Adverse childhood experiences (ACEs), such as abuse, neglect, and family dysfunction, are associated with a myriad of poor physical and mental health outcomes in adults (Anda et al., Reference Anda, Felitti, Bremner, Walker, Whitfield, Perry and Giles2006; Dong et al., Reference Dong, Giles, Felitti, Dube, Williams, Chapman and Anda2004; Li, D'Arcy, & Meng, Reference Li, D'Arcy and Meng2016; Lindert et al., Reference Lindert, von Ehrenstein, Grashow, Gal, Braehler and Weisskopf2014) and also with higher rates of internalizing and externalizing behavior problems in their offspring (Min, Singer, Minnes, Kim, & Short, Reference Min, Singer, Minnes, Kim and Short2012; Miranda, Granero, & Ezpeleta, Reference Miranda, Granero and Ezpeleta2013; Plant, Jones, Pariante, & Pawlby, Reference Plant, Jones, Pariante and Pawlby2017; Rijlaarsdam et al., Reference Rijlaarsdam, Stevens, Jansen, Ringoot, Jaddoe, Hofman and Tiemeier2014). Behavior problems in childhood are risk factors for future psychopathology (Mesman & Koot, Reference Mesman and Koot2001), substance use (King, Iacono, & McGue, Reference King, Iacono and McGue2004), and disability into adulthood (Narusyte, Ropponen, Alexanderson, & Svedberg, Reference Narusyte, Ropponen, Alexanderson and Svedberg2017). To date, the majority of studies that have evaluated the intergenerational transmission of parental ACEs to child emotional and behavioral problems have focused on postnatal environmental risk factors including parental psychopathology, parenting practices, and offspring maltreatment (Miranda et al., Reference Miranda, Granero and Ezpeleta2013; Plant, Barker, Waters, Pawlby, & Pariante, Reference Plant, Barker, Waters, Pawlby and Pariante2013; Plant et al., Reference Plant, Jones, Pariante and Pawlby2017). A growing area of research that is informed by the developmental origins of health and disease (DOHaD) hypothesis postulates that prenatal biological factors might also play a role. For example, ACEs are associated with alterations in maternal stress biomarkers (e.g., cortisol, the product of the hypothalamic–pituitary-adrenal (HPA) axis; Thomas, Magel, et al., Reference Thomas, Magel, Tomfohr-Madsen, Madigan, Letourneau, Campbell and Giesbrecht2018), and prenatal exposure to cortisol affects child development (Buss et al., Reference Buss, Davis, Shahbaba, Pruessner, Head and Sandman2012). Accordingly, cortisol may operate as a mechanism that transmits the effects of ACEs to child development and/or an effect modifier that alters the strength and/or direction of the association between maternal exposure to ACEs and child development. The plausibility of a biological pathway through cortisol is supported by the ability of cortisol to cross both the placenta and the blood–brain barrier and thereby directly affect fetal brain development and increase the risk of cognitive and mental disorders in childhood (Lupien, McEwen, Gunnar, & Heim, Reference Lupien, McEwen, Gunnar and Heim2009; Moisiadis & Matthews, Reference Moisiadis and Matthews2014). To our knowledge, no study has evaluated the mediating or moderating effects of prenatal HPA-axis function on the association between maternal ACEs and child emotional and behavioral problems. Therefore, the purpose of the current study was to evaluate the role of prenatal maternal cortisol in the intergenerational transmission of maternal ACEs to child internalizing and externalizing problems at 4 years of age.
Adverse childhood experiences are prevalent in the general population. A large observational study of over 17,000 adults found that 64% of the participating individuals reported at least one ACE and 12.5% reported four or more ACEs (Anda et al., Reference Anda, Felitti, Bremner, Walker, Whitfield, Perry and Giles2006). This study also identified a strong, graded relationship between ACEs exposure and risk for psychopathology. For individuals reporting ≥ 4 ACEs, the risk of panic reactions, depressed affect, and anxiety increased 2.5-, 3.6-, and 2.4-fold, respectively. Beyond the risk for poor behavioral and health outcomes in individuals who were directly exposed to ACEs, there is now growing evidence that some of these risks extend to individuals that are not directly exposed, for example, to the offspring of the exposed individuals. For instance, large observational studies of more than 4,500 families observed that maternal childhood maltreatment history was associated with child internalizing and externalizing problems at 6 years of age (Rijlaarsdam et al., Reference Rijlaarsdam, Stevens, Jansen, Ringoot, Jaddoe, Hofman and Tiemeier2014) and in preadolescence (Plant et al., Reference Plant, Jones, Pariante and Pawlby2017). These findings suggest that maternal ACEs may have a lingering effect on the next generation by increasing risk for psychopathology.
An objective of studies that have examined the intergenerational transmission of stress has been to elucidate the pathways by which maternal childhood adversity affects offspring emotional and behavioral outcomes, with a primary focus on postnatal environmental factors. The postnatal environment is a key developmental context for children, and it is vulnerable to the influence of parents’ own experiences of early life adversity. The associations between ACEs and poor mental health outcomes including depression, anxiety, and psychosis are well documented in a number of recent meta-analyses (Li et al., Reference Li, D'Arcy and Meng2016; Lindert et al., Reference Lindert, von Ehrenstein, Grashow, Gal, Braehler and Weisskopf2014; Varese et al., Reference Varese, Smeets, Drukker, Lieverse, Lataster, Viechtbauer and Bentall2012), and ACEs have been shown to account for approximately 30% of mental health disorders worldwide (Kessler et al., Reference Kessler, McLaughlin, Green, Gruber, Sampson, Zaslavsky and Williams2010). The negative psychological consequences of ACEs that persist into adulthood are thought to interfere with optimal parenting and parent–child relationships, and these environmental pathways have been the primary focus of studies that have investigated the links that explain the intergenerational transmission of ACEs. Several studies have demonstrated that the association between maternal ACEs and child emotional and behavioral difficulties is mediated by maternal psychological distress (Min et al., Reference Min, Singer, Minnes, Kim and Short2012; Roberts, O'Connor, Dunn, & Golding, Reference Roberts, O'Connor, Dunn and Golding2004), particularly depression (Letourneau et al., Reference Letourneau, Dewey, Kaplan, Ntanda, Novick and Thomas2018; Miranda et al., Reference Miranda, Granero and Ezpeleta2013; Plant et al., Reference Plant, Jones, Pariante and Pawlby2017). Interestingly, these studies have also highlighted the unique and important contributions of prenatal depression in the pathways that link maternal ACEs to child psychopathology (Letourneau et al., Reference Letourneau, Dewey, Kaplan, Ntanda, Novick and Thomas2018; Pawlby, Hay, Sharp, Waters, & Pariante, Reference Pawlby, Hay, Sharp, Waters and Pariante2011; Plant et al., Reference Plant, Barker, Waters, Pawlby and Pariante2013, Reference Plant, Jones, Pariante and Pawlby2017; Plant, Pariante, Sharp, & Pawlby, Reference Plant, Pariante, Sharp and Pawlby2015). For example, in a study of 9,397 mother–child dyads, Plant et al. (Reference Plant, Jones, Pariante and Pawlby2017) demonstrated that prenatal depression mediated the association between maternal childhood maltreatment and offspring behavior problems even after accounting for the effects of postpartum depression, which the authors posited might be explained in part by fetal programming during pregnancy.
The DOHaD hypothesis, referred to above, is also known as the “fetal programming” hypothesis because of the proposed alterations in physiological, neuroendocrine, and/or metabolic development that result from exposure to environmental stress in utero (Kwon & Kim, Reference Kwon and Kim2017). The DOHaD field was born out of observations from large epidemiological studies that have demonstrated associations between low birth weight (a proxy for poor fetal growth and malnutrition) and adult health outcomes including coronary heart disease, hypertension, obesity, and type II diabetes mellitus (Barker, Reference Barker1995, Reference Barker1998; Barker, Osmond, Kajantie, & Eriksson, Reference Barker, Osmond, Kajantie and Eriksson2009; Hales & Barker, Reference Hales and Barker1992). Barker (Reference Barker1995) proposed that these alterations, which enable the fetus to adapt to its intrauterine environment, result in permanent programming in tissues and organ systems with the potential for pathological consequences in adulthood. Subsequent to the pioneering work of Barker et al., which primarily focused on the effects of prenatal undernutrition, researchers expanded their lens to consider the potential for other environmental factors, such as experiences of psychological stress, to “program” birth and developmental outcomes.
