INTRODUCTION
An understanding of host-parasite interactions requires us to explore the nature of the host-parasite interface. It is assumed that the surface membrane of the parasite is the most extensive interface (Skelly and Wilson, Reference Skelly and Wilson2006; Kusel et al. Reference Kusel, Al Adhami and Doenhoff2007), but this assumption has been recently modified. The influx of fluorescent membrane-impermeant molecules of up to 20 kDa molecular weight into cercariae as they penetrate mouse skin has been described. This was reproduced in vitro by transformation in the presence of the membrane-impermeant molecules. One implication of this observation is that there may exist a host-parasite interface at the skin stage of parasite development which is unexpected and unique (Thornhill et al. Reference Thornhill, Coelho, McVeigh, Maule, Jurberg and Kusel2009), and resides within the parasite rather than at the surface.
During transformation from the water-adapted cercaria to the skin schistosomulum, the surface membrane undergoes extensive loss of glycocalyx attached to cercarial membrane, and there is replacement of this membrane by the double-membrane characteristic of the schistosomulum (Hockley and McLaren, Reference Hockley and McLaren1973). The membrane components are incorporated into the surface by fusion of cytoplasmic multilaminate vesicles (Hockley and McLaren, Reference Hockley and McLaren1973). This dynamic remodelling of the surface may alter the membrane permeability of the surface at this stage of development.
Although the process of influx into the cercaria as it transforms into the schistosomulum was shown by fluorescence and confocal microscopy, the mechanism by which the influx occurred was not examined (Thornhill et al. Reference Thornhill, Coelho, McVeigh, Maule, Jurberg and Kusel2009). Membrane-impermeant molecules, such as Lucifer Yellow, propidium iodide and a variety of dextrans, might enter the schistosomulum through the surface membrane during the remodelling described above, or it may be that pores which allow passage of such molecules exist in the membrane. Photomicrographs of labelled schistosomula show labelling of the surface membrane consistent with this route of entry. Other routes of uptake might be the apertures of the oesophagus, the head glands and the pre- and post-acetabular glands, but inspection of the photomicrographs shows that these apertures are not regularly associated with the fluorescently labelled compound that has entered the parasite. One aperture, however, that always shows the presence of the fluorescent compound is the nephridiopore. This aperture is the orifice through which waste products from the excretory system of the parasite are voided from the organism (Wilson and Webster, Reference Wilson and Webster1974; Dorsey et al. Reference Dorsey, Cousin, Lewis and Stirewalt2002). The nephridiopore, together with other excretory orifices (Dorsey et al. Reference Dorsey, Cousin, Lewis and Stirewalt2002), may be partially exposed to the environment during vigorous tail movement and completely when the tail is removed during initial penetration of the skin.
In this paper, we present evidence from in vitro transformation of cercaria to schistosomulum that there is a major route of uptake of macromolecules through the nephridiopore. This paper uses the macromolecule fluorescently labelled poly-L-lysine to demonstrate this. We also show that the skin component linoleic acid has an effect on the very early schistosomulum to increase uptake of Lucifer Yellow into the pre-acetabular gland ducts, whereas when applied to older schistosomula, a dramatic uptake into the parasite occurs into the excretory/subtegumental region.
MATERIALS AND METHODS
Origin of parasite material, skin penetration and in vitro culture
A Puerto Rican isolate (PR) of Schistosoma mansoni was maintained in our laboratory in Biomphalaria glabrata snails and TO mice (Jones et al. Reference Jones, Breeze and Kusel1989).
Fluorescent and other chemicals
Lucifer Yellow CH lithium and potassium salt (molecular weight 457 Da), Hoechst 33258 (molecular weight 624 Da) and propidium iodide (molecular weight 668 Da) were purchased from Invitrogen (UK); FITC-labelled dextrans of molecular weights 4, 10, 20 and 70 kDa were purchased from Sigma (UK) and Invitrogen. FITC-labelled poly-L-lysine (10 kDa molecular weight) was obtained from Sigma-Aldrich (UK). All fluorescent chemicals are water soluble and were dissolved (1 mg per ml) in RPMI 1640 medium.
Paraformaldehyde and linoleic acid (L1376>99%) were obtained from Sigma-Aldrich.
Exposure of cercariae to fluorescent compounds during in vitro transformation
This has been fully described by Thornhill et al. (Reference Thornhill, Coelho, McVeigh, Maule, Jurberg and Kusel2009). Two experimental situations were described in which the cercariae were transformed in the presence of the fluorescent compounds (a), and in which the fluorescent compounds were added to transformed cercariae which had been incubated as schistosomula for up to 2 h (b).
