Introduction
Prepulse inhibition (PPI) is a measure of inhibitory control of information processing in which a weak sensory stimulus (the prepulse) inhibits the startle response to a subsequent sudden intense stimulus (pulse). PPI is thought to reflect ‘sensorimotor gating’, a form of central nervous system inhibition wherein distracting sensory information is filtered out during the early stages of processing so that attention can be focused on more salient features of the environment (Braff et al. Reference Braff, Stone, Callaway, Geyer, Glick and Bali1978; Braff & Geyer, Reference Braff and Geyer1990).
PPI is a widely used surrogate measure of psychosis in animal models. It is also considered a candidate endophenotype for schizophrenia (Braff & Light, Reference Braff and Light2005; Calkins et al. Reference Calkins, Dobie, Cadenhead, Olincy, Freedman, Green, Greenwood, Gur, Gur, Light, Mintz, Nuechterlein, Radant, Schork, Seidman, Siever, Silverman, Stone, Swerdlow, Tsuang, Tsuang, Turetsky and Braff2007) because of its high heritability (Anokhin et al. Reference Anokhin, Heath, Myers, Ralano and Wood2003) and the presence of PPI deficits in the unaffected first-degree relatives of probands (Cadenhead et al. Reference Cadenhead, Swerdlow, Shafer, Diaz and Braff2000; Kumari et al. Reference Kumari, Das, Zachariah, Ettinger and Sharma2005). However, the genetic architecture of the PPI endophenotype is in its infancy. To date, only one study has reported PPI deficits in both schizophrenia and healthy control populations with a missense mutation on rs3924999 of the neuregulin 1 gene, one of the leading candidate genes in schizophrenia (Hong et al. Reference Hong, Wonodi, Stine, Mitchell and Thaker2007).
Animal studies show that PPI involves a widely distributed cortico-striato-pallido-pontine network (Swerdlow et al. Reference Swerdlow, Caine, Braff and Geyer1991, Reference Tunbridge, Harrison and Weinberger2001a) potently regulated by dopaminergic neurotransmission (Swerdlow et al. Reference Swerdlow, Caine, Braff and Geyer1992; Koch & Schnitzler, Reference Koch and Schnitzler1997; Geyer et al. Reference Geyer, Krebs-Thompson, Braff and Swerdlow2001). Dopamine (DA) agonists disrupt PPI in rats (Mansbach et al. Reference Mansbach, Geyer and Braff1988; Swerdlow et al. Reference Swerdlow, Taaid, Oostwegel, Randolph and Geyer1998, Reference Swerdlow, Platten, Shoemaker, Pitcher and Auerbach2001b, Reference Swerdlow, Stephany, Shoemaker, Ross, Wasserman, Talledo and Auerbach2002, Reference Swerdlow, Stephany, Wasserman, Talledo, Shoemaker and Auerbach2003) and this DA-stimulated loss of PPI has been proposed as an animal model with face, predictive and construct validity for the loss of sensorimotor gating in schizophrenia (Swerdlow et al. Reference Swerdlow, Braff, Taaid and Geyer1994). There is now abundant evidence (for reviews, see Harrison & Weinberger, Reference Harrison and Weinberger2005; Tunbridge et al. Reference Tunbridge, Harrison and Weinberger2006) that COMT impacts critically on dopaminergic transmission. A polymorphism in the COMT gene, leading to an amino acid substitution [valine (Val) to methionine (Met)], results in the Met/Met variant showing 40% less enzymatic activity than the Val/Val (Chen et al. Reference Chen, Lipska, Halim, Ma, Matsumoto, Melhem, Kolachana, Hyde, Herman, Apud, Egan, Kleinman and Weinberger2004). This COMT polymorphism determines basal cortical DA neurotransmission levels that, in turn, regulate striatal DA activity. Indeed, higher COMT activity, as conferred by the Val158 allele, is associated with elevated midbrain DA synthesis (Meyer-Lindenberg et al. Reference Meyer-Lindenberg, Kohn, Kolachana, Kippenhan, McInerney-Leo, Nussbaum, Weinberger and Berman2005). This suggests that the Val158 allele may indirectly increase striatal dopaminergic function, thus reducing PPI. The COMT Val158Met polymorphism has been associated with auditory information gating deficits (Lu et al. Reference Lu, Martin, Edgar, Smith, Lewis, Escamilla, Miller and Cañive2007), and this is the first study to test the effects of this polymorphism on PPI in healthy male subjects.