Maternal ACEs are associated with alterations in the intrauterine environment, and these changes may confer risk for alterations in fetal development (Gustafsson, Doyle, Gilchrist, Werner, & Monk, Reference Gustafsson, Doyle, Gilchrist, Werner and Monk2017; Sancho-Rossignol et al., Reference Sancho-Rossignol, Schilliger, Cordero, Serpa, Epiney, Hüppi and Schechter2018). During pregnancy, mother and fetus communicate via the placenta and psychological stress may be transmitted to the fetus via placental exchange of stress hormones. Alterations in maternal HPA-axis function and elevated levels of its end product, cortisol, are thought to be one of the primary biological mediators of the effects of prenatal stress and child outcomes (Huizink, Mulder, & Buitelaar, Reference Huizink, Mulder and Buitelaar2004; Talge, Neal, & Glover, Reference Talge, Neal and Glover2007). Similar to evidence from studies that evaluate current experiences of stress during pregnancy, there is evidence that past experiences of childhood adversity may leave a “signature” of stress within the HPA axis, both within the individuals who directly experienced them or intergenerationally. In individuals, for instance, studies have shown that ACEs are associated with reduced waking levels of cortisol (Shea et al., Reference Shea, Streiner, Fleming, Kamath, Broad and Steiner2007), a higher cortisol awakening response (CAR; Bublitz & Stroud, Reference Bublitz and Stroud2012; Thomas, Magel, et al., Reference Thomas, Magel, Tomfohr-Madsen, Madigan, Letourneau, Campbell and Giesbrecht2018), and a flatter diurnal cortisol slope (Thomas, Magel, et al., Reference Thomas, Magel, Tomfohr-Madsen, Madigan, Letourneau, Campbell and Giesbrecht2018). Intergenerationally, prenatal cortisol exposure may alter fetal development through tissue remodeling or by epigenetic DNA modification, whereby cortisol exposure alters the structure and function of the rapidly developing fetal physiological systems to result in permanent changes in infant neurophysiology (Sandman, Davis, Buss, & Glynn, Reference Sandman, Davis, Buss and Glynn2011), which in turn may increase risk for behavior problems. Indeed, human studies have demonstrated that exposure to elevated maternal cortisol during pregnancy is associated with greater behavioral reactivity and negative emotionality in infancy (Braithwaite et al., Reference Braithwaite, Pickles, Sharp, Glover, O'Donnell, Tibu and Hill2017; Davis et al., Reference Davis, Glynn, Dunkel Schetter, Hobel, Chicz-DeMet and Sandman2007; de Weerth, van Hees, & Buitelaar, Reference de Weerth, van Hees and Buitelaar2003), affective problems in childhood (Buss et al., Reference Buss, Davis, Shahbaba, Pruessner, Head and Sandman2012), and increased risk for anxiety in preadolescence (Davis & Sandman, Reference Davis and Sandman2012). Taken together with the observation that ACEs are associated with changes in HPA-axis function during pregnancy (Bublitz, Parade, & Stroud, Reference Bublitz, Parade and Stroud2014; Bublitz & Stroud, Reference Bublitz and Stroud2012, Reference Bublitz and Stroud2013; Shea et al., Reference Shea, Streiner, Fleming, Kamath, Broad and Steiner2007; Thomas, Magel, et al., Reference Thomas, Magel, Tomfohr-Madsen, Madigan, Letourneau, Campbell and Giesbrecht2018), these findings suggest that prenatal cortisol is a plausible biological mediator in the intergenerational transmission of maternal ACEs to child internalizing and externalizing problems.
In addition to maternal ACEs leading to alterations in the HPA axis and prenatal alterations in the HPA axis leading to more child behavior problems (mediation), the strength of the association between maternal ACEs and child behavior problems may change as a function of prenatal cortisol exposure (moderation). The effect sizes that have been reported for direct and indirect associations between maternal ACEs and offspring behavior problems are modest, which is consistent with the proposal that the effects of ACEs on child outcomes operate through multiple pathways (Madigan, Wade, Plamondon, Maguire, & Jenkins, Reference Madigan, Wade, Plamondon, Maguire and Jenkins2017; Plant et al., Reference Plant, Jones, Pariante and Pawlby2017). The fact that some but not all offspring of mothers that are exposed to ACEs develop behavior problems, or alternately, that the severity of behavior problems that are displayed by the offspring of exposed mothers varies greatly, suggests that effect modifiers may be operating. That is, the current evidence leads us to hypothesize that the strength of the association between maternal ACEs and offspring behavior problems is moderated by other factors, specifically by prenatal cortisol. The plausibility of this hypothesis is supported by two observations. First, there may be threshold or cumulative exposure effects by which lesser exposure to ACEs result in essentially no correlation between ACEs and the factors that contribute to offspring behavior problems, whereas greater exposures result in strong associations (Madigan et al., Reference Madigan, Wade, Plamondon, Maguire and Jenkins2017). Second, factors such as supportive social relationships can buffer the effects of ACEs on the maternal HPA axis (Thomas, Letourneau, Campbell, & Giesbrecht, Reference Thomas, Letourneau, Campbell and Giesbrecht2018), which implies that ACEs do not have unitary effects on the HPA axis. Third, factors such as social buffering result in both between-person and within-person variability in the HPA-axis response to ACEs (Thomas, Magel, et al., Reference Thomas, Magel, Tomfohr-Madsen, Madigan, Letourneau, Campbell and Giesbrecht2018), which may in turn result in variability in offspring behavior. Taken together, these factors suggest that ACEs may have a variety of effects on the maternal HPA axis, with the potential for this array of effects to cascade to child behavior. Moderator analyses allow for the identification of potential interaction effects between maternal ACEs and prenatal cortisol exposure on child behavioral outcomes. To the best of our knowledge, no studies have evaluated the potential mediating and/or moderating role of prenatal HPA-axis function in explaining the association between maternal ACEs and child behavioral problems.
A review of the fetal programming literature and the field of developmental psychopathology (more generally) suggest that there may be sex differences that need to be considered in examining the intergenerational transmission of ACEs. With respect to developmental psychopathology, females are at higher risk for developing internalizing problems, whereas males are at increased risk of externalizing problems (Crick & Zahn-Waxler, Reference Crick and Zahn-Waxler2003). Within the fetal programming literature, there is also strong evidence of sexually dimorphic effects of prenatal stress and cortisol exposures on child development (Sandman, Glynn, & Davis, Reference Sandman, Glynn and Davis2013). Specifically, Braithwaite et al. (Reference Braithwaite, Pickles, Sharp, Glover, O'Donnell, Tibu and Hill2017) observed that higher maternal prenatal cortisol levels at 32 weeks gestation was associated with more negative emotionality in female infants at 5 weeks of age but less negative emotionality in male infants. Another study of 907 mother–child dyads demonstrated that a maternal history of ACEs was indirectly associated with internalizing and externalizing problems in children at 2 years of age via increases in perinatal anxiety and depression. However, the intergenerational effects of ACEs on child behavior problems were more pronounced in male children (Letourneau et al., Reference Letourneau, Dewey, Kaplan, Ntanda, Novick and Thomas2018). These and many other well-documented sex differences (Bale, Reference Bale2011; Giesbrecht, Letourneau, & Campbell, Reference Giesbrecht, Letourneau and Campbell2017; Maxwell, Fineberg, Drabick, Murphy, & Ellman, Reference Maxwell, Fineberg, Drabick, Murphy and Ellman2018) indicate that prenatal exposure to maternal ACEs and cortisol may have sexually dimorphic effects on child risk for behavior problems.
Current Study
Although previous studies have evaluated prenatal depression and/or anxiety as predictors of the associations between maternal ACEs and child psychopathology (Letourneau et al., Reference Letourneau, Dewey, Kaplan, Ntanda, Novick and Thomas2018; Pawlby et al., Reference Pawlby, Hay, Sharp, Waters and Pariante2011; Plant et al., Reference Plant, Pariante, Sharp and Pawlby2015, Reference Plant, Jones, Pariante and Pawlby2017), to our knowledge no previous studies have evaluated the role of prenatal maternal HPA-axis function to explain these associations. A recent commentary highlighted the need for “longitudinally collected, reliable, and noninvasive biological markers of stress” to establish support for the causal inferences that are implied by models that have tested the intergenerational transmission of stress (Gelaye & Koenen, Reference Gelaye and Koenen2018, p. 92). The present study addressed the need for such research by examining the biological pathways that link maternal ACEs to offspring psychopathology. Specifically, we examined the potential mediating and/or moderating role of maternal prenatal HPA-axis function on the association between maternal ACEs history and child behavioral problems.
The first objective was to evaluate whether the associations between maternal ACEs and child internalizing and externalizing problems at 4 years of age were mediated by maternal HPA-axis function during pregnancy. Building on our previous work, which demonstrated that maternal ACEs are associated with alterations in the maternal CAR and the diurnal cortisol slope during pregnancy (Thomas, Magel, et al., Reference Thomas, Magel, Tomfohr-Madsen, Madigan, Letourneau, Campbell and Giesbrecht2018), we hypothesized that these alterations in HPA-axis function would be associated with greater internalizing and externalizing problems in children. The second objective was to evaluate whether prenatal HPA-axis function moderated the association between maternal ACEs and child behavior problems at 4 years of age. We hypothesized that prenatal cortisol exposure would interact with maternal ACEs history to increase the risk of child behavior problems. There is evidence that suggests sex differences in the association between maternal ACEs and prenatal cortisol and child behavior problems. Therefore, a third objective was to test whether sex moderates the association between prenatal cortisol exposure and child behavior problems and to evaluate the possibility of sex differences in the first and second objectives that were tested. We hypothesized that maternal ACEs and prenatal cortisol exposure would be associated with more internalizing problems in female children and more externalizing problems in male children. Given the potential for timing effects of prenatal cortisol exposure on fetal development, these objectives were investigated separately at 6–26 weeks gestation and 27–37 weeks gestation. To test these hypotheses, maternal ACEs and HPA-axis function were assessed during pregnancy and child internalizing and externalizing behavior problems were assessed at 4 years of age.
