Introduction
Reduction spots (or reduction spheroids) occur within red beds as grey, green or mottled spherical areas of sediment. These spheroids transect depositional boundaries and are therefore thought not to be depositional features, but zones of secondary reduction (Turner Reference Turner1980). The grains in the surrounding reddened sediment in red beds are coated with pigmentary iron oxides, such as haematite and/or goethite, which precipitated onto the grains' surface in the presence of oxidizing groundwater. Grains in reduction spots lack surface iron-oxide pigment.
A distinguishing feature of reduction spots is the common occurrence of dark cores in their centres. The dark cores in reduction spots commonly contain minerals rich in metallic and rare-earth elements (REEs) and/or organic matter (Hofmann Reference Hofmann1990).
Some typical minerals found in these cores are chalcopyrite, vanadian mica (roscoelite), chalcocite, niccolite, native copper, native silver, maucherite (Harrison Reference Harrison1975) and pyrite (Hofmann Reference Hofmann1990; this paper). These minerals account for the concentrations of elements such as As, V, Ni, Cu, U and S in the cores (Hofmann Reference Hofmann1990).
The spheroidal morphology of reduction spots indicates that they were not formed at the sediment surface during deposition. In some instances, the spheroids appear to have been vertically shortened, suggesting they were formed pre-compaction during the early stages of diagenesis. However, many show no evidence of vertical shortening and exhibit almost complete sphericity, suggesting they were formed at depth post-compaction.
The occurrence of reduction spots in red beds can be seen throughout the geological record from the late Proterozoic age and throughout the Phanerozoic age, but are observed widely in the Permo-Triassic age.
In Scotland there are various exposures of red beds containing reduction spots: the Devonian of Banffshire (Van Panhuys-Sigler et al. Reference Van Panhuys-Sigler, Trewin and Still1996) and Dingwall (Parnell Reference Parnell1985; Parnell & Eakin Reference Parnell and Eakin1987), the Carboniferous of North Berwick (Hofmann Reference Hofmann1993), the Triassic of Gruinard Bay (author's observation; Evans Reference Evans1982) and the Mesoproterozoic lower Torridonian rocks of NW Scotland (this paper).
Various mechanisms responsible for the formation or reduction spots have been proposed. Many workers have related their formation to the presence of detrital organic material within the cores (Hartmann Reference Hartmann1963; Van de Poll & Sutherland Reference Van de Poll and Sutherland1976; Durrence et al. Reference Durrence, Meads, Ballard and Walsh1978; Myakura & Hampton Reference Myakura and Hampton1984), migrating hydrocarbons from leaking reservoirs (Parnell & Eakin Reference Parnell and Eakin1987) and the reducing potential of vanadium present within the cores (Schreiter Reference Schreiter1929; Eichhoff & Reineck Reference Eichhoff and Reineck1952). These models, however, present problems, as we will discuss in the following sections. A very likely cause of the formation of reduction spots is the activity of bacteria or microbes, which was first proposed by Keller (Reference Keller1929) and was highlighted by Hofmann (Reference Hofmann1990). Using new isotopic data from sulphides present in Triassic reduction spot cores used as an analogue, we present the following findings under the proposed model of microbial synthesis of reduction spots. Here we document the occurrence of reduction spots in the Mesoproterozoic Stoer Group of NW Scotland, discuss their potential microbial origin and explore the significance of potential evidence for life in terrestrial rocks of this age.
Sample Location
Mesoproterozoic
The Mesoproterozoic Torridonian rocks of NW Scotland (Fig. 1) contain breccias, pebbly sandstones, sandstones and shales. The Torridonian ‘Supergroup’ unconformably overlies Archaean Lewisian Gneiss, which forms the basement of much of NW Scotland. The Torridonian ‘Supergroup’ has been divided into three groups: the Stoer Group, the Sleat Group and the Torridon Group (Stewart Reference Stewart2002), the oldest being the Stoer Group (~1150–1200 Ma). The Torridonian is only slightly metamorphosed, offering an excellent opportunity to study the depositional palaeoenvironment and diagenesis.

Fig. 1. Geological map of the Mesoproterozoic Torridonian rocks of NW Scotland. Sample location at Stoer Bay is indicated. Map of Scotland shown inset.
Stratigraphically, the Stoer Group can be divided into three formations (Fm): the basal Clachtoll Fm, the Bay of Stoer Fm and the Meall Dearg Fm (Fig. 2).