(a) Cercariae were exposed to the fluorescent compounds during syringe transformation as follows. They were concentrated on ice for 30 min and reduced to a volume of 0·5 ml. Added to this suspension were 1·5 ml of RPMI 1640 and 100 μl of 1 mg per ml fluorescent Lucifer Yellow, FITC dextrans or FITC poly-L-lysine containing Hoechst 33258 and propidium iodide added to a final concentration of 10 μg per ml. Syringe transformation, with a 21 G needle, was carried out at 4°C and the mixture incubated at 4°C or at 37°C. (b) Lucifer Yellow, FITC dextrans or FITC poly-L-lysine, propidium iodide and Hoechst 33258 were also added to in vitro-transformed schistosomula after transformation had been completed. The freshly transformed schistosomula were washed and kept in RPMI 1640 in 10% foetal calf serum for up to 2 h at 37°C. During this incubation period, the schistosomula were washed 3 times in RPMI 1640 to remove the foetal calf serum and then they were exposed to 100 μl of 1 mg per ml fluorescent Lucifer Yellow or poly-L-lysine containing Hoechst 33258 and propidium iodide added to a final concentration of 10 μg per ml for 15 min at 37°C.
The use of propidium iodide to detect changes in the permeability of the surface membrane
The uptake of propidium iodide by cells or parasites is indicative of an increase in membrane permeability (Gillan et al. Reference Gillan, Evans and Maxwell2005). The use of propidium iodide as an indicator of increased membrane permeability in cells need not mean irreversible damage. It can mean an increase in membrane permeability at a region in the membrane (Goksor et al. Reference Goksor, Diez, Enger, Hanstorp and Nystrom2003; Shiu et al. Reference Shiu, Barbier, Di Cello, Choi and Stins2007). This has also been shown for mammalian cells being subjected to electroporation (Kennedy et al. Reference Kennedy, Ji, Hedstrom, Booske and Hagness2008) and for bacteria (Graca et al. Reference Graca, Vitoria, Loureiro-Dias, Rombouts and Abee2002) and uptake through calcium-dependent hemi-channels (Valiunas, Reference Valiunas2002). Propidium iodide used in the multicellular schistosome can reveal damage, which results in a rapid massive uptake of propidium iodide labelling many nuclei. With the results reported here, we are measuring a slight increase in surface and internal membrane permeability, where only several nuclei are labelled.
Incubation of cercariae and cultured schistosomula with linoleic acid
(a) Cercariae were sedimented on ice for 30 min, the supernatant reduced to a volume of 0·5 ml and RPMI 1640 added to a volume of 1·5 ml. A volume of 100 μl of RPMI 1640 containing 100 μg linoleic acid was added to one sample and 100 μl of RPMI 1640 to another (control). Lucifer Yellow and propidium iodide were then added as previously, and transformation to schistosomula was carried out and the schistosomula incubated at 4°C or 37°C. (b) Schistosomula were produced by syringe transformation. The freshly transformed schistosomula were washed and kept in RPMI 1640 in 10% foetal calf serum for up to 2 h at 37°C. During this incubation period, the schistosomula were washed 3 times in RPMI 1640 to remove the foetal calf serum and then divided into 2 tubes each containing 100 μl of 1 mg per ml fluorescent Lucifer Yellow, dextrans or poly-L-lysine containing Hoechst 33258 and propidium iodide added to a final concentration of 10 μg per ml. To one tube was added 100 μg linoleic acid in 100 μl of RPMI 1640 and the control tube received 100 μl of RPMI 1640. Both tubes were incubated for 15 min at 37°C.
In both experimental situations (transformation in the presence of labelled compounds (a) or transformation and then addition of labelled compounds (b)), all parasite samples were washed 3 times in RPMI 1640 before observation under a fluorescence microscope.
Fixation of schistosomula
Labelled schistosomula were fixed at 4°C for 4 h in 4% paraformaldehyde. The 4% paraformaldehyde was prepared by dissolving the solid in RPMI 1640 at 60°C.
Examination of parasites by fluorescence microscopy
All labelled parasites were viewed and photographed using a Leitz Orthoplan Laborlux S microscope. This allowed the variability of the labelling of the parasite population to be observed, counted and recorded. Photographs were taken after observation of the parasites under the 40× or 10× objectives. Further detail in the distribution of the labelled compounds was sought using a Zeiss microscope (Axioplan 2). All specimens in Laborlux and Zeiss microscopes were unfixed. All specimens examined in the confocal microscope were fixed in 4% paraformaldehyde. Confocal microscopy was carried out with the Leica TCS NT AOBS SP2 confocal microscope.
RESULTS
Uptake of FITC poly-L-lysine (10 kDa) during transformation at 4°C
The access of dextrans (3–20 kDa) to the parasite at 4°C appeared to occur through the nephridiopore (see Introduction section). Such molecules have no net charge at pH 7·4 (Invitrogen, UK). We therefore used a macromolecule of a molecular weight in this range, poly-L-lysine, which carries a net positive charge at pH 7·4. Poly-L-lysine has been used as a cationic protein to bind to schistosome surface membranes (McLaren et al. Reference McLaren, Peterson and Venge1984; Jones et al. Reference Jones, Helm and Kusel1988; Tan et al. Reference Tan, Thornhill, Al Adhami, Akhkha and Kusel2003).