Method
Participants
The participants were a subsample of women who enrolled in an ongoing prospective cohort study, the Alberta Pregnancy Outcomes and Nutrition (APrON) study, which is a community sample of volunteers that were recruited from prenatal clinics between 2009 and 2012 (Kaplan et al., Reference Kaplan, Giesbrecht, Leung, Field, Dewey and Bell2014). Women were included if they had a singleton pregnancy, were less than 27 weeks of gestation at the first study visit, and were 18 years of age or older. Women were excluded if they smoked or consumed alcohol during pregnancy, were being treated with a synthetic glucocorticoid, or had known fetal complications at time of study entry. The study measurement points were as follows: Time 1 (T1; 6–26 weeks gestation, mean = 15.4 weeks±3.5); Time 2 (T2; 27–37 weeks gestation, mean = 32.4 weeks±1.1); and Time 3 (T3 = 4 years postpartum, mean = 4.3 years±0.54). Gestational age (GA) at each measurement point in pregnancy was determined based on the last reported menstrual period and confirmed by at least one ultrasound. As is typical for longitudinal studies, there was participant attrition over the course of the study. Of the 356 women in the initial study sample, 6 participants dropped out, 12 were not suitable to test (i.e., aged out), 22 declined to participate, 64 were unable to be reached or moved, and 4 did not complete the child behavior problems questionnaire. Thus, the final study sample was comprised of 248 dyads that completed at least one diurnal cortisol measurement during pregnancy and the final assessment at T3. With respect to attrition, the participants differed in terms of maternal age, F (1, 354) = 12.3, p = .001, with women that completed the study being older (M = 32.3, SD = 3.7) than those who dropped out (M = 30.8, SD = 3.6). The participants did not differ on any other demographic variable including marital status, education, income, parity, ethnicity, or any of the primary study variables (all ps > .05).
Descriptive information for the final study sample is shown in Table 1. The study sample represents a relatively low-sociodemographic-risk population of women and infants, as the majority were mature, married or in common-law relationships, White, had university-level education, and had middle to upper-middle class household annual income. Fifteen infants included in the study were identified as being born preterm (i.e., < 37 weeks GA). Although the study sample underrepresents young (i.e., under 20) and low-income pregnant women, as compared with a nationally representative sample (Leung, McDonald, Kaplan, Giesbrecht, & Tough, Reference Leung, McDonald, Kaplan, Giesbrecht and Tough2013; Public Health Agency of Canada Team, 2009), the sample is largely consistent with the sociodemographics of families that are living within the recruitment region (Calgary, Canada) and may be comparable to low-sociodemographic-risk families that are living in Canada and other high-income countries.
Table 1. Sociodemographic characteristics for the study sample (n=248)

Note. SES = socioeconomic status; ACEs = adverse childhood experiences.
Data Collection
Maternal history of ACEs was assessed by using a retrospective self-report measure of adverse experiences prior to the age of 18. Maternal self-reported depression was assessed during pregnancy (T1 and T2) by using a standardized self-report questionnaire. Prenatal maternal cortisol was assessed from saliva samples that were collected by the mothers over multiple days within each measurement point (excluding weekends to rule out potential weekend–weekday differences in stress and diurnal cortisol; Schlotz, Hellhammer, Schulz, & Stone, Reference Schlotz, Hellhammer, Schulz and Stone2004). The women self-collected saliva samples on two consecutive days at two measurement points during pregnancy (T1 and T2). During the first study visit, the participants were instructed on the use of a personal digital assistant (PDA), which was used to facilitate saliva collection at waking, 30 min after waking, at 1100 hours, and at 2100 hours. The timing of each assessment was recorded by the PDA, permitting precise modeling of diurnal patterns. The participants were asked to refrain from consuming food, caffeine, citric drinks, and dairy and to avoid vigorous exercise or brushing teeth in the 30 min prior to saliva collection and to report adherence to these guidelines via questions that were administered by the PDA after each saliva collection. The responses to the adherence questions on the PDA were used to evaluate whether nonadherence was associated with cortisol levels. The analysis revealed that adherence to the protocol was excellent (85% of samples) and nonadherence was not associated with maternal cortisol levels. Accordingly, adherence indicators were not included in the models as covariates. At the 4-year postnatal assessment (T3), the mothers completed a standardized parent report of child behavior. Prior to data collection, the participants provided informed consent to the procedures, which were approved by the University of Calgary Conjoint Health Research Ethics Board.
Measures
Maternal history of ACEs
The Adverse Childhood Experiences (ACEs) questionnaire consists of 10 yes or no questions (Felitti et al., Reference Felitti, Anda, Nordenberg, Williamson, Spitz, Edwards and Marks1998) that assess early life adversity in three domains: abuse (emotional, physical, and sexual), neglect (physical and emotional), and household challenges (mother treated violently, substance abuse, mental illness, parental separation/divorce, and incarcerated household member). The ACEs questionnaire is a widely used measure that has demonstrated good reliability and internal consistency (α = .81; Bruskas & Tessin, Reference Bruskas and Tessin2013) as well as adequate test-retest reliability (weighted κ = .64; Dube, Williamson, Thompson, Felitti, & Anda, Reference Dube, Williamson, Thompson, Felitti and Anda2004). In the current study, the occurrence of individual ACEs was summed to create the ACE scores (range = 0–9). Because scores of 4 or more occurred infrequently, and in keeping with previous methods (e.g., Anda et al., Reference Anda, Felitti, Bremner, Walker, Whitfield, Perry and Giles2006), scores above 4 were recoded to a value of 4 so that the final ACEs scores ranged from 0 to 4.
Prenatal maternal HPA-axis function
After self-collection according to the schedule described above, the saliva samples were stored in home freezers (1–3 days) until they could be shipped on freezer packs to the lab, where they were stored at −80°C at the university laboratory until they were shipped frozen to Salimetrics, State College, PA, for assay. All of the maternal samples were assayed for salivary cortisol by using the Salimetrics enzyme immunoassay. It has a lower limit of sensitivity of 0.007 μg/dL, standard curve range from 0.012 to 3.0 μg/dL, and average intra- and interassay coefficients of variation 3.5% and 5.1%, respectively. The method accuracies, which were determined by serial dilution, were 100.8% and 91.7%, respectively. A random 25% of the samples were assayed in duplicate to confirm reliability, and the intra-assay coefficient of variation was 4.2%. The mean values from duplicate samples were used for analysis. Several HPA-axis parameters were calculated from the saliva samples as indicators of HPA-axis function. The CAR was calculated by using the trapezoid method for area under the curve increase (AUCi) as a measure of the morning increase in cortisol (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, Reference Pruessner, Kirschbaum, Meinlschmid and Hellhammer2003). To ensure that our measure of the CAR was valid, samples were excluded if they were taken more than 15 min after waking (for the waking sample) or more than 50 min after waking for the second sample (Okun et al., Reference Okun, Krafty, Buysse, Monk, Reynolds, Begley and Hall2010), resulting in a total of 14 CAR samples (1 from T1 and 13 from T2) that were not available for data analysis. Because the area under the curve increase is dependent on the amount of time between baseline and the +30-min sample, CAR was standardized to reflect 30 min of output in order to account for individual differences in the time between the waking and waking +30-min samples. Total cortisol secretion over the day was estimated as area under the curve with respect to ground (AUCg; Pruessner et al., Reference Pruessner, Kirschbaum, Meinlschmid and Hellhammer2003). As with the AUCi, AUCg is sensitive to the total amount of time between the waking and bedtime samples, and because individuals had different amounts of time between these samples, AUCg was standardized to the average time between waking and 2100-hours samples (853 min). The diurnal cortisol slope was calculated as 2100 hours - waking / time (in hours) to describe the decline in cortisol concentration across the day (Fekedulegn et al., Reference Fekedulegn, Andrew, Burchfiel, Violanti, Hartley, Charles and Miller2007). The descriptive statistics for the cortisol data are provided in Supplementary Table 1.
Child behavioral problems
The mothers reported on child behavior at 4 years of by age using the Parent Rating Scale–Preschool (PRS-Preschool) on the Behavior Assessment System for Children–Second Edition (BASC-2; Reynolds & Kamphaus, Reference Reynolds and Kamphaus2004), which is a multidimensional behavioral measure for children aged 2 to 5. The PRS-Preschool consists of 134 phrases that describe positive and negative behaviors (e.g., “Adjusts easily to new surroundings”) and assesses behavior frequency on a 4-point Likert-type scale ranging from (0) Never to (3) Almost Always. The BASC-2 is a standardized, norm-referenced measure that provides T-scores (i.e., standard scores with M = 50, SD = 10) for each scale, reflecting level of adjustment. For the current study, we were interested in the T-scores from the Internalizing and Externalizing Problems composites. The Internalizing Problems composite is comprised of the anxiety, depression, and somatization subscales. The Externalizing Problems composite on the preschool version is comprised of the hyperactivity and aggression subscales. The BASC-2 scales and composites demonstrate good psychometric properties, with reliability alpha coefficients of the internalizing and externalizing scales for the preschool form being .85 and .87, respectively (Reynolds & Kamphaus, Reference Reynolds and Kamphaus2004).