Method
Subjects
One hundred and fifteen subjects were recruited from a pooled volunteer list of university students. We restricted the sample to men to avoid PPI variability related to gender (Swerdlow et al. Reference Swerdlow, Auerbach, Monroe, Harston, Geyer and Braff1993; Aasen et al. Reference Aasen, Kolli and Kumari2005) and menstrual cycle (Swerdlow et al. Reference Swerdlow, Hartman and Auerbach1997) and to avoid the regulatory effect on the expressed activity of the COMT enzyme by the circulatory oestrogens in women (Salama et al. Reference Salama, Ho, Wang, Tenhunen, Tilgmann and Al-Hendy2006). Inclusion criteria included right-handedness, absence of personal history of head trauma, medical and neurological conditions, or use of prescribed and recreational drugs; absence of personal or family (up to second-degree relatives) history of DSM-IV Axis I disorders and hearing threshold greater than 40 dB at 1 kHz. All subjects underwent psychiatric assessment using the Mini-International Neuropsychiatric Interview (Sheehan et al. Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller1998) and physical assessment including a urine toxicology screening and audiometry using a Kamplex AC30 Clinical Audiometer (PC Werth Ltd, London, UK). Eight subjects were excluded because of a family history of psychiatric illness, 10 subjects were startle non-responders (mean startle amplitude <10 μV) and four had a positive drug screen. Ninety-three Greek/Caucasian healthy males aged 18–35 years (mean±s.d., 26.2±4.0) entered and completed the study. The study was approved by the Ethics Committee of the University of Crete. All participants gave written informed consent before screening. Participants were seen and assessed on a single occasion.
Genotyping
Genomic DNA was extracted from venous blood samples. The COMT Val158Met genotype was determined by restriction fragment length polymorphism (RFLP) after polymerase chain reaction (PCR) amplification and digestion with NlaIII, similarly to a previously described methodology (Lachman et al. Reference Lachman, Papolos, Saito, Yu, Szumlanski and Weinshilboum1996).
Measurement of the startle response
A commercially available electromyographic (EMG) startle system (EMG SR-LAB, San Diego Instruments, San Diego, CA, USA) was used to examine the eye-blink component of the acoustic startle response from the right orbicularis oculi muscle. Equipment descriptions and set-up have been described previously in detail (Bitsios et al. Reference Bitsios, Giakoumaki and Frangou2005). Pulses consisted of 40-ms, 115-dB white noise bursts, and prepulses consisted of 20-ms, 75-dB and 85-dB white noise bursts over 70-dB background noise. Recording began with 3 min of acclimation when only background noise was present. The recording period comprised 12 pulse-alone trials and 24 prepulse–pulse trials. Two lead intervals (onset to onset) were used (60 ms, 120 ms). For each interval, there were six trials with the 75-dB prepulse and six with the 85-dB prepulse. All trials were presented in pseudo-random order with the constraint that no two identical trials occurred in succession. The intertrial interval varied between 9 s and 23 s (average 15 s). The entire test session lasted approximately 15 min. No specific instructions were given to the subjects with regards to the prepulses (passive PPI paradigm).
The EMG data were at first inspected on a trial-to-trial basis (to exclude erroneous trials for a particular subject) and then scored by the system's analytic programme for response amplitude and latencies. Trials were rejected (<5%) if excessive EMG activity (>20 digital units) was observed during the first 20 ms of recording or when onset latencies (defined by a shift of 20 digital units from the baseline value, occurring within 20–85 ms after the onset of the pulse stimulus) and peak latencies differed by more than 95 ms (Braff et al. Reference Braff, Grillon and Geyer1992, Reference Braff, Swerdlow and Geyer1999). The maximum absolute amplitude of the raw EMG data occurring in the 20–150-ms time window of the non-rejected trials was scored offline and stored for averaging and data analysis.
Statistical analysis
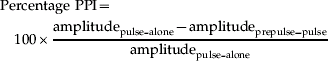
was calculated for each trial type. Kolmogorov–Smirnov tests showed normal distributions of startle and PPI data. Separate mixed model ANOVAs with genotype as the grouping factor and prepulse and interval as the within-subject factors were used to analyse %PPI and latency data. Partial η2 values are reported.
