactory striae, which terminate in the amygdala. The olfactory tract also has connections with the frontal olfactory cortex, subthalamic nuclei, thalamus and stria medullaris. These tracts are not visualised on conventional MRI techniques.
Causes of olfactory dysfunction demonstrable on imaging
Nasal sinusitis and nasal polyposis
Rhinosinusitis is one of the most common causes of olfactory dysfunction. While it is usually a clinical diagnosis, imaging has an important role to play in the pursuit of acute complications and, more commonly, in the investigation of treatment resistant chronic rhinosinusitis with or without nasal polyps.Reference Higgins and Lane6
The classical computed tomography (CT) appearance of chronic rhinosinusitis is of mucosal thickening and soft tissue opacification of a non-expanded sinus (Figure 2). Computed tomography has an advantage over endoscopy in its ability to detect luminal sinus disease and an advantage over MRI in its ability to demonstrate bony detail well. A further disadvantage of MRI in chronic rhinosinusitis is that as the protein content within sinus secretions approaches 30 per cent, both the T1- and T2-weighted signals decrease considerably, such that the sinus secretions may mimic a normal aerated sinus. This is a major pitfall in the diagnosis of inflammatory disease. With nasal polyps, there are often multiple, variable density polypoid soft tissue masses in the nasal cavity and sinuses. The adjacent bone may be expanded and/or remodelled, which is in contradistinction to chronic rhinosinusitis (Figure 3).
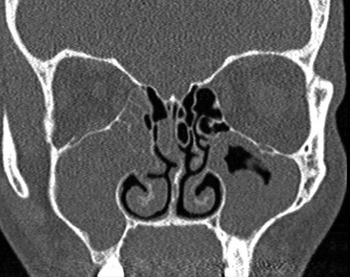
Fig. 2 Coronal computed tomography scan showing opacified right maxillary antrum and moderately marked mucosal thickening in left maxillary antrum in a patient with chronic rhinosinusitis.

Fig. 3 Coronal computed tomography scan showing complete opacification of the paranasal sinuses and nasal cavity, with associated hyperostoses and slight irregularity to the maxillary antral walls.
Head trauma
Although patients with frontal contusions post-trauma often have more immediate problems, olfactory dysfunction is common in survivors.Reference Wise, Moonis and Mirza7 This is due to direct injury to the fila olfactoria from cribriform plate fractures, or injury to the olfactory bulbs or tracts as part of the primary injury of frontobasal contusions. In the acute phase, CT is the imaging modality of choice to identify recent haemorrhage, but following this period, MRI is more appropriate as it demonstrates the integrity of the olfactory apparatus (Figure 4).
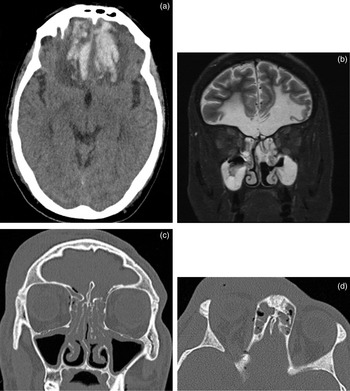
Fig. 4 (a) Axial computed tomography (CT) scan showing intraparenchymal haemorrhage in the inferior frontal lobes bilaterally, with surrounding vasogenic oedema. (b) Coronal short tau inversion recovery magnetic resonance imaging scan showing extensive bifrontal gliosis secondary to trauma, and no identifiable olfactory bulbs or tracts. Also note incidental sinus opacification. (c) Coronal and (d) axial CT scans showing cribriform fracture and pneumocephalus.
Congenital hypoplasia or aplasia
Kallmann syndrome is a genetic condition characterised by delayed or absent puberty and an impaired sense of smell. It is a form of hypogonadotrophic hypogonadism accompanied by the absence of olfactory bulbs and tracts on MRI.Reference Abolmaali, Hietschold, Vogl, Huttenbrink and Hummel8 The olfactory sulcus is also absent, with a single gyrus formed by the gyrus rectus and medial orbital gyrus (Figure 5).

Fig. 5 Coronal (a) T2-weighted and (b) T1-weighted magnetic resonance imaging scans of a patient with Kallmann syndrome, with the characteristic absence of normal olfactory bulbs and tracts. The gyri recti fill the subfrontal cisterns (arrows). Note also the lack of a normal olfactory sulcus.
Tumours
Any tumour involving the roof of the nasal cavity, cribriform plate and inferior frontal lobes may cause olfactory dysfunction (Figure 6). Two types in particular, olfactory neuroblastomas and planum sphenoidale meningiomata, may present with olfactory dysfunction alone.