Covariates
Gestational age at each prenatal assessment was included to account for individual differences in the exact timing of the prenatal assessments. Additionally, we included waking cortisol at each measurement point as a covariate in the analyses to account for initial daytime cortisol levels and to detect relative changes in cortisol level throughout the day. In order to rule out other potential confounders as the basis for any observed effects, additional covariates were investigated for inclusion in the analyses by conducting bivariate correlations with the primary study variables. Sociodemographic characteristics (i.e., socioeconomic status [SES], maternal age) were self-reported and investigated as potential covariates given that they have previously been associated with ACEs, maternal cortisol, and child behavior problems (Bøe et al., Reference Bøe, Sivertsen, Heiervang, Goodman, Lundervold and Hysing2014; Cohen, Doyle, & Baum, Reference Cohen, Doyle and Baum2006; Madigan, Wade, Tarabulsy, Jenkins, & Shouldice, Reference Madigan, Wade, Tarabulsy, Jenkins and Shouldice2014; Metzler, Merrick, Klevens, Ports, & Ford, Reference Metzler, Merrick, Klevens, Ports and Ford2017). We also investigated maternal prenatal and postpartum psychological distress, stressful life events, and birth outcome factors (birthweight and gestational age at birth) given their associations with maternal ACEs and child behavior problems (Arpi & Ferrari, Reference Arpi and Ferrari2013; Christiaens, Hegadoren, & Olson, Reference Christiaens, Hegadoren and Olson2015; Miller et al., Reference Miller, Culhane, Grobman, Simhan, Williamson, Adam and Borders2017; Plant et al., Reference Plant, Barker, Waters, Pawlby and Pariante2013, Reference Plant, Jones, Pariante and Pawlby2017; Smith, Gotman, & Yonkers, Reference Smith, Gotman and Yonkers2016). Maternal age and birthweight were not associated with any of the primary study variables, all ps > .05, and were therefore not included as covariates.
Socioeconomic status
A composite SES variable was created by summing the z-scores of self-reported family income (1 = < $20,000/year, 2 = $20,000–$39,999, 3 = $40,000–$69,000, 4 = $70,000–$99,999, 5 = ≥ $100,000), maternal education (1 = Less than high school, 2 = High school, 3 = Trade or technical school, 4 = Undergraduate, 5 = Graduate degree), and ethnicity (0 = non-White, 1 = White). The distribution of the composite SES scores for the study sample were negative skewed, so the scores were reflected and log transformed, as is recommended (Tabachnick & Fidell, Reference Tabachnick and Fidell2012). Final composite SES scores were then mean-centered with higher values indicating greater sociodemographic risk (i.e., lower annual income, less education, non-White). Socioeconomic status was included as a covariate because it was associated with maternal ACEs, r (246) = .154, p = .015.
Stressful life events
The Stressful Life Events Questionnaire (SLEQ) is a measure of exposure to stressful life events, adapted from (Barnett, Hanna, & Parker, Reference Barnett, Hanna and Parker1983). We used a seven-item yes or no questionnaire that was designed for use with pregnant women (Bergman, Sarkar, O'Connor, Modi, & Glover, Reference Bergman, Sarkar, O'Connor, Modi and Glover2007). The scores on this measure ranged from 0 to 7, with higher scores indicating exposure to more stressful life events. The predictive validity of the SLEQ is supported by the finding that antenatal levels of stress as measured by the SLEQ predict cognitive development and fearfulness in offspring (Bergman et al., Reference Bergman, Sarkar, O'Connor, Modi and Glover2007). Respondents indicated which stressful events occurred during pregnancy at each prenatal study visit and which events occurred during the past year at the T3 visit. Maternal stressful life events at T3 were associated with ACEs, r (246) = .150, p = .02, and child externalizing problems, r (246) = .15, p = .02.
Maternal psychological distress
Maternal psychological distress during pregnancy and at the postnatal assessment was evaluated by combining the measures of maternal depression and anxiety that were obtained at each study visit. Maternal depressive symptoms were assessed by using the Edinburgh Postnatal Depression Scale (EPDS; Bergink et al., Reference Bergink, Kooistra, Lambregste-van den Berg, Wijnen, Bunevicius, van Baar and Pop2011), a 10-item self-report measure that is designed to identify depression in the perinatal period. This instrument has demonstrated excellent reliability and validity among pregnant and postpartum women (Bergink et al., Reference Bergink, Kooistra, Lambregste-van den Berg, Wijnen, Bunevicius, van Baar and Pop2011; Cox, Holden, & Sagovsky, Reference Cox, Holden and Sagovsky1987; Jomeen & Martin, Reference Jomeen and Martin2007). In addition, maternal symptoms of anxiety were assessed by using the 10-item anxiety scale from the Symptom Checklist-90 Revised (SCL-90-R; Derogatis, Reference Derogatis1992), a multidimensional self-report measure that is designed to evaluate a broad range of symptoms of psychopathology. The anxiety scale has demonstrated good convergent and divergent validity (Morgan, Wiederman, & Magnus, Reference Morgan, Wiederman and Magnus1998) and adequately discriminates between clinical and nonclinical samples (Bonicatto, Dew, Soria, & Seghezzo, Reference Bonicatto, Dew, Soria and Seghezzo1997; Holi, Sammallahti, & Aalberg, Reference Holi, Sammallahti and Aalberg1998). The scores from the two instruments were z-score transformed and summed to create a composite measure of psychological distress during pregnancy and at the 4-year postnatal assessment. Maternal psychological distress during pregnancy was associated with ACEs, r (246) = .26, p <.001, and child internalizing problems, r (246) = .22, p <.001. Maternal psychological distress at T3 was associated with child internalizing problems, r (232) = .31, p <.001, and externalizing problems, r (232) = .22, p = .001.
Sex
Child sex was obtained from the medical birth record and coded as follows: males = 0 and females = 1. Child sex was included as a covariate in the first and second objectives because it was associated with child internalizing problems, r (246) = .135, p = .034. Sex was investigated as a moderating factor in the third objective to determine whether there may be sex effects.
Gestational age at birth
Gestational age at birth was obtained from the medical birth record and was based on last reported menstrual period. Gestational age at birth was included as a covariate because it was associated with ACEs, r (246) = -.161, p = .011, and child internalizing problems, r (246) = -.222, p < .001.
Data Analytic Strategy
The preliminary analyses were conducted by using SPSS 22.0 software (IBM Inc., 2013) and involved conducting bivariate correlations between the primary study variables of interest. Tests of all of study hypotheses were conducted by using Hayes’ PROCESS macro (Hayes, Reference Hayes2012). For the first objective we assessed parallel mediation models to determine whether maternal HPA-axis function (i.e., CAR, diurnal slope, and total cortisol output) mediated the association between maternal ACEs and child behavior problems at 4 years of age. The PROCESS macro implements a product of coefficients approach where the parameter estimates are bootstrapped (n = 10,000 resamples) to derive 95% confidence intervals (CIs) for all of the indirect paths (Preacher, Rucker, & Hayes, Reference Preacher, Rucker and Hayes2007). This approach accounts for the possibility of nonnormality and/or asymmetry for the indirect effect (Hayes & Preacher, Reference Hayes, Preacher, Hancock and Mueller2013). Mediation is supported when the CIs do not contain zero.
For the second objective we tested moderation models with prenatal HPA-axis function as the moderator of the association between maternal ACEs history and child internalizing and externalizing behavior problems. Moderation was supported if the interaction term between maternal ACEs and cortisol was significant. Significant moderation (interaction) effects were probed by examining the conditional effects for two-way interactions between the predictors (Aiken & West, Reference Aiken and West1991). The Johnson–Neyman technique was used to derive regions of significance (ROS) for the conditional effects of maternal ACEs at varying levels of prenatal cortisol (Aiken & West, Reference Aiken and West1991; Johnson & Neyman, Reference Johnson and Neyman1936).
For the third objective, we conducted moderation analyses to evaluate sex as a moderator of the association between prenatal HPA-axis function and child behavior problems. Additionally, we added sex as a moderator of the prenatal effects, according to the steps that were outlined by Hayes and Preacher (Reference Hayes, Preacher, Hancock and Mueller2013), to evaluate whether the models that were run in the first and second objectives were conditional on child sex.
Each objective was evaluated separately for prenatal HPA-axis function at T1 and T2 to allow for the possibility that exposure timing may have differentially affected child developmental outcomes (Davis & Sandman, Reference Davis and Sandman2010; Ellman et al., Reference Ellman, Schetter, Hobel, Chicz-Demet, Glynn and Sandman2008; Laurent, Ablow, & Measelle, Reference Laurent, Ablow and Measelle2011). All of the models included the following covariates: gestational age at which maternal cortisol was collected, waking cortisol concentration, prenatal distress, prenatal stressful life events, SES, postnatal distress, postnatal stressful life events, infant GA at birth, and child sex.
Results
Missing Value Analysis
Of the 248 participants that were included in the current study, 235 completed the T1 assessment (6–26 weeks), 213 completed the T2 assessment (27–37 weeks), and 248 completed the T3 assessment (4 years). All of the primary study variables had less than 5% missing data at each measurement point.
Preliminary Analyses
Bivariate correlations were calculated to gain a preliminary understanding of the associations between the study variables (Table 2). The mean and median exposure to ACEs in the current study were 1 and 0, respectively. Maternal ACEs were positively associated with the CAR at T1 and T2 but not with diurnal slope or total cortisol output. With respect to the primary child outcome variables, greater maternal ACEs and a flatter diurnal slope at T1 were associated with higher child internalizing problems at 4 years of age. Child externalizing problems were not associated with any of the study predictors.