Results
Thirty subjects were homozygous for Val/Val, 48 were heterozygous for Val/Met, and 15 were homozygous for Met/Met, a distribution consistent with Hardy–Weinberg expectations (χ2=0.33, df=2, p=0.85). There were no differences in demographic and startle variables between the three genotype groups (Table 1) and polynomial contrasts failed to show a significant linear trend in baseline startle amplitude from the Val/Val to the Met/Met group. As an additional control to rule out gross stratification effects, genotyping was also performed for five unrelated gene polymorphisms. A contingency table approach (Raymond & Rousset, Reference Raymond and Rousset1995; Roussos et al. in press) was used to test for differences in the allelic distributions of these additional markers for COMT Val/Val and Met/Met subjects. This analysis revealed no significant differences at p<0.05 in allele frequencies for each locus (Table 2). This finding makes genetic inhomogeneity of the tested population unlikely. Figure 1 shows the %PPI of the three genotype groups. The ANOVA revealed significant main effects of genotype [F(2, 90)=14.95, p<0.001, η2=0.25], prepulse intensity [F(1, 90)=143.1, p<0.001, η2=0.61] and interval [F(1, 90)=38.1, p<0.001, η2=0.30] but no interactions (F's<2.1, p>0.1). These effects remained significant (all p values <0.001) after covarying for both baseline startle and smoking status. Post-hoc Bonferroni comparisons revealed that PPI of the Met/Met group was greater than PPI of the Val/Met (p<0.01) and the Val/Val group (p<0.001); PPI of the Val/Met group was also greater than PPI of the Val/Val group (p<0.003). There were no significant correlations between baseline startle and PPI for the entire sample or within the separate groups (all p values >0.1). ANOVAs of the latency data (Table 3) revealed prepulse and interval but not genotype main effects or interactions (F values <2.8, p>0.08). ANOVAs with identical factorial design showed that none of the polymorphisms shown in Table 2 had a significant effect on PPI in our sample, as evidenced by lack of significant genotype main effects or interactions involving genotype (all F values <1). Table 4 shows the %PPI in each genotype group for these polymorphisms and their distribution in our sample.
Discussion
We found that in healthy males COMT polymorphism is associated with PPI levels. Specifically, we observed a linear relationship between PPI levels and Val allele load; Val homozygotes had the lowest PPI, Met homozygotes the highest, and heterozygotes were intermediate for both prepulse intensities and both lead interval conditions. Importantly, our findings were obtained from a homogeneous cohort of healthy male subjects and cannot be attributed to differences in demographic characteristics or genetic inhomogeneity because the genotype groups did not differ in that respect (Tables 1 and 2). Our observations resonate closely with results from recent functional magnetic resonance imaging (fMRI) literature showing that, relative to Met-loading subjects, Val homozygotes underperform in prefrontal cortex (PFC)-related tasks and/or have prefrontal hyperactivation (Egan et al. Reference Egan, Goldberg, Kolachana, Callicott, Mazzanti, Straub, Goldman and Weinberger2001; Bilder et al. Reference Bilder, Volavka, Czobor, Malhotra, Kennedy, Ni, Golgman, Hoptman, Sheitman, Lindenmayer, Citrome, McEnvoy, Kunz, Chakos, Cooper and Lieberman2002; Malhotra et al. Reference Malhotra, Kestler, Mazzanti, Bates, Goldberg and Goldman2002; Mattay et al. Reference Mattay, Goldberg, Fera, Hariri, Tessitore and Egan2003; Bertolino et al. Reference Bertolino, Caforio, Blasi, De Candia, Latorre, Petruzzella, Altamura, Nappi, Papa, Callicott, Mattay, Bellomo, Scarabino, Weinberger and Nardini2004; Winterer et al. Reference Winterer, Egan, Kolachana, Goldberg, Coppola and Weinberger2006). The lower PPI levels in our Val loading subjects are also suggestive of less efficient information processing and increased prefrontal neuronal ‘noise’ in individuals with the COMT Val allele (Egan et al. Reference Egan, Goldberg, Kolachana, Callicott, Mazzanti, Straub, Goldman and Weinberger2001; Winterer & Goldman, Reference Winterer and Goldman2003; Winterer et al. Reference Winterer, Egan, Kolachana, Goldberg, Coppola and Weinberger2006).