When 10 kDa poly-L-lysine was used in the medium during transformation at 4°C, the images shown in Figs 1, 2 and 3 were seen. Under the Laborlux microscope it was observed that all schistosomula were labelled at the nephridiopore and there was some labelling of the internal region, probably the excretory bladder. Two of the 10 examined specimens showed labelling of tubules (Fig. 3b) which lead into the bladder. These may be excretory tubules.

Fig. 1. Binding of poly-L-lysine to schistosomula. Cercariae were transformed at 4°C in RPMI 1640 in the presence of 10 kDa poly-L-lysine. After 15 min on ice, the parasites were washed in RPMI 1640 and examined under the Laborlux S fluorescence microscope. Strong binding of poly-L-lysine was observed in all specimens at the nephridiopore. The fluorescence could, in some specimens, also be observed in the internal tissues of the schistosomula, which appeared to represent the excretory bladder and the excretory tubules. EB, excretory bladder; N, nephridiopore. Scale bars=25 μm.

Fig. 2. Binding of poly-L-lysine to schistosomula. The sample from Fig. 1 was examined in the Zeiss fluorescence microscope to show the binding at the nephridiopore and in the excretory bladder. EB, excretory bladder; N, nephridiopore.
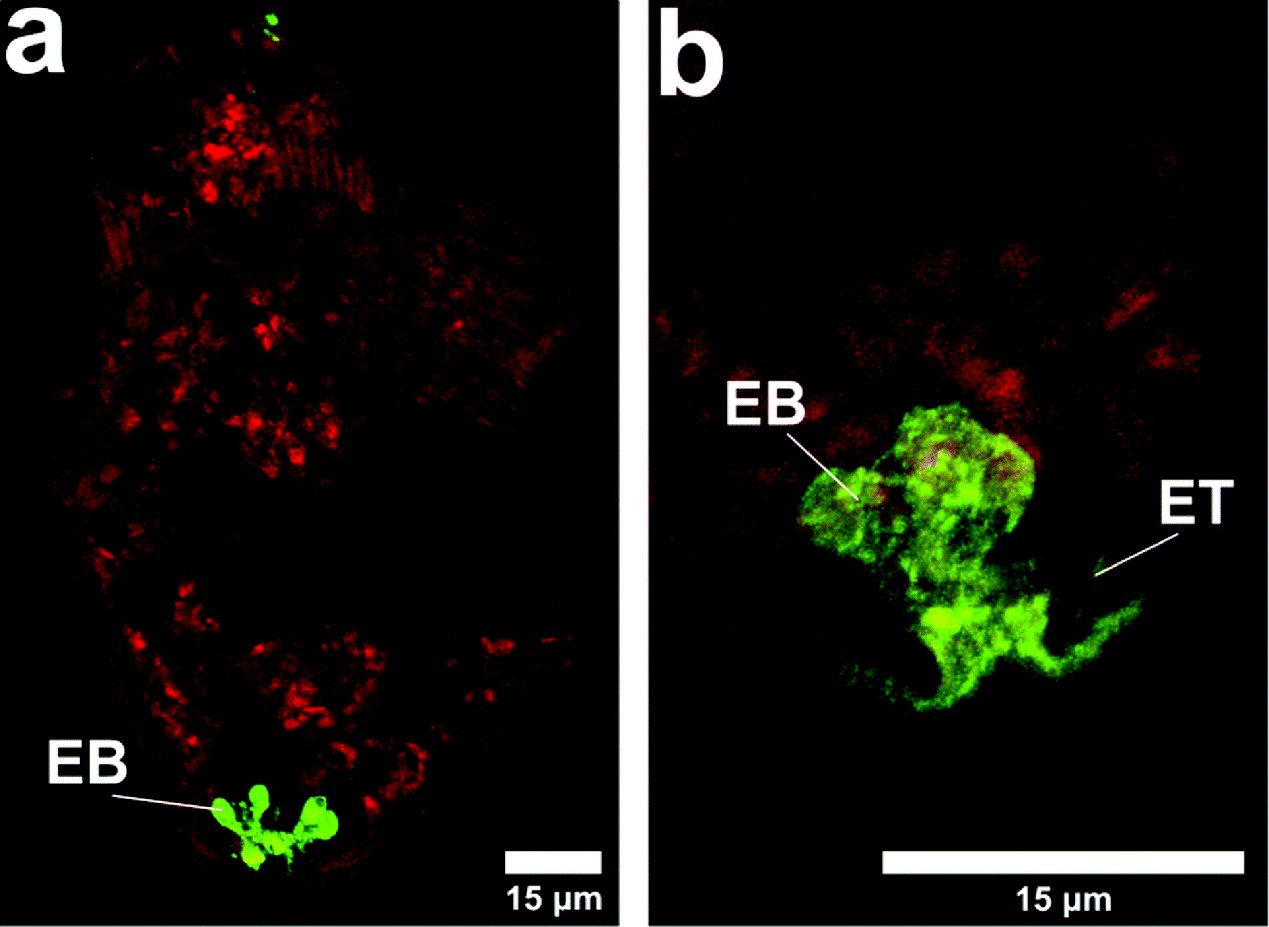
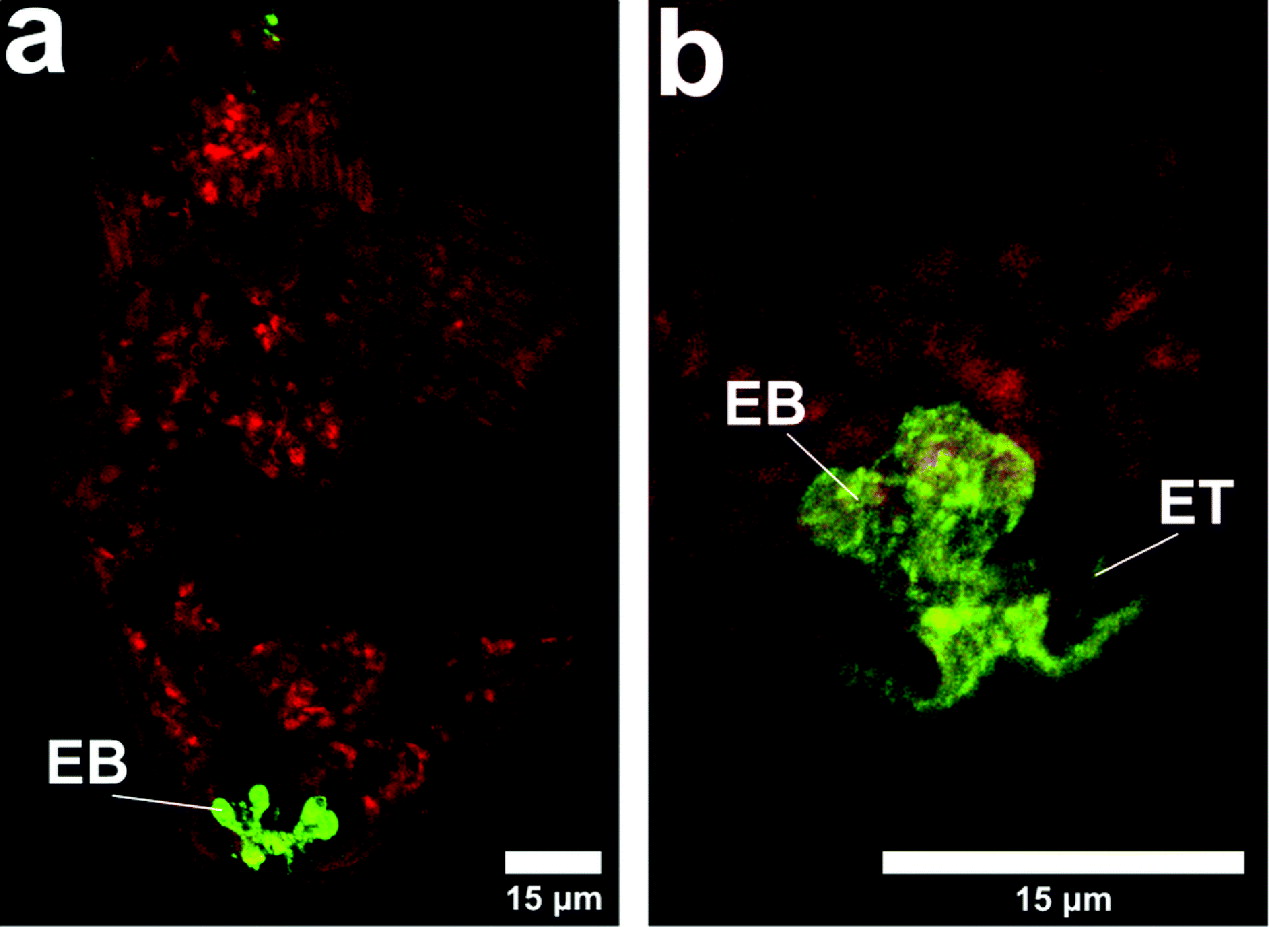
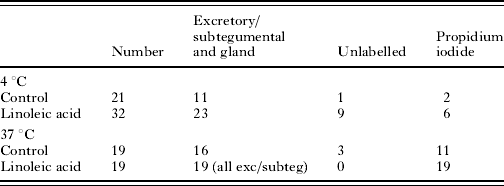
Fig. 3. Binding of poly-L-lysine to schistosomula. The sample from Fig. 1 was examined by confocal microscopy after fixation of the parasites with paraformaldehyde. The presence of the fluorescent poly-L-lysine could be seen at the nephridiopore and in the excretory bladder and in some specimens in the excretory tubules. The red background shows the presence of TRITC dextran (10 kDa). EB, excretory bladder; ET, excretory tubule.