Table 2. Bivariate correlation coefficients between primary study variables

Note: T1 = 6–26 weeks gestation; T2 = 27–37 weeks gestation; T3 = 4 years postpartum; ACEs = adverse childhood experiences; CAR = cortisol awakening response; AUCg = total cortisol output; * = p ≤ .05. ** = p < .01.
Objective 1.1: Mediating Effect of Diurnal Cortisol at T1 in the Association Between Maternal ACEs and Child Behavior Problems
Objective 1.1 tested whether the association between maternal ACEs and child behavior problems was mediated by maternal CAR, diurnal slope, and total cortisol levels at T1, which was examined in parallel mediation models (Supplementary Table 2). There was a significant negative indirect association between maternal ACEs and child internalizing problems via maternal CAR at T1, B = −0.21, 95% CI [−0.53, −0.04]. Specifically, ACEs were positively associated with maternal CAR at T1, B = 0.51, p = .002, and maternal CAR at T1 was negatively associated with child internalizing problems, B = −0.40, p = .02 (Figure 1a). Neither diurnal slope nor total cortisol at T1 mediated the association between maternal ACEs and child internalizing problems. After accounting for the mediators, a positive direct association remained between maternal ACEs and child internalizing problems, B = 0.79, p = .05, while the total effect was nonsignificant, B = 0.57, p = .14. There were no direct or indirect associations between maternal ACEs and child externalizing problems via diurnal cortisol at T1, and the total effect was nonsignificant, B = 0.06, p = .89 (Figure 1b). There was, however, a significant direct association between the cortisol slope at T1 and child externalizing problems, B = 18, p = .03. Adding child sex as a moderator in the model did not yield any further findings.
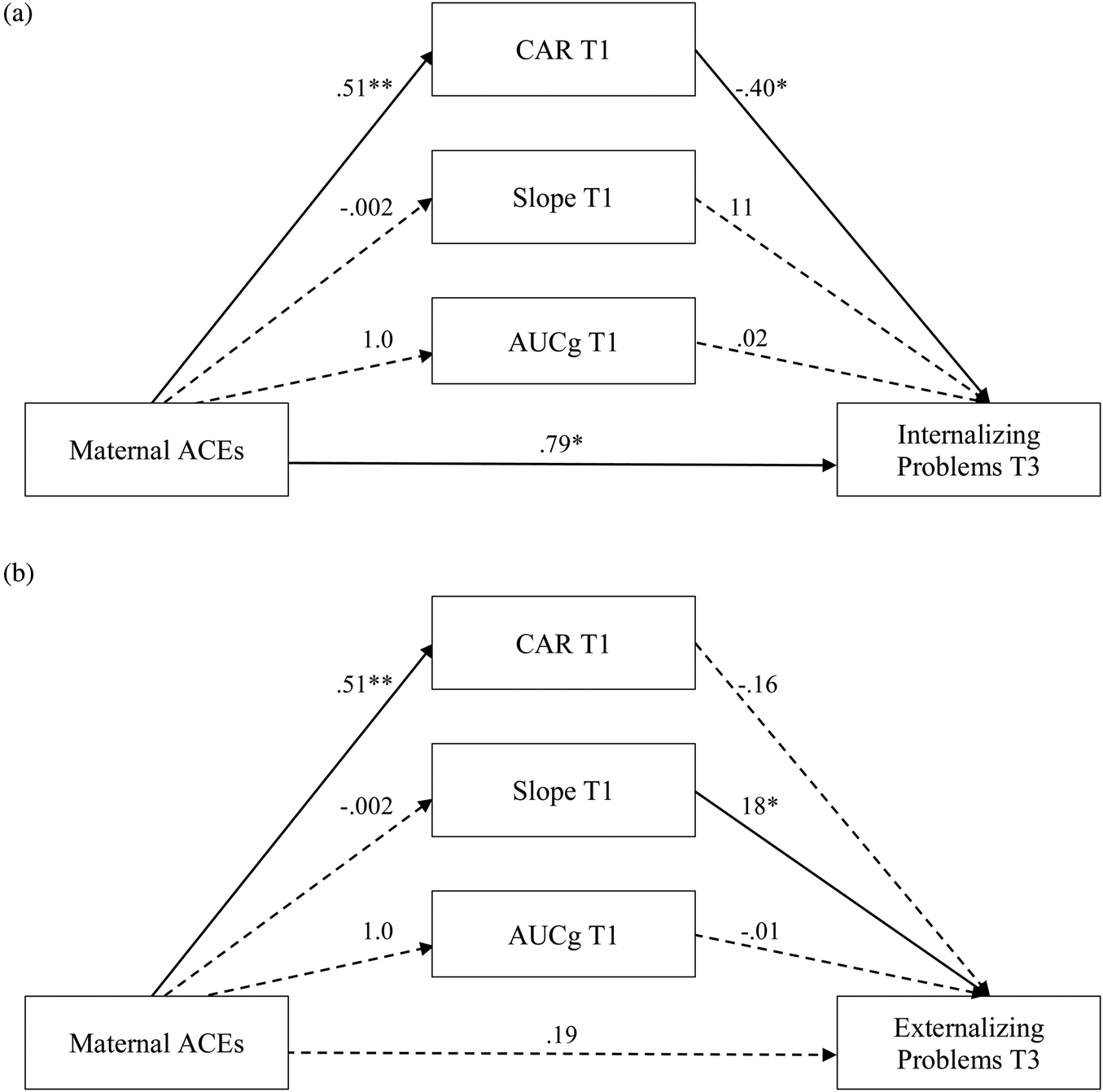
Figure 1. Parallel indirect effects of maternal ACEs on child behavior problems via maternal CAR, diurnal slope, and total cortisol output at T1. (a) Maternal CAR at T1 mediated the association between maternal ACEs and child internalizing problems. (b) No mediation effects were observed between maternal ACEs and child externalizing problems. ACEs = adverse childhood experiences; CAR = cortisol awakening response; AUCg = total cortisol; T1 = 6–26 weeks gestation; T3 = 4 years. Significant associations are indicated by solid lines and nonsignificant associations are indicated by dotted lines. *p ≤ .05. **p < .01.
Objective 1.2: Mediating Effect of Diurnal Cortisol at T2 in the Association Between Maternal ACEs and Child Behavior Problems
Objective 1.2 tested whether the association between maternal ACEs and child behavior problems was mediated by diurnal cortisol at T2 (Supplementary Table 3). There was a significant negative indirect association between maternal ACEs and child internalizing problems via maternal CAR at T2, B = −0.14, 95% CI [−0.48, −0.002]. Specifically, ACEs were positively associated with maternal CAR at T2, B = 0.42, p = .03, and maternal CAR at T2 was negatively associated with child internalizing problems, B = −0.33, p = .05 (Figure 2a). There was a marginally significant direct association between maternal ACEs and child internalizing problems, B = 0.86, p = .06, but the total effect was nonsignificant, B = 0.73, p = .12. There were no direct or indirect effects of ACEs on child externalizing problems via diurnal cortisol at T2, and the total effect was nonsignificant, B = 0.48, p = .28. Adding child sex to the model did not yield any further findings.

Figure 2. Parallel indirect effects of maternal ACEs on child behavior problems via maternal CAR, diurnal slope, and total cortisol output at T2. (a) Maternal CAR at T2 mediated the association between maternal ACEs and child internalizing problems. (b) No mediation effects were observed between maternal ACEs and child externalizing problems. ACEs = adverse childhood experiences; CAR = cortisol awakening response; AUCg = total cortisol; T2 = 27–37 weeks gestation; T3 = 4 years. Significant associations are indicated by solid lines and nonsignificant associations are indicated by dotted lines. *p ≤ .05.
Objective 2.1: Moderating Effect of Diurnal Cortisol at T1 on the Association Between Maternal ACEs and Child Behavior Problems
Objective 2.1 tested whether diurnal cortisol at T1 moderated the association between maternal ACEs and child behavior problems (Supplementary Table 4). Moderator effects were observed only for the diurnal slope, and only for child externalizing problems, B = 4.2, p = .03 (Figure 3). A flatter diurnal slope at T1 was associated with greater child externalizing problems but only in women with a history of ACEs. The Johnson–Neyman ROS test indicated that there was a negative association between maternal ACEs and child externalizing problems when the diurnal slope at T1 was steeper than −1.03 (99th percentile) and a positive association when it was flatter than −0.18 (4th percentile).

Figure 3. The diurnal cortisol slope at T1 moderated the association between maternal ACEs history and child externalizing problems at age 4. The association between maternal ACEs and child externalizing problems was negative when the diurnal slope at T1 was steeper than −1.03 and became positive when the diurnal was flatter than –0.18. The graph depicts the diurnal slope at T1 at the region of significance. ACEs = adverse childhood experiences.
Adding child sex revealed sexually dimorphic effects in the diurnal slope model. Specifically, there was a significant interaction between maternal ACEs and diurnal slope at T1 to predict child internalizing problems for female children, B = 4.7, p = .03, but not male children, B = −0.22, p = .95. The opposite was true for the moderating effect of the diurnal slope at T1 on the association between maternal ACEs and child externalizing problems such that the effect was significant for male children, B = 6.8, p = .05, but not female children, B = 2.8, p = .22. No sex effects were observed for the CAR or total cortisol models.