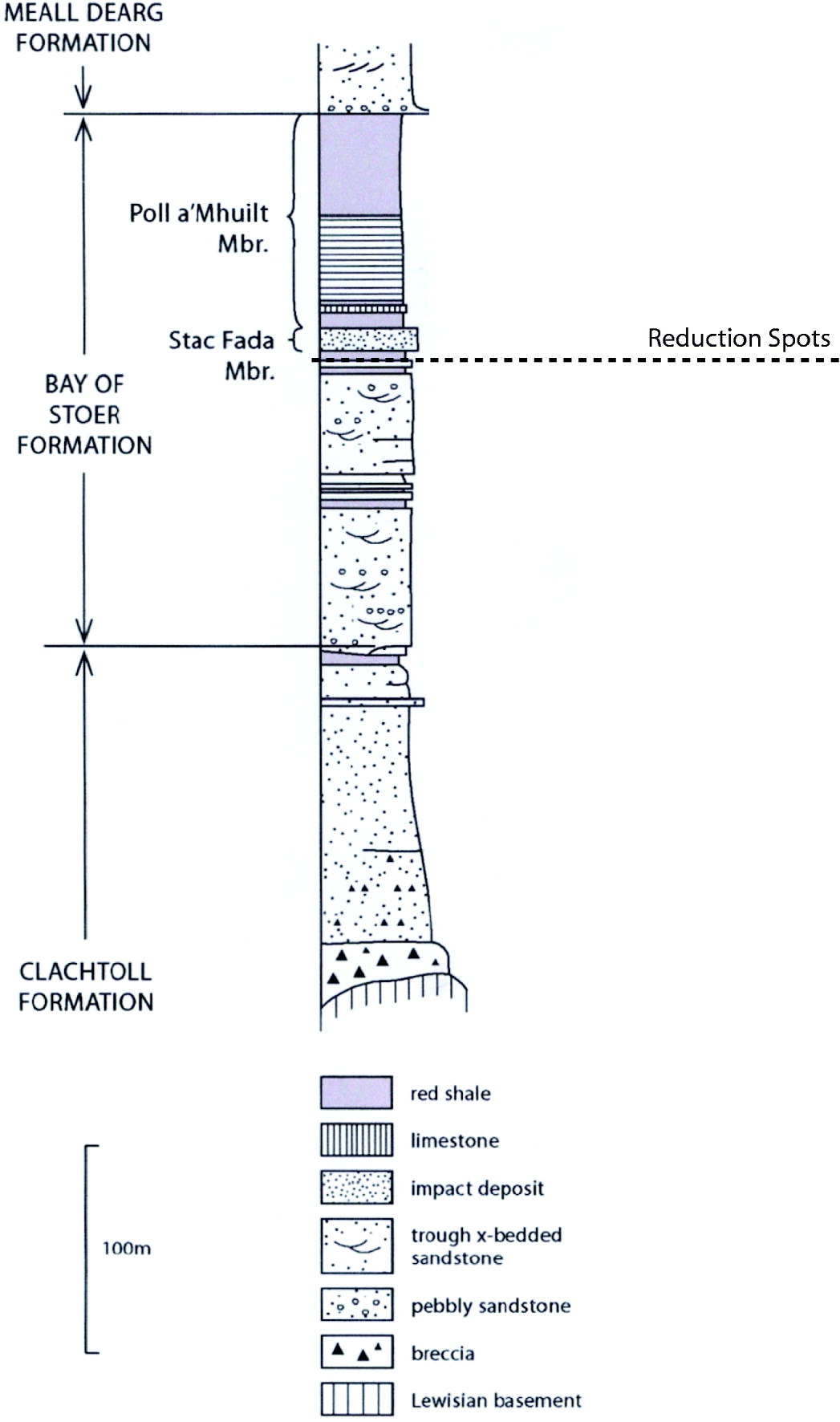
Fig. 2. Simplified stratigraphy of the Stoer Group at the type locality at Bay of Stoer. Sample location is highlighted by the dashed line.
The Bay of Stoer Fm, which is further subdivided into various stratigraphic members (Mbr), consisting of trough cross-bedded fluviatile sandstones and overlying lacustrine siltstones and shales. A defining boundary between these facies is a distinctive ~20 m thick impact ejecta deposit named the Stac Fada member (Amor et al. Reference Amor, Hesselbo, Porcelli, Thackrey and Parnell2008).
Immediately below the Stac Fada Mbr at Stoer Bay are silty and fine sandstone red beds with some localized zones of reduced sediment. Areas of reduced sediment are evident in the form of spheroids and undulating sub-horizontal zones. These areas of reduced sediment are grey or green in colour and contrast markedly against the surrounding reddened sediment.