Fig. 6 Coronal short tau inversion recovery magnetic resonance imaging scan showing a large sinonasal tumour occupying the entire nasal cavity, ethmoidal air cells and left maxillary antrum, with extension extradurally via a destroyed cribriform plate region. The patient had presented with nasal blockage and anosmia. Histology revealed adenocarcinoma.
Olfactory neuroblastomas (aesthesioneuroblastomas) arise from the nasal olfactory epithelium. They have a bimodal distribution, being more common in the second and sixth decades of life. Classically, they are dumbbell-shaped masses with wasting at the cribriform plate, low or intermediate intensity on T1-weighted imaging, and intermediate or hyperintensity on T2-weighted imaging. They enhance avidly and homogeneously (Figure 7).

Fig. 7 (a) Axial T2-weighted magnetic resonance imaging (MRI) scan and (b) coronal T1-weighted, post-contrast MRI scan of a patient with olfactory neuroblastoma, showing an enhancing soft tissue mass destroying the cribriform plate, and filling and expanding the superior nasal cavity, with intracranial extension superiorly (arrows).
Planum sphenoidale meningiomata are tumours more common with increasing age. They are usually isointense or hypointense on T1-weighted imaging, isointense or hyperintense on T2-weighted imaging, and enhance avidly and homogeneously post-contrast (Figure 8). They may be partially calcified on CT.
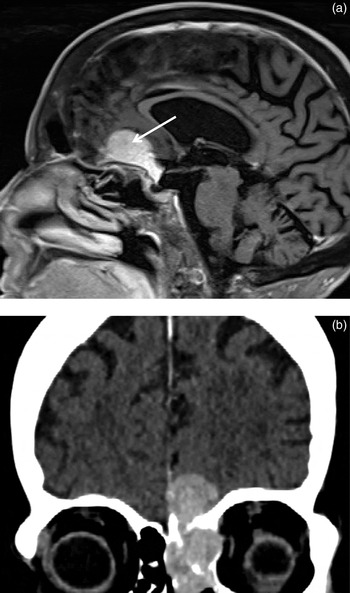
Fig. 8 (a) Sagittal T1-weighted, post-contrast magnetic resonance imaging scan demonstrating a planum sphenoidale meningioma (arrow). (b) Coronal post-intravenous contrast computed tomography scan showing histologically proven meningioma with nasal and extradural components.
Neurodegenerative diseases
There is an association between olfactory dysfunction and several neurodegenerative diseases,Reference Bitter, Gudziol, Burmeister, Mentzel, Guntinas-Lichius and Gaser9, Reference Peng, Gu, Xiao, Si, Wang and Wang10 most notably Parkinson's. Crucially, the development of olfactory dysfunction may precede the onset of other neurological symptoms by several years.
Magnetic resonance imaging is the first investigation of choice in cases where the clinicians are suspicious of a neurological cause of olfactory dysfunction. However, in elderly patients with tremor, in whom imaging fails to reveal a structural cause, and where the diagnosis is uncertain, other imaging modalities such as DaTscan™ can be considered.
DaTscan is a nuclear medicine scan that uses small amounts of a radioactive drug, ioflupane iodine 123. This is injected intravenously to assess dopamine-containing neurones involved in controlling movement. A special detector in the form of a gamma camera then measures the amount and location of the drug in the brain. This reflects the amount of healthy dopamine neurones in the basal ganglia. The DaTscan is primarily designed to differentiate Parkinsonian syndrome from essential tremor. However, it cannot differentiate Parkinson's disease, the commonest form of Parkinsonian syndrome, from other diseases that have similar symptoms, such as multiple system atrophy or progressive supranuclear palsy. Classically in Parkinsonian syndrome, cell loss occurs initially in the posterior aspect of one side of the basal ganglia and this manifests itself as dark areas on the DaTscan (Figure 9).

Fig. 9 DaTscan demonstrating absent tracer uptake in the putamina and decreased uptake in the caudate heads, more marked on the right, in a patient with Parkinson's disease.
Conclusion
Olfactory dysfunction is a cause of morbidity in its own right, but can also be the first symptom of a number of potentially serious intracranial pathologies. We have reviewed the normal anatomy and potentially identifiable causes of olfactory dysfunction detectable on radiological imaging. We have also highlighted the need for an appropriate choice of scan technique, depending on the clinical question, and describe what the abnormalities would look like with these modalities.