During experiments with poly-L-lysine, TRITC dextran (10 kDa) (Figs 3a and b) was included in the labelling mixture and internal labelling by the dextran indicated that, although binding in this region of the nephridiopore, the poly-L-lysine did not obstruct the passage of other macromolecules.
Variability and the binding of poly-L-lysine
Thornhill et al. (Reference Thornhill, Coelho, McVeigh, Maule, Jurberg and Kusel2009) showed that in vitro transformation of cercariae in the presence of fluorescent compounds gave very variable percentage uptake into schistosomula when the results of different experiments were compared and a large proportion of schistosomula was poorly labelled. This was in contrast to the skin-derived forms, most of which were brightly labelled. When the images (Figs 1, 2 and 3) from the transformation in vitro in the presence of poly-L-lysine were considered, it was immediately apparent that influx into the nephridiopore was not variable. This suggests that although there is access to molecules via the nephridiopore, in a proportion of cercariae (in vitro transformed with Lucifer Yellow or dextrans) these membrane-impermeant molecules cannot be internalised further. Skin-derived forms, however, may receive signals from skin molecules which enable internalisation to take place. Signals received during skin penetration have been identified by Haas and colleagues (Haas et al. Reference Haas, Haeberlein, Behring and Zoppelli2008; Haeberlein and Haas, Reference Haeberlein and Haas2008). One of these, linoleic acid, was tested to ascertain whether it could stimulate uptake of membrane-impermeant molecules into schistosomula.
Effect of linoleic acid at 4°C and 37°C on the initial transformation of cercariae
Cercariae in ice were transformed with Lucifer Yellow and propidium iodide in the presence and absence of 60 μg ml−1 linoleic acid and then incubated at 4°C for a further 30 min or 1 h at 37°C. After washing in RPMI 1640, the parasites were examined under a fluorescence microscope. Table 1 shows the data from 2 experiments.
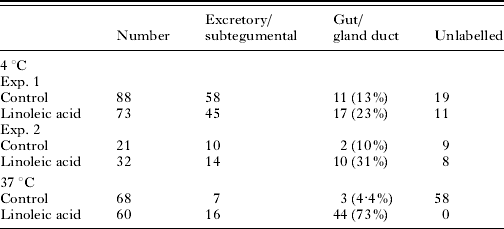
Table 1. The effect of linoleic acid on the uptake of Lucifer Yellow when present during in vitro transformation of cercariae to schistosomula
(Cercariae were transformed at 4°C into schistosomula in RPMI 1640 and Lucifer Yellow in the presence and absence (control) of 60 μg ml−1 linoleic acid. One group remained at 4°C for 30 min and the other was incubated for 1 h at 37°C. All parasites were then washed and observed under the fluorescence microscope. The total numbers of schistosomula are shown and the number of schistosomula in each category is shown.)
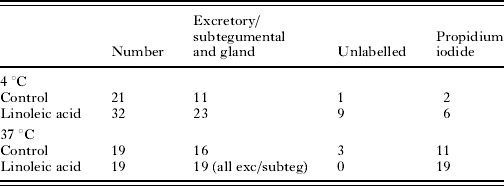
The schistosomula kept at 4°C showed that in the presence of linoleic acid, there was a significant effect on the accumulation of the Lucifer Yellow into the pre-acetabular gland ducts and gut (Fig. 4). In those cercariae which were transformed and incubated for a further 1 h at 37°C in RPMI 1640, the linoleic acid had a greater effect than was detected at 4°C. At 4°C, of 68 schistosomula examined, 3 had gland/gut labelling. At 37°C, 44 out of 60 schistosomula examined had gland/gut labelling. Thus, there was considerable increase in the labelling of the gut and gland ducts. Linoleic acid had only a slight effect on the incorporation into the excretory/subtegumental region at this early stage in transformation (Table 1).

Fig. 4. Binding of probes to schistosomula, showing location of the gut and pre-acetabular gland tubules. The preacetabular gland ducts are shown stained with alizarin (c and d) specific for calcium and with 10 kDa FITC dextran (b). Image (a) shows the appearance of the gut and oesophagus after Lucifer Yellow labelling. During and after transformation, cercariae could be labelled in these ducts, especially in the presence of linoleic acid (Table 1). DPrAc, ducts of preacetabular glands; Es, oesophagus. Scale bars=25 μm.