Triassic
As an analogue, reduction spots from the Triassic Mercia Mudstone Group, which outcrop at Larne, Northern Ireland, were collected. The Permo-Triassic red bed sequences of Northern Ireland contain reduction spots in several localities. The spots range from 1 to 3 cm in diameter and a small proportion exhibit small cores 1–3 mm in diameter (Parnell Reference Parnell1988). Many of these reduction spots contain macroscopic pyrite (locally microscopic) within their cores. Framboidal pyrite has also been identified in the cores of these reduction spots.
Secondary reduction in sediment
Areas of reduced sediment within red beds occur in various forms: spheroids (spots) millimetres to decimetres in size, non-continuous boundaries which show a rough relationship to bedding, and whole units of stratigraphic thickness. The former are attributed to the localized reducing nature of an in situ medium and the migration of reducing groundwater, respectively, whereas the latter are attributed to the larger-scale, long-term presence of reducing groundwater (Turner Reference Turner1980).
Reduction spots that occur at the Bay of Stoer are sub-spherical and ~3 cm in diameter (Fig. 3(a) and (c)) with dark cores, which have a metallic-like lustre. The cores of these spots contain the vanadium-rich mica roscoelite. Roscoelite has commonly, but not ubiquitously, been found in the cores of reduction spots throughout the Phanerozoic age (Hofmann Reference Hofmann1993).

Fig. 3. (a) Reduction spot with dark ‘metallic’ core. Box highlights the field of view in B (5 mm×5 mm). (b) SEM X-ray element map of vanadium concentration within the core of reduction spot A. Bright areas show high vanadium concentration (roscoelite). (c) Reduction spot with rhomb-shaped core. The box highlights the field of view in (d) (5 mm×5 mm). (d) SEM X-ray element map of vanadium concentration within the core of reduction spot (c). Bright areas show high vanadium concentration (roscoelite).
Analyses
Bay of Stoer (Mesoproterozoic)
Scanning Electron Microscopic (SEM) X-ray analysis was conducted on the reduced sediment in the reduction spots and their ‘metallic’ cores, and minerals were identified by analysis of elemental spectra. In addition to the expected siliciclastic sedimentary rock-forming minerals, a significant component of the reduction spot is the barium sulphate, barite. Xenotime, an yttrium phosphate mineral, is also widely evident within the reduced sediment. Within the core there is a widespread abundance of the vanadium-rich mica, roscoelite. Roscoelite was identified by comparison of an X-ray backscatter spectrum (Fig. 4) with peaks in Al, Si, K, V and Fe to spectra of known occurrences of roscoelite. An additional occurrence of a mineral with a vanadium constituent is the vanadium-rich end member of xenotime, wakefieldite.

Fig. 4. SEM X-ray spectrum of the mineral roscoelite in the core of a reduction spot found at Stac Fada. The spectrum is consistent with other known occurrences of roscoelite.

Fig. 5. SEM backscatter micrograph of framboidal pyrite present in the core of a reduction spot found at Larne. The bright minerals indicate pyrite, identified by X-ray spectrum analysis.
Elemental X-ray mapping was conducted on the reduction spot cores. The element maps for V (Fig. 3(b) and (d)) show clear concentration in the cores of the reduction spots. No vanadium (roscoelite) is present in the sediment surrounding the cores, suggesting a localized formation mechanism within the cores.
Larne (Triassic)
Reduction spots with dark cores were analysed with SEM X-ray backscatter. The composition of the cores is similar to the Mesoproterozoic reduction spot cores. However, there is a high concentration of the vanadium oxide, montroseite, identified by comparison of elemental X-ray spectra to other known occurrences of the mineral. There are no other vanadium phases evident in the cores analysed from this location. Montroseite has been identified previously in Triassic reduction spots (e.g. Weibel & Friis Reference Weibel and Friis2004) and should therefore not be regarded as unusual.