Objective 2.2: Moderating Effect of Diurnal Cortisol at T2 on the Association Between Maternal ACEs and Child Behavior Problems
Objective 2.2 tested whether maternal cortisol at T2 moderated the association between maternal ACEs and child behavior problems (Supplementary Table 5). Effects were observed only for the diurnal slope, and only for child internalizing problems, B = 4.8, p = .01 (Figure 4). A flatter slope at T2 was associated with greater child internalizing problems but only in women reporting a history of ACEs. Specifically, the Johnson–Neyman ROS test indicated that the association between maternal ACEs and child internalizing problems was positive when the diurnal slope at T2 was flatter than −0.40, which corresponds to the 35th percentile. Adding child sex to the models did not yield any further findings.

Figure 4. The diurnal cortisol slope at T2 moderated the association between maternal ACEs history and child internalizing problems at age 4. The association between maternal ACEs and child internalizing problems was positive when the diurnal slope at T2 was flatter than –0.40. The graph depicts the mean diurnal slope at T2 (–.45 ug/dL decline/day) and the region of significance (–.4 ug/dL decline/day). ACEs = adverse childhood experiences.
Objective 3: Moderating Effect of Child Sex on the Association Between Diurnal Cortisol and Child Behavior Problems
Objective 3 tested whether child sex moderated the association between diurnal cortisol exposure at T1 and T2 and child behavior problems (Supplementary Tables 6 and 7). There were no interactions observed between prenatal diurnal cortisol and child sex to predict child internalizing or externalizing problems.
Discussion
The current study illustrates the role of the maternal HPA axis during pregnancy in transmitting the effects of maternal adverse childhood experiences (ACEs) to offspring behavior. First, prenatal HPA-axis function was a mediator between maternal ACEs history and child internalizing problems at age 4. Specifically, maternal ACEs were associated with a higher CAR during pregnancy, and a higher CAR was associated with lower internalizing problems. Second, maternal diurnal slope was a moderator of the association between maternal ACEs history and child internalizing and externalizing behavior problems. These effects were sex dependent such that the interaction between maternal ACEs and prenatal diurnal slope at T1 was associated with internalizing behavior in female children and externalizing behavior in male children. The mediating and moderating effects that are described in the present study were strongest at T1, indicating that early to middle gestation may be a sensitive period for prenatal cortisol exposures to affect offspring behavior. To our knowledge, this study represents the first examination of prenatal diurnal cortisol as a biological mechanism in the association between maternal ACEs and offspring risk for developmental psychopathology in the preschool years. This research also demonstrates that risk for the intergenerational transmission of ACEs may begin as early as the prenatal period.
Mediating Effects
Consistent with previous studies of pregnant and postpartum women (Bublitz & Stroud, Reference Bublitz and Stroud2012, Reference Bublitz and Stroud2013; Gonzalez, Jenkins, Steiner, & Fleming, Reference Gonzalez, Jenkins, Steiner and Fleming2009), the present study identified associations between maternal ACEs and an elevated CAR during pregnancy. This suggests that ACEs leave a signature of stress on the maternal HPA axis, which may represent a biological pathway for the intergenerational transmission of stress (Thomas, Magel, et al., Reference Thomas, Magel, Tomfohr-Madsen, Madigan, Letourneau, Campbell and Giesbrecht2018). Changes in the HPA axis that increase vigilance and readiness to respond to potential threats are an adaptive response under conditions of early life stress (Del Giudice, Ellis, & Shirtcliff, Reference Del Giudice, Ellis and Shirtcliff2011). Although these changes in the HPA axis are adaptive in that they enable an individual to respond appropriately to harsh and unpredictable environments, they may also have associated costs over the long term such as increased risk for psychopathology including major depressive disorder, posttraumatic stress disorder, and anxiety (Cowen, Reference Cowen2010; Ehlert, Gaab, & Heinrichs, Reference Ehlert, Gaab and Heinrichs2001).
Generally, a larger CAR in late pregnancy has been associated with adverse child outcomes including shorter gestational length (Buss et al., Reference Buss, Entringer, Reyes, Chicz-DeMet, Sandman, Waffarn and Wadhwa2009) and lower birth weight (Bolten et al., Reference Bolten, Wurmser, Buske-Kirschbaum, Papousek, Pirke and Hellhammer2011). Although we expected a cascade of negative effects from ACEs to some form of maternal HPA-axis dysregulation to increases in child problem behavior, we observed exactly the opposite. The increase in maternal CAR consequent to ACEs exposure was subsequently associated with reduced internalizing symptoms in children. Overall, there was inconsistent mediation, whereby the indirect effect of the CAR on child internalizing problems was negative and the direct effect of maternal ACEs on child internalizing was positive. This suggests that the prenatal CAR was a suppressor variable (MacKinnon, Krull, & Lockwood, Reference MacKinnon, Krull and Lockwood2000), and thereby it may represent a resilience factor. Suppressor variables are important because they help to unmask associations that appear to be null (i.e., a null total effect) when in fact opposite direct and indirect effects are operating.
There are a number of possible explanations for the unexpected association between an elevated maternal CAR and lower child internalizing problems. First, in this healthy low-sociodemographic-risk sample, an elevated CAR (consequent to ACEs exposure) may represent a biological resilience factor in pregnant women that promotes child development. This possibility is supported by previous studies that have demonstrated associations between mild to moderate levels of prenatal stress (i.e., anxiety and depressive symptoms) and advanced motor and mental development in children (DiPietro, Novak, Costigan, Atella, & Reusing, Reference DiPietro, Novak, Costigan, Atella and Reusing2006) as well as accelerated brain maturation (Lebel et al., Reference Lebel, Walton, Letourneau, Giesbrecht, Kaplan and Dewey2016). In nonhuman primates, early life stress that is challenging but not overwhelming promotes the development of behavioral and physiological arousal and resilience (for a review see Lyons, Parker, Katz, & Schatzberg, Reference Lyons, Parker, Katz and Schatzberg2009), indicating that a certain degree of early life stress may be positive for development. Nonetheless, it has also been hypothesized that early brain maturation (e.g., premature myelination and synaptic pruning) resulting from prenatal stress exposure may be at the expense of extended brain plasticity, with the potential to affect cognitive and behavioral outcomes later in life (Lebel et al., Reference Lebel, Walton, Letourneau, Giesbrecht, Kaplan and Dewey2016). Therefore, although an elevated maternal CAR may be a resilience factor that reduces the risk of child internalizing problems at 4 years of age, these children may present with affective or behavioral problems later in development. The possibility that an elevated CAR may represent a short-term resilience factor for child emotional development with long-term consequences that emerge later in development should be evaluated in future longitudinal studies.
Another possible explanation is that there is a U-shaped association between maternal CAR and child outcomes such that extremely low (blunted) or extremely high (exaggerated) CARs may be associated with poorer child outcomes. Both blunted and exaggerated diurnal patterns have been observed in clinical populations and may therefore be considered maladaptive (Fries, Dettenborn, & Kirschbaum, Reference Fries, Dettenborn and Kirschbaum2009). For example, a blunted CAR has been observed in patients with chronic pain (Geiss, Varadi, Steinbach, Bauer, & Anton, Reference Geiss, Varadi, Steinbach, Bauer and Anton1997), functional gastrointestinal disorders (Böhmelt, Nater, Franke, Hellhammer, & Ehlert, Reference Böhmelt, Nater, Franke, Hellhammer and Ehlert2005), and psychiatric disorders such as posttraumatic stress disorder (Wessa, Rohleder, Kirschbaum, & Flor, Reference Wessa, Rohleder, Kirschbaum and Flor2006). In contrast, exaggerated CARs have been observed in women with fibromyalgia who report physical or sexual abuse experiences in childhood (Weissbecker, Floyd, Dedert, Salmon, & Sephton, Reference Weissbecker, Floyd, Dedert, Salmon and Sephton2006) and in patients with depression (Bhagwagar, Hafizi, & Cowen, Reference Bhagwagar, Hafizi and Cowen2005). We tested for a curvilinear association between maternal CAR squared and child internalizing problems in the present study (results not shown), but this association was not significant. However, this may be because we examined these associations in a nonclinical, low-sociodemographic-risk sample, which may have undersampled the highest and lowest range of CARs. Given that there are no reference norms available for diurnal cortisol levels in pregnant women, we are unable to definitively discern whether the relative distribution of maternal CARs in the present study under-represents the highest and lowest range of CARs. Therefore, the possibility of a U-shaped association between prenatal maternal CAR and child internalizing problems should be evaluated in samples of women that are at higher risk (e.g., those meeting criteria for major depressive disorder during pregnancy).