Effect of linoleic acid on schistosomula after a 2-hour incubation in RPMI 1640 and 10% foetal calf serum
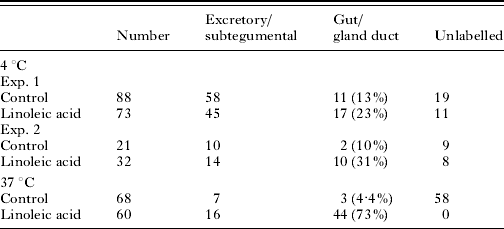
After transformation, schistosomula were cultured for 2 h at 37°C and thereafter exposed to membrane-impermeant Lucifer Yellow. It was found that 60 μg ml−1 linoleic acid significantly stimulated the uptake of this molecule into the excretory/subtegumental tubule region of the schistosomula (Table 2), but not into the glands as was found in the above experiments with freshly transformed parasites (Table 1). Variable numbers were unlabelled between experiments.
Table 2. The effect of linoleic acid on the uptake of Lucifer Yellow by schistosomula
(Schistosomula were cultured for 2 h at 37°C without Lucifer Yellow. Lucifer Yellow was added in the presence and absence (control) of linoleic acid. The labelling recorded is that which occurred in the excretory tubule subtegumental region. The total numbers of schistosomula counted are shown.)
* Student's t-test (two tailed); P<0·001.
Uptake of propidium iodide during and after transformation of cercariae
We have used propidium iodide as a marker for any change in surface membrane permeability of individual parasites during their incubations under different conditions. The uptake of Lucifer Yellow has been recorded in the same parasite and was located in either the excretory/subtegumental region of the body or the pre-acetabular gland ducts and gut region.
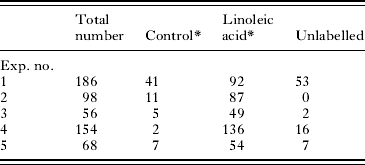
We have recorded the uptake of propidium iodide at 4°C during syringe transformation and after subsequent incubation at 37°C. At 4°C, propidium iodide remained in the region of the nephridiopore and Lucifer Yellow was widely dispersed within the parasite (data in Fig. 4, Thornhill et al. Reference Thornhill, Coelho, McVeigh, Maule, Jurberg and Kusel2009). After a 1-h incubation of schistosomula at 37°C in the presence of Lucifer Yellow and propidium iodide, uptake of Lucifer Yellow was often, but not always, accompanied by uptake of propidium iodide (Table 3). We conclude from this that in a variable proportion of parasites, uptake of Lucifer Yellow occurs without the membrane permeability change indicated by propidium iodide uptake.
Table 3. Uptake of propidium iodide by cercariae transformed in Lucifer Yellow and propidium iodide and incubated in RPMI 1640 for 1 h
(Cercariae were transformed on ice in the presence of Lucifer Yellow (in excretory/subtegumental region) and propidium iodide and incubated at 37°C for 1 h in RPMI 1640. The parasites were washed and counted under the fluorescence microscope.)

Uptake of propidium iodide during and after transformation of cercariae in the presence and absence of linoleic acid
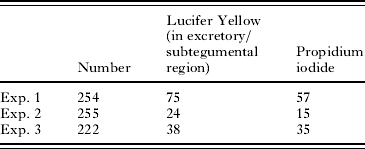
Incubation of schistosomula for 2 h after transformation was followed by exposure to Lucifer Yellow and propidium iodide in the presence and absence of linoleic acid. The schistosomula were exposed at 4°C (to slow membrane metabolism) and 37°C. In the absence of linoleic acid at 4°C, few of the schistosomula which showed labelling of Lucifer Yellow were propidium iodide positive. After linoleic acid treatment at 4°C, 11/16 labelled parasites were propidium iodide positive. At 37°C, linoleic acid induced all of the schistosomula to show excretory/subtegumental labelling and 100% showed propidium iodide labelling. These results strongly suggest that linoleic acid stimulates cross-membrane transit of Lucifer Yellow (Table 4).
Table 4. The uptake of propidium iodide by 2-h-old schistosomula in the presence of Lucifer Yellow
(Schistosomula were incubated on ice or at 37°C. Cercariae were transformed on ice and incubated for 2 h in RPMI 1640 plus 10% foetal calf serum at 37°C. The parasites were washed and Lucifer Yellow and propidium iodide added in the presence and absence of linoleic acid.)
DISCUSSION
The observation that macromolecules up to 20 kDa molecular weight entered the schistosomulum either during skin penetration or in vitro transformation (Thornhill et al. Reference Thornhill, Coelho, McVeigh, Maule, Jurberg and Kusel2009) has been examined with the objective of determining the route by which the molecules enter the parasites. Molecules might enter the tissues of the parasite through the surface membrane or through several apertures in the surface. The orifices of the excretory pore (nephridiopore), those leading to the pre- and post-acetabular glands, that leading to the oesophagus and those leading to the head glands are all possible entry points. When Lucifer Yellow or any of the different molecular weight fluorescent dextrans were used during in vitro transformation, all schistosomula were labelled in the surface membrane and the nephridiopore. The oesophagus, ducts of the pre-acetabular glands and head glands were sometimes labelled. We therefore formed the hypothesis that molecules entered the parasite either through the surface membrane or through the nephridiopore or both.