Samples with evident sulphides (pyrite) present in their cores were selected for sulphur isotope analysis. The samples were crushed to a fine powder using a tungsten mill. The powder was then processed through heavy liquid mineral separation to separate the pyrite. The pyrite-rich mass was processed in an ultrasonic bath to achieve further isolation of the pyrite by removal of other material adherent to the pyrite. The pyrite was then analysed by the mass-spectrometry techniques outlined by Robinson & Kusakabe (Reference Robinson and Kusakabe1975) and Coleman & Moore (Reference Coleman and Moore1978). Analysis was conducted on a VG Isotech SIRA II mass spectrometer. δ34S values (34S/32S) of sulphides were determined as −2.1‰ and –2.2‰ (mean=–2.15‰). A value for sulphate in the approximate stratigraphic equivalent, the Norian Trent Formation; Mercia Mudstone Group in the English East Midlands, was taken as δ34S=+17.6‰ (Taylor Reference Taylor1983). Isotopic fractionation between sulphate and sulphide in the Upper Triassic Mercia Mudstone Group (expressed as Δ34S=δ34Ssulphate – δ34Ssulphide) reduction spot core pyrite is Δ34S 19.75‰.
Discussion
The formation mechanism of reduction spots is debated. It was widely thought that the presence of organic matter in the cores of reduction spots was the cause of the localized reducing conditions (Miller Reference Miller1910; Mempel Reference Mempel1960; Prest et al. Reference Prest, Steacy and Bottrill1969; Wu Reference Wu1971; Manning Reference Manning1975; Durrence et al. Reference Durrence, Meads, Ballard and Walsh1978; Turner Reference Turner1980), but Hofmann (Reference Hofmann1993) showed that much of this was based on an assumption that the dark colour present in the cores was due to organic matter. However, roscoelite appears dark brown in the cores of reduction spots when weathered, much like the appearance of organic-rich rocks. There are arguments against a detrital organic carbon (OC) component causing adequate reducing conditions to reduce the sediment observed in reduction spots. The calculations summarized in Hartmann (Reference Hartmann1963) and Hofmann (Reference Hofmann1990) show that the volume of OC required to adequately reduce the surrounding sediment, as is commonly observed in reduction spots, is far greater than is contained in the cores. Evidence of primary sedimentary lamination transecting the cores excludes the possibility that the cores represent large organic fragments (Turner Reference Turner1980). Therefore, reduction is unlikely to be a result of the oxidation of detrital OC.
The analyses in Hofmann (Reference Hofmann1993) show that the majority of reduction spot cores are not enriched in reduced OC; however, roscoelite is a very common mineral present in the cores. Cores that do show enrichment of OC, however, do not contain roscoelite. This may suggest that reduction spots with cores enriched in OC formed by a different mechanism to those with little or no OC.
The role of microorganisms in the secondary reduction of oxidized sediment is a possibility. Hofmann (Reference Hofmann1990) demonstrates that the formation of reduction spots requires a reductant, which is kinetically inert and catalytically sustained, arguing for microorganisms as such a catalyst.
The presence of sulphides within the cores of reduction spots is probably the result of the reduction of in situ sulphate. The formation temperatures for reduction spots are deduced to be low/moderate temperatures (<100°C) (Hofmann Reference Hofmann1990), at which temperatures the only possible fractionation mechanism is through the activity of sulphate-reducing microbes (Trudinger et al. Reference Trudinger, Chambers and Smith1985; Machel Reference Machel and Marshall1987). Based on the minimal compaction the reduction spots have suffered, it is likely that the temperature during the formation of the reduction spots was below 100°C.
Sulphate fractionated by non-biological kinetic mechanisms has a maximum Δ34S of up to ~20‰ (Machel Reference Machel2001). Maximum fractionation by non-biological means occurs under high temperatures (up to ~170°C). Biological sulphate reduction fractionates by 4–46‰ with higher values occurring due to repeated phases of oxidation and reduction (Canfield & Teske Reference Canfield and Teske1996). The cores of the reduction spots at Larne contain sulphides with a Δ34S value of about 20‰. This is at the upper limit of thermofractionation of sulphate, but is well within the range of fractionation possible through the action of sulphate reducing microbes. Whilst we are unable to completely discount the possibility of a kinetic formation mechanism, in this case a microbial formation mechanism for the sulphides in the cores of the reduction spots is more likely.
In addition, the presence of framboidal pyrite in the cores of the Triassic reduction spots may support a microbial formation mechanism. Framboids are crystal aggregates often composed of pyrite and display a raspberry-like morphology. There are believed to be inorganic formation mechanisms of framboids (Wilkin & Barnes Reference Wilkin and Barnes1996), but the abundance of framboids in areas below the redox transition zone suggests that there may also be a biogenic formation mechanism (e.g. Kohn et al. Reference Kohn, Riciputi, Stakes and Orange1998; Schrieber Reference Schrieber2002; Popa et al. Reference Popa, Kinkle and Badescu2004). The morphology of framboids is believed to have been inherited from a pre-existing spherical body (Rickard Reference Rickard1970), such as microbial cells or clusters of microbes (Scheiderhöhn Reference Scheiderhöhn1923). The pyrite framboids present in the reduction spot cores at Larne (Fig. 5) may therefore be the mineralized remnants of microbes present in the sediment. If so, it is very likely that the presence of microbes caused the secondary reduction in the sediment.