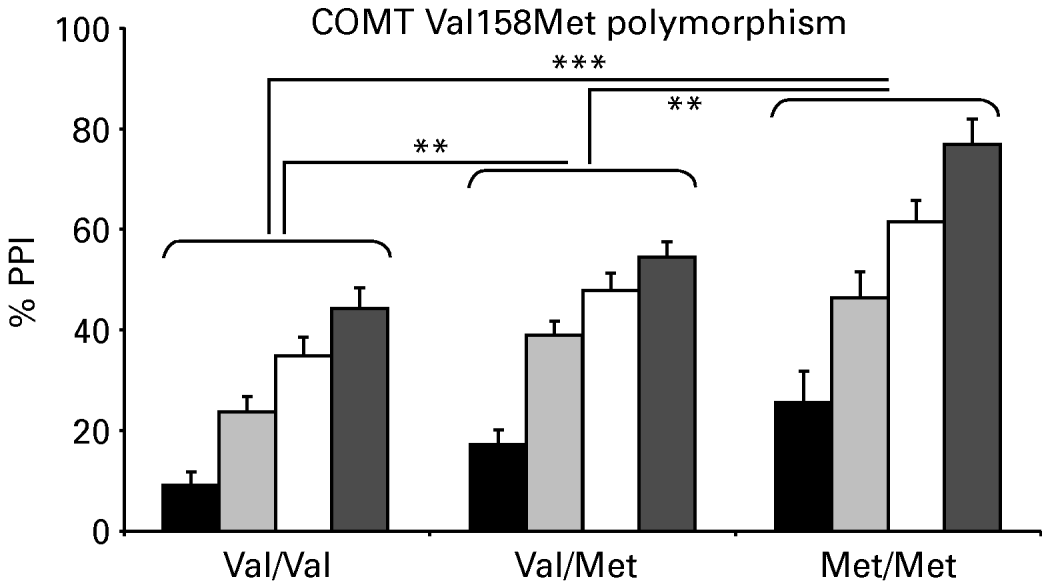
Fig. 1. Group means and standard error of the mean (s.e.m.) for percentage prepulse inhibition (%PPI) for the three genotype groups with 75-dB and 85-dB prepulses, at 60-ms and 120-ms prepulse–pulse intervals. ■, 75 dB, 60 ms;![]() , 75 dB, 120 ms; □, 85 dB, 60 ms;
, 75 dB, 120 ms; □, 85 dB, 60 ms;![]() , 85 dB, 120 ms (** p<0.01; *** p<0.001).
, 85 dB, 120 ms (** p<0.01; *** p<0.001).
Table 1. Demographic and startle characteristics for each genotype group and the entire sample (mean±standard deviation)
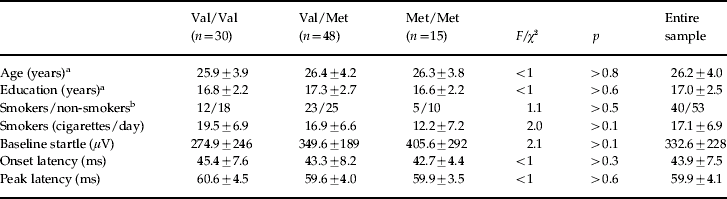
a For this measure, the overall distribution of the score differed from normality and the equivalent non-parametric Kruskal–Wallis procedure was applied.
b χ2 comparison.
Table 2. Test for significant differences in allele frequencies for each locus when dividing samples by COMT Val158Met

COMT, catechol O-methyltransferase; BDNF rs6265, brain-derived neurotrophic factor Val66Met polymorphism; DRD2A1 rs1800497, dopamine receptor D2TaqIA restriction fragment length polymorphism; DAT1 rs28363170, a 40-bp tandem repeat polymorphism in the 3′ region of the SLC6A3 gene; DRD1 rs4532, dopamine receptor D1 A-48G polymorphism in the 5′ untranslated region of the DRD1 gene; ZDHHC8 rs175174, zinc finger DHHC domain-containing protein 8 A/G polymorphism; df, degrees of freedom.