Thornhill et al. (Reference Thornhill, Coelho, McVeigh, Maule, Jurberg and Kusel2009) showed the pattern for Lucifer Yellow, TRITC and FITC dextrans and propidium iodide that was obtained when parasites were transformed on ice (4°C) and examined after 3 min. Much of the propidium iodide was concentrated in the posterior region close to the nephridiopore. Lucifer Yellow and dextrans had a more interior distribution. These images provide evidence that, in the very early stages of transformation at least, macromolecules and membrane-impermeant propidium iodide enter the parasite through the aperture of the excretory system. Staining by propidium iodide is used to detect small changes in membrane or tubule permeability and not a massive effect seen during damage as was explained in the Materials and Methods section.
We sought a molecule which, because it carried a net positive charge at pH 7·4, might be delayed in its uptake into the parasite due to binding to negatively charged groups. We chose FITC poly-L-lysine as it has been used as a model for eosinophil cationic protein, and has been shown to bind to schistosome membranes (Jones et al. Reference Jones, Helm and Kusel1988; Tan et al. Reference Tan, Thornhill, Al Adhami, Akhkha and Kusel2003). All the schistosomula could be seen to be labelled in the nephridiopore itself, but many showed heavy labelling of what seemed to be the excretory bladder. In some specimens, tubules leading into the bladder could be seen. This is firm evidence that 10 kDa poly-L-lysine entered the nephridiopore and was rarely found bound to other membranes under these conditions of transformation. TRITC dextran 10 kDa, co-incubated with the poly-L-lysine during transformation entered the parasite and distributed to various tissues. TRITC dextran was not as clear as poly-L-lysine, since its lack of amino groups (Leypoldt and Henderson, Reference Leypoldt and Henderson1993) allowed limited preservation by paraformaldehyde. Nevertheless, the TRITC dextran was seen in the nephridiopore and bladder region, as well as internal tissues.
The conclusions from this work are that under the syringe transformation conditions, chosen to be carried out at 4°C to limit the activity of the surface membrane in transport activity and endocytosis (Ribeiro et al. Reference Ribeiro, Coelho, Vieira, Powell and Kusel1998), macromolecules and other membrane-impermeant molecules can enter the parasite and be distributed to internal tissues through the nephridiopore and excretory bladder. The role of the surface membrane in molecule uptake at 4°C is assumed in our arguments not to be important, but there is a possibility that any permeability increase in the surface membrane during its remodelling might be enhanced at 4°C. We think this unlikely because poly-L-lysine was not found bound at the surface and propidium iodide did not enter the parasite in the subtegumentary position under these conditions.
The original observation that penetration of the skin by cercariae allowed uptake of macromolecules (Fig. 2 of Thornhill et al. Reference Thornhill, Coelho, McVeigh, Maule, Jurberg and Kusel2009 ) may be partially explained by their passage into the excretory system and from that location their rapid diffusion into body tissue spaces, perhaps through gap junctions (Dorsey et al. Reference Dorsey, Cousin, Lewis and Stirewalt2002). These macromolecules may also pass through the surface membrane, since increased permeability to Hoechst 33258 and propidium iodide was observed in some parasites during culture at 37°C. The addition of linoleic acid to schistosomula in culture in the presence of Lucifer Yellow significantly increased the uptake of Lucifer Yellow into the subtegumental/excretory tubule region of the parasite. The linoleic acid may interact with the surface membrane of the schistosomulum and increase cell membrane permeability (Haas, Reference Haas1984) as unsaturated fatty acids can do in mammalian cells (Oliveira et al. Reference Oliveira, Nascimento, Freire, Moreira, Scofano, Barrabin and Mignaco2008). The interesting work of El Ridi and colleagues gives a different explanation for the effects of unsaturated fatty acids on the surface membrane involving their effects on sphingomyelin metabolism. Increased hydrolysis of sphingomyelin reveals surface antigens and possibly transporters (Tallima et al. Reference Tallima, Salah and El Ridi2005).
One unexpected effect of linoleic acid on freshly transformed schistosomula was its mediation of a very large increase in the uptake of Lucifer Yellow by the pre-acetabular gland ducts. The ability of linoleic acid to stimulate the holocrine secretion (Curwen et al. Reference Curwen, Ashton, Sundaralingam and Wilson2006) of these glands may be the explanation for this enhanced labelling (Fusco et al. Reference Fusco, Salafsky and Delbrook1986; Mikes et al. Reference Mikes, Zidkova, Kasny, Dvorak and Horak2005). The pronounced muscular activity during secretion of these glands might accelerate uptake under these conditions.