Mesoproterozoic life was limited to microbial colonies, most of which were ocean-borne. Evidence of terrestrial life during this time is relatively rare and is also restricted to traces of microbes. Sulphate Reducing Microbes (SRM) are known to have existed during this time (e.g. Canfield et al. Reference Canfield, Habicht and Thamdrup2000; Johnston et al. Reference Johnston, Farquhar and Canfield2007). Sulphides formed by the microbially induced reduction of sulphates have been well documented in the Proterozoic record (e.g. Guo et al. Reference Guo2009). However, most of the occurrences have been in marine sediments.
The Stoer Group rocks are well established as terrestrial in origin (Stewart Reference Stewart2002). Terrigenous sulphide minerals readily oxidize to soluble sulphate in oxic environments. Sulphate is integrated into the hydrosphere where it is reduced to sulphides, such as pyrite, by SRM. Sulphate accumulation in the open oceanic system is influenced by both terrigenous and volcanic sources and represents a mean global sulphate level. Terrestrial hydrologic systems, however, are influenced by the sulphur content of the surrounding rock. The lower level of sulphate in many terrestrial environments, in comparison to marine environments with higher sulphate concentrations, likely inhibited mass colonization by sulphate-reducing microbes. The presence of reduction spots in the Stoer Group may indicate the presence of microbial life in a 1.2 Ga terrestrial environment.
The depth of formation of the Stoer reduction spots may have implications of a deep biosphere during the Mesoproterozoic age. There is widespread evidence for an extensive deep biosphere today (e.g. Parkes & Wellsbury Reference Parkes, Wellsbury and Bull2004; Wilcock Reference Wilcock2004; Jørgensen & Boetius Reference Jørgensen and Boetius2007), but relatively little evidence in the deep geological record. Many of the reduction spots exhibit almost complete sphericity, indicating that they formed in the sediment post-compaction. If we assume a microbial formation mechanism, we can place constraints on the depth of formation. The upper temperature limit for microbial inhabitancy is ~125°C (Kashefi & Lovley Reference Kashefi and Lovley2003). This equates to a maximum depth of around 4000 m. As some spots show evidence of vertical shortening, it may be possible that there was some reduction spot formation before complete compaction. Reduction spot formation at depth implies that microbes had not only colonized the terrestrial environment in the Mesoproterozoic age, but also the deep subsurface.
Conclusions
Reduction spots in the Stoer Group are not likely to have formed due to the presence of detrital organic matter in the sediment. The role of microbes in the reduction of oxidized sediment is far more likely. Isotopic fractionation of sulphur in sulphides in the cores of analogue Triassic reduction spots supports a microbial formation mechanism.
The abundance of roscoelite in the cores of these reduction spots is comparable with occurrences in younger rocks. Roscoelite is observed in the cores of reduction spots, lacking organic matter throughout the Palaeozoic and Phanerozoic ages, when organic material was much more abundant than in the Mesoproterozoic age, suggesting long-term continuity in the formation mechanism from the Proterozoic age. Other formation mechanisms, such as migrating hydrocarbons, are known from Archaean and Proterozoic rocks (Rasmussen Reference Rasmussen2005), but are unlikely to have been present during the formation of the Stoer Group sediments.
The sulphate concentrations present in the Stoer Group sediment were sufficient to support an active biota. Sulphur isotope fractionation and framboidal pyrite in the cores of reduction spots are consistent with a microbial formation mechanism.
Sphericity of reduction spots implies formation at depth in the subsurface. Assuming a microbial formation mechanism for reduction spots, formation at depth in the subsurface has implications of a deep biosphere present in the terrestrial environment during the Mesoproterozoic age.
Acknowledgements
Thanks go to those whom contributed to making this project possible. They include, but are not limited to, John Still for invaluable technical assistance and guidance and Barry Fulton for helpful IT and graphical support, both of the University of Aberdeen Department of Geology & Petroleum Geology. Credit goes to Adrian Boyce of the Scottish Universities Environmental Research Centre for contributing the sulphur isotope work. Special thanks also go to the Astrobiology Society of Britain for support and constructive discussion.