Another possible explanation for the unexpected finding invokes the concept of biobehavioral asynchrony, whereby observed behavioral functioning does not align with physiological indicators of stress. This possibility was demonstrated in a previous study of 107 mother–infant dyads that showed that a higher maternal CAR in late pregnancy was associated with less infant crying and fussing in response to maternal separation at 9 months of age but elevated cortisol reactivity that did not habituate in response to repeated separations (de Weerth, Buitelaar, & Beijers, Reference de Weerth, Buitelaar and Beijers2013). This suggests that children who are exposed to an elevated maternal CAR during pregnancy may appear to be well adjusted behaviorally (i.e., they present with low internalizing problems) while still demonstrating an elevated physiological response to stress. This pattern of low biobehavioral coherence, wherein children display no behavioral indications of negative mood but elevated cortisol reactivity, has also been observed in children with insecure attachment (Ahnert et al., Reference Ahnert, Gunnar, Lamb, Barthel, Anhert, Gunnar and Barthel2004). When the quality of mother–child interactions are of lower quality and when insecure attachment is evident, children may develop behavioral strategies that reduce the need for the attachment figure to intervene, for example reduced fussing and crying (Cassidy, Reference Cassidy1994; Sroufe, Reference Sroufe2000), while still mounting a cortisol response to stress (Ahnert et al., Reference Ahnert, Gunnar, Lamb, Barthel, Anhert, Gunnar and Barthel2004). This should be further evaluated in future studies, particularly in investigations of the possibility that these children may lack biobehavioral coherence and are therefore at risk for future health problems via physiological stress-related risk factors such as elevated HPA-axis reactivity.
Moderating Effects
Maternal diurnal cortisol slope moderated the association between ACEs and child internalizing and externalizing behavior at 4 years of age. Specifically, maternal ACEs history was associated with higher child internalizing and externalizing behavior when exposed to a flatter diurnal cortisol slope during pregnancy. These findings suggest that maternal ACEs may interact with prenatal cortisol exposure to have downstream effects on fetal development and subsequent child behavior. The ways in which maternal ACEs may interact with prenatal cortisol to affect child development are not known. However at least one study has demonstrated that ACEs may alter placental-fetal stress physiology. Specifically, Moog et al. (Reference Moog, Buss, Entringer, Shahbaba, Gillen, Hobel and Wadhwa2016) demonstrated that maternal childhood trauma exposure was associated with an almost 25% increase in placental corticotropin-releasing hormone (CRH) towards the end of pregnancy. Given that the placenta plays a central role in buffering the fetus from maternal cortisol, factors that modify placental function may alter the amount of cortisol that reaches the developing fetus, potentiating the effects of prenatal cortisol exposure on child outcomes (Togher et al., Reference Togher, O'Keefe, Khashan, Gutierrez, Kenny and O'Keefe2014). More research is needed to elucidate the effect of maternal ACEs on placental-fetal stress processes to understand the ways in which early life adversity may amplify the effects of maternal cortisol on fetal development.
Interestingly, the moderating effects were conditional on child sex such that maternal ACEs and diurnal cortisol interacted to predict higher internalizing behavior in female children and higher externalizing behavior in male children. This is consistent with a number of other well-documented sex differences in the developmental psychopathology literature, which indicate that females are at relatively higher risk of developing internalizing problems, whereas males are at increased risk of externalizing problems (Crick & Zahn-Waxler, Reference Crick and Zahn-Waxler2003). While the neurobiological processes by which prenatal cortisol exposure alters male and female neurobiological development remain to be fully elucidated, there is some evidence that sexually dimorphic prenatal programming of the fetal HPA axis may be involved. Specifically, in our previous work we demonstrated that prenatal cortisol exposure had opposite effects on male and female infant cortisol reactivity at 3 months of age such that a smaller CAR or flatter daytime slope was associated with blunted cortisol responses in female infants, whereas a larger CAR and steeper daytime slope was associated with blunted cortisol responses in male infants (Giesbrecht et al., Reference Giesbrecht, Letourneau and Campbell2017). There is also evidence that fetal sex moderates the association between prenatal cortisol exposure and fetal neurological development (Glynn & Sandman, Reference Glynn and Sandman2012). A recent study found that elevated maternal cortisol levels during pregnancy were associated with higher internalizing symptoms in girls at 24 months of age and this association was mediated by stronger neonatal amygdala connectivity (Graham et al., Reference Graham, Rasmussen, Entringer, Ward, Rudolph, Gilmore and Buss2019). These sexually dimorphic alterations in the effects of prenatal cortisol exposure on fetal development may explain some of the sex differences that are observed in the incidence and presentation of stress-related psychopathology, including that females are at higher risk of internalizing disorders such as anxiety and depression (Altemus, Sarvaiya, & Neill Epperson, Reference Altemus, Sarvaiya and Neill Epperson2014). Nonetheless, further study is needed to fully understand the neurobiological underpinnings of the sexually dimorphic effects of maternal ACEs on child behavior.
As in previous work, this study found that the effects of maternal ACEs and prenatal cortisol exposure on child behavior problems were dependent on the specific index of HPA-axis function that was measured. Different metrics of maternal cortisol not only appear to play different roles in the relationship between maternal ACEs and child behavior but also appear to be in opposite directions. Whereas the CAR mediated the association between maternal ACEs and lower child internalizing problems, the diurnal slope interacted with maternal ACEs to predict higher child internalizing and externalizing problems in a sex-dependent manner. Total cortisol levels did not mediate or moderate the association between maternal ACEs and child behavior. These different findings for the CAR and diurnal slope, which are under separate regulatory control, suggest that the effects of the maternal HPA axis on child outcomes depend both on the specific component of the HPA axis and the specific child outcome (Zijlmans, Riksen-Walraven, & de Weerth, Reference Zijlmans, Riksen-Walraven and de Weerth2015). It is important to note that mediation and moderation analyses answer different kinds of questions, with the former interrogating the “how” of the association and the latter the “when,” or how the strength of the association changes at different levels of the moderator. Accordingly, these results are compatible with each other because they address different aspects of the relationship between ACEs and children's behavior. Nevertheless, a specific explanation for these differing associations is not yet clear. It is known that different components of HPA-axis function are differentially affected by physical and mental health conditions (Adam et al., Reference Adam, Quinn, Tavernier, McQuillan, Dahlke and Gilbert2017; Fries et al., Reference Fries, Dettenborn and Kirschbaum2009), and subsequently these prenatal exposures are likely to have different programming effects on fetal development. Furthermore, fetal programming by prenatal HPA-axis function is a complex process given the unique contributions of the fetal-placental stress response systems that develop and affect the circulation of cortisol over the course of gestation (e.g., the production of placental CRH; Sandman, Reference Sandman2018). Our findings highlight the complexity of the associations between maternal ACEs, prenatal HPA-axis function, and child behavioral outcomes and indicate the need to consider both mediator and moderator models. The results also suggest that dynamic indicators of HPA-axis function may be more sensitive measures for evaluating fetal programming effects than are gross measures of total cortisol exposure. This is in keeping with the notion that the pattern of parental signals carries important information for the developing brain that is not captured by the overall level of function (Glynn et al., Reference Glynn and Baram2019).
Timing Effects
The mediating and moderating effects of prenatal HPA-axis function that were identified in the present study were strongest in early to middle gestation (~15 weeks) versus later (~32 weeks) gestation. The pattern of findings that was observed in the present study indicates that the first two trimesters of pregnancy may be a critical period for the development of the fetal neurobiological systems that underlie child behavioral outcomes. This possibility is supported by one study that found that higher prenatal cortisol levels in early gestation were associated with alterations in child amygdala volume (in girls), and these brain alterations were subsequently associated with more affective problems (Buss et al., Reference Buss, Davis, Shahbaba, Pruessner, Head and Sandman2012). It is important to note that the effects of prenatal cortisol exposure during prenatal development are complex and those that are observed depend both on the timing of the exposure as well as the child outcomes of interest (Zijlmans et al., Reference Zijlmans, Riksen-Walraven and de Weerth2015). With respect to infant cognitive outcomes, for example, prenatal maternal cortisol levels have opposite effects depending on the timing of exposure (Davis & Sandman, Reference Davis and Sandman2010). Specifically, one study showed that infants who were exposed to low maternal cortisol in early gestation and higher levels towards the end of pregnancy demonstrated accelerated cognitive development over the first year, whereas those who were exposed to higher cortisol levels early in gestation had a slower rate of cognitive development (Davis & Sandman, Reference Davis and Sandman2010). The timing effects that were observed in the present study suggest that intervention and prevention efforts that are directed at reducing the effects of maternal ACEs on child outcomes should target early pregnancy and perhaps even preconception.
The present study supports the need for psychosocial interventions that specifically target HPA-axis function to improve not only the health and developmental outcomes of those directly affected by ACEs but also those of their offspring. Early intervention studies have demonstrated the utility of psychosocial interventions that are directed at reducing the physiological effects of ACEs in children (Dozier, Peloso, Lewis, Laurenceau, & Levine, Reference Dozier, Peloso, Lewis, Laurenceau and Levine2008; Fisher, Stoolmiller, Gunnar, & Burraston, Reference Fisher, Stoolmiller, Gunnar and Burraston2007; Fisher, Van Ryzin, & Gunnar, Reference Fisher, Van Ryzin and Gunnar2011). Although no studies have evaluated prenatal interventions for ACEs in pregnant women, a few studies have evaluated the utility of psychosocial treatments for current experiences of stress during pregnancy (Chen et al., Reference Chen, Chou, Yang, Tsai, Chang and Liaw2017; Matvienko-Sikar & Dockray, Reference Matvienko-Sikar and Dockray2017; Richter et al., Reference Richter, Bittner, Petrowski, Junge-Hoffmeister, Bergmann, Joraschky and Weidner2012; Urizar Jr. & Munoz, Reference Urizar and Munoz2011; Zhang & Emory, Reference Zhang and Emory2015). For example, a randomized-controlled trial of cognitive behavioral therapy for subclinical stress, depression, and anxiety during pregnancy found that women who received the intervention (n = 21) exhibited posttreatment reductions in the CAR in comparison with a treatment as usual group (n = 40; Richter et al., Reference Richter, Bittner, Petrowski, Junge-Hoffmeister, Bergmann, Joraschky and Weidner2012). The findings of the current study support the need for intervention studies to evaluate whether psychosocial interventions that target maternal HPA-axis function may ameliorate the intergenerational transmission of maternal ACEs to offspring behavior.