We have used the uptake of propidium iodide to decide whether the membrane-impermeant Lucifer Yellow enters the parasite through the surface membrane. At 4°C, uptake of Lucifer Yellow occurs without propidium iodide uptake into the parasite, except at the nephridiopore. In the absence of linoleic acid at 37°C, a proportion of parasites shows uptake of Lucifer Yellow without propidium iodide uptake, and we conclude that this cannot take place through the surface membrane in these parasites unless transporters exist (Cao et al. Reference Cao, Steinberg, Neu, Cohen, Horwitz, Hickman and Silverstein1993). At 37°C in the presence of linoleic acid, all parasites which take up Lucifer Yellow, also show uptake of propidium iodide. As described in the Materials and Methods section, we interpret this uptake as a physiological change in membrane permeability, and not as damage to the membrane.
During skin penetration, many molecules such as ceramides and glycolipids as well as linoleic acid (Haas et al. Reference Haas, Haeberlein, Behring and Zoppelli2008) will have effects on the permeability of the surface membranes and the results we have obtained from skin (Thornhill et al. Reference Thornhill, Coelho, McVeigh, Maule, Jurberg and Kusel2009) can be explained by an influx through both the nephridiopore and the surface membrane. A summary of these results is shown in Fig. 5.

Fig. 5. This diagram summarizes the results in this paper and in Thornhill et al. (Reference Thornhill, Coelho, McVeigh, Maule, Jurberg and Kusel2009). Cercariae are shown penetrating skin into which fluorescent macromolecules have been injected (Thornhill et al. Reference Thornhill, Coelho, McVeigh, Maule, Jurberg and Kusel2009). Cercariae penetrating the skin (left) experience influx of Lucifer Yellow (LY; green) to label the subtegumental region close to the excretory tubules within the schistosomulum (S/E). Tissues become labelled with propidium iodide (PI; 15 min to 2 h; pink spots). This process continues for at least 2 h. To reproduce this in vitro, syringe transformation is carried out in the presence of the macromolecules and at 4°C a proportion of schistosomula shows the same pattern of uptake as the skin forms (subtegumental/excretory tubule region), but the propidium iodide remains at the region of the nephridiopore (N). A proportion becomes only very weakly labelled (D; diffuse green). At 4°C in the presence of linoleic acid (LA), the weakly labelled forms show pronounced uptake into the pre-acetabular gland ducts (DPrAc) and the oesophagus. At 37°C, transformation in the presence of the macromolecules shows a percentage of schistosomula labelled in the subtegumental/excretory tubule region and also diffusely labelled forms. The propidium iodide can be seen in internal tissues in some parasites. The presence of linoleic acid increases the proportion of parasites labelled in the pre-acetabular gland ducts. After 2 h, schistosomula previously unexposed to fluorescent macromolecules are incubated with these molecules and there is a mixture of forms labelled in the subtegumental/excretory tubule region and diffusely labelled. Addition of linoleic acid yields a very high proportion labelled in the subtegumental/excretory tubule region.
It is possible that large molecules can enter the surface membrane through pores (Braschi and Wilson, Reference Braschi and Wilson2006). Pores in membranes of mammalian cells have been demonstrated by the uptake of a variety of dextrans (Dong et al. Reference Dong, Patel, Saikumar, Weinberg and Venkatachalam1998), and fenestrations in blood capillaries by transmission electron microscopy (Milici et al. Reference Milici, L'Hernault and Palade1985). It is also possible that the remodelling of the parasite surface which occurs during skin penetration could contribute to an increased permeability.
We can also consider the effects of stress during tissue migration on the properties and permeability of the surface membrane of schistosomes and indeed any tissue-dwelling or migrating parasite. In mammalian cells, deformation of the plasma membrane can greatly affect the synthesis of the surface (Vlahakis and Hubmayr, Reference Vlahakis and Hubmayr2003; LaPlaca et al. Reference LaPlaca, Prado, Cullen and Irons2006; Booth et al. Reference Booth, Edwards, Black, Schumann and Miller2007) and its permeability (Silver and Siperko, Reference Silver and Siperko2003).
In conclusion, the uptake of macromolecules by cercariae as they transform into schistosomula can occur through the nephridiopore and excretory tubules and the surface membrane. Surface membrane pores may be formed during the massive expenditure of energy during skin penetration and surface changes during remodelling may contribute to the increase in permeability. Further work is needed to discover both the causes of permeability change and the consequences for the parasite of molecule uptake.
ACKNOWLEDGEMENTS
We are immensely grateful to Professor Paul Hagan for continued support and encouragement, Dr Andrew MacDonald for provision of infected mouse livers, Dr Catherine Clark for superb editing, Caroline Morris for the artwork in Fig. 5 and Mr Joseph Ndabahweje whose vision was in this work.
FINANCIAL SUPPORT
This work was supported by The Leverhulme Trust (J.A.T. and J.R.K., grant number EM20252); John Robertson Bequest (J.A.T. and J.R.K., grant number JR08/06); Fundação para a Ciência e a Tecnologia (A.D.J., grant number SFRH/BD/33562/2008).