Strengths and Limitations
The present study demonstrated that prenatal maternal HPA-axis function plays a role in explaining the association between maternal ACEs and child behavior problems, highlighting a possible target for intervention efforts to prevent or reduce the intergenerational transmission of ACEs. Importantly, these findings also indicate that the effects of maternal ACEs may be transmitted to the next generation as early as pregnancy (and this likely even extends to preconception), which supports the need for early interventions for individuals with ACEs. This study has several strengths, including a relatively large sample size; the availability of longitudinally collected, reliable, and noninvasive biological markers of stress; and repeated assessments of women during pregnancy. To our knowledge, this is the first study to evaluate the maternal HPA axis as a prenatal biological factor that links maternal ACEs to child behavioral outcomes and therefore represents a novel contribution to this growing field. Nevertheless, several limitations require consideration.
First, although we had a diverse sample, the majority of the participants in this study represented a relatively low-sociodemographic-risk sample of women because most of the participants were married or in common-law relationships, White, had a university-level education, and had a relatively high household annual income. Therefore, it may not be appropriate to generalize the findings to sociodemographically diverse populations. We also did not enroll women who reported consuming tobacco, nonprescription drugs, or alcohol during pregnancy, which may have excluded women with exposure to more severe forms of early life adversity given the well-documented associations between ACEs and poor health behaviors (Anda et al., Reference Anda, Croft, Felitti, Nordenberg, Giles, Williamson and Giovano1999; Dube, Anda, Felitti, Edwards, & Croft, Reference Dube, Anda, Felitti, Edwards and Croft2002; Dube et al., Reference Dube, Felitti, Dong, Chapman, Giles and Anda2003). Despite the demographic homogeneity, a substantial proportion of women reported a history of at least one ACE (44%), which, consistent with Felitti et al., (Reference Felitti, Anda, Nordenberg, Williamson, Spitz, Edwards and Marks1998), indicates that ACEs are pervasive, even among populations of low sociodemographic risk. More importantly, this demonstrates that the intergenerational effects of ACEs on child behavior problems persist despite the apparent psychosocial advantages of the women in this study. It also highlights the need to examine these associations in groups that are at higher sociodemographic risk to determine whether such effects are stronger (we predict there would be greater variability and therefore stronger effects) and whether the pathways are the same or different from those that are found in low-sociodemographic-risk groups. For example, we suspect that the relative resilience that is afforded by the higher prenatal CAR for reduced internalizing problems in children in the present study may not hold in groups that are at higher sociodemographic risk.
Second, we were unable to disentangle whether the prenatal effects of cortisol on child behavior problems may in fact represent heritable factors as opposed to prenatal environmental programming effects. Recent studies that have used adoption-biological family designs offer unique insights into the environmental versus heritable influence of maternal trauma, depression, and parenting on child internalizing and externalizing problems (Grabow et al., Reference Grabow, Khurana, Natsuaki, Neiderhiser, Harold, Shaw and Leve2017; Marceau et al., Reference Marceau, Laurent, Neiderhiser, Reiss, Shaw, Natsuaki and Leve2015). Additionally, although we adjusted for postnatal environmental factors including maternal psychological distress and stressful life events, we did not account for other important postnatal factors including child exposure to ACEs and parenting behavior. We did, however, have an observational measure of maternal sensitivity (the Parent Child Interaction Teaching Scale; Oxford & Finlay, Reference Oxford and Finlay2013), which was completed at 6 months of age on a subset of 155 women and infants that were enrolled in the present study. When we reran the analyses including maternal sensitivity as a covariate on this subset of participants (not shown), the mediating effect of the CAR at T1 and the moderating effect of the slope at T2 predicting child internalizing behavior remained significant. This subanalysis provided further confidence in our conclusions that the associations that were observed in the present study may be attributable to the prenatal environment as opposed to postnatal factors. Future studies should consider evaluating prenatal maternal HPA-axis functioning by using adoption-biological family designs to further disentangle the combined influences of genes, prenatal exposures, and the postpartum environment.
Third, this study used cortisol, the end product of the HPA axis, as an indicator of HPA-axis function during pregnancy. Although this is a reasonable approach, it neglects other biomarkers of HPA-axis function (such as adrenocorticotropic hormone or corticotropin-releasing hormone), which could lend insight into specific aspects of HPA-axis function that are associated with exposure to ACEs. Pregnancy is a dynamic and complex process, particularly with respect to the HPA axis, given the increasing contributions of the placenta as a neuroendocrine organ with advancing gestation. Further study is needed, specifically with respect to placental corticotropin-releasing hormone, to determine what role the placenta may play in explaining the association between maternal ACEs and child developmental outcomes (e.g., see Moog et al., Reference Moog, Buss, Entringer, Shahbaba, Gillen, Hobel and Wadhwa2016). Some studies have failed to detect associations between ACEs and maternal cortisol during pregnancy (Deuschle et al., Reference Deuschle, Hendlmeier, Witt, Rietschel, Gilles, Sánchez-Guijo and Hellweg2018), which is consistent with the broader literature that evaluates associations between psychosocial stress and cortisol during pregnancy (Beijers, Buitelaar, & de Weerth, Reference Beijers, Buitelaar and de Weerth2014). Given that stress is a complex phenomenon with diverse effects on physiology, behavior, and emotions, there are likely many different mechanisms by which maternal ACEs may affect fetal development and there is a need to expand the literature to look beyond the HPA axis (Beijers et al., Reference Beijers, Buitelaar and de Weerth2014). For example, there is evidence to suggest that prenatal sleep disturbance may be a mediator of the association between maternal abuse history and fetal parasympathetic nervous system functioning (Gustafsson et al., Reference Gustafsson, Doyle, Gilchrist, Werner and Monk2017).
Finally, we used a maternal report of child behavior as a measure of child internalizing and externalizing problems, and maternal perceptions of child behavior problems may be influenced by current maternal mental health (Min et al., Reference Min, Singer, Minnes, Kim and Short2012; Najman et al., Reference Najman, Williams, Nikles, Spence, Bor, O'Callaghan and Andersen2000). We addressed this issue by adjusting the analysis for maternal psychological distress and stressful life events at the postpartum assessment. Nonetheless, the state of the literature would be further strengthened by investigating the intergenerational effects of maternal ACEs on biological markers in children that are unbiased by maternal perceptions, such as brain anatomy (Moog et al., Reference Moog, Entringer, Rasmussen, Styner, Gilmore, Kathmann and Buss2018) and HPA reactivity (Thomas, Letourneau, et al., Reference Thomas, Magel, Tomfohr-Madsen, Madigan, Letourneau, Campbell and Giesbrecht2018), to characterize the neurobiological underpinnings of risk for internalizing and externalizing behavior in children more effectively.
Conclusion
The present study offers unique insights into the prenatal biological processes by which maternal early life adversity may “get under the skin” of the next generation to increase or decrease the risk for internalizing and externalizing problems. Specifically, we found that a higher maternal CAR was a mediator of the association between maternal ACEs and lower child internalizing problems and appeared to be an unexpected resilience factor. We also found that maternal prenatal diurnal cortisol slope moderated the effects of the maternal ACEs on child behavior problems in a sex-dependent manner, such that a flatter slope was associated with more internalizing behavior in female children and more externalizing behavior in male children. The present study contributes to the growing recognition of the intergenerational influence of ACEs and highlights various potential targets for treatment and/or risk factors to identify individuals for early intervention. Future research should focus not only on further elucidating the mechanisms that are involved in the intergenerational transmission of ACEs but also on examining moderating factors that may buffer this transmission and improve maternal and child outcomes. Promising new research points to the potential for social support, and particularly the role of supportive partners in the perinatal period, for buffering the intergenerational transmission of ACEs to the next generation (Thomas, Letourneau, et al., Reference Thomas, Letourneau, Campbell and Giesbrecht2018).
While the best approach to reducing the burden of ACEs on society is clearly to prevent the occurrence of ACEs entirely or to intervene shortly after exposure, the perinatal period offers another exciting window of opportunity for improving both maternal and child health outcomes and breaking the cycle of adversity. Evidence from experimental studies supports the notion that maternal HPA-axis function is amenable to change through psychosocial interventions during pregnancy. Future studies should evaluate whether these findings also extend to pregnant women with a history of ACEs and whether improvements in prenatal HPA-axis function have downstream benefits for child health.
Acknowledgments
This research was supported by grants from the Alberta Children's Hospital Research Institute, Alberta Innovates Health Solutions, the Canadian Child Health Clinician Scientist Program, the Canadian Institutes of Health Research, and the Alberta Centre for Child, Family, and Community Research.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579419001767.