Table 3. Onset and peak latency data (mean±standard deviation) at each trial type for the three genotype groups
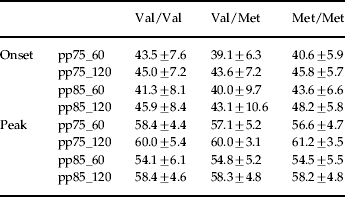
Table 4. %PPI (mean±s.d.) at all trial types, when the sample was regrouped for BDNF (M/M n=2, V/M n=34, V/V n=57), DRD2A1 (A1/A1 n=3, A1/A2 n=19, A2/A2 n=71), DAT1 (9/9 n=11, 9/10 n=37, 10/10 n=45), DRD1 (A/A n=47, A/G n=41, G/G n=5), and ZDHHC8 (A/A n=6, A/G n=73, G/G n=14) gene polymorphisms
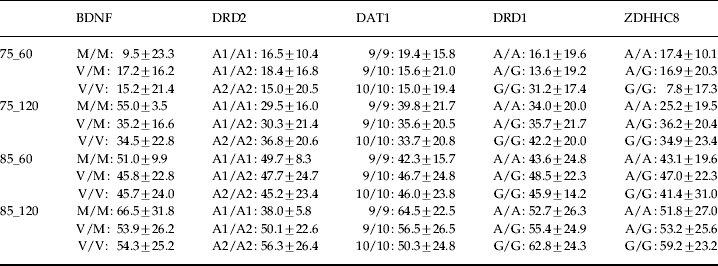
PPI, Prepulse inhibition; s.d., standard deviation.
PPI in rodents is modulated by activity in cortico-striato-pallido-pontine circuitry (Swerdlow et al. Reference Swerdlow, Caine, Braff and Geyer1991, Reference Swerdlow, Geyer and Braff2001a) of which the PFC is a crucial node (Koch & Bubser, Reference Koch and Bubser1994; Swerdlow et al. Reference Swerdlow, Lipska, Weinberger, Braff, Jaskiw and Geyer1995; Ellenbroek et al. Reference Ellenbroek, Budde and Cools1996; Broersen et al. Reference Broersen, Feldon and Weiner1999; Japha & Koch, Reference Japha and Koch1999; Zavitsanou et al. Reference Zavitsanou, Cranney and Richardson1999; Lacroix et al. Reference Lacroix, Spinelli, White and Feldon2000; Yee, Reference Yee2000). Our observations suggest that PFC DA transmission may be an important neural mechanism that modulates PPI in humans. The findings of the present study extend recent reports from our laboratory (Bitsios et al. Reference Bitsios, Giakoumaki, Theou and Frangou2006; Giakoumaki et al. Reference Giakoumaki, Bitsios and Frangou2006) and others (Csomor et al. Reference Csomor, Stadler, Feldon, Yee, Geyer and Vollenweider2008) showing (a) an association between PPI levels and performance on PFC-related tasks of working memory and planning, and (b) changes in PPI levels following administration of DA agonists or antagonists depending on basal PPI (Swerdlow et al. Reference Swerdlow, Stephany, Wasserman, Talledo, Shoemaker and Auerbach2003; Bitsios et al. Reference Bitsios, Giakoumaki and Frangou2005; Csomor et al. Reference Csomor, Stadler, Feldon, Yee, Geyer and Vollenweider2008). Graham (Reference Graham1975) proposed that PPI reflects automatic pre-attentive processes but recent evidence suggests that, even at this early stage of information processing, PPI is associated with cognitive processes controlled in a ‘top-down’ fashion by the PFC (Hazlett et al. Reference Hazlett, Dawson, Schell and Nuechterlein2001). This is supported by functional imaging studies in healthy individuals showing increased frontal and parietal cortical activation in attentionally modulated (Hazlett et al. Reference Hazlett, Buchsbaum, Haznedar, Singer, Germans, Schnur, Jimenez, Buchsbaum and Troyer1998) and passive PPI paradigms (Kumari et al. Reference Kumari, Gray, Geyer, ffytche, Soni, Mitterschiffthaler, Vythelingum, Simmons, Williams and Sharma2003, Reference Kumari, Antonova, Geyer, ffytche, Williams and Sharma2007; Postma et al. Reference Postma, Gray, Sharma, Geyer, Mehrotra, Das, Zachariah, Hines, Williams and Kumari2006; Campbell et al. Reference Campbell, Hughes, Budd, Cooper, Fulham, Karayanidis, Hanlon, Stojanov, Johnston, Case and Schall2007). Animal studies also support a close link between PPI and PFC DA activity. Reductions in DA activity in the PFC after local injection of selective D2 or D1 antagonists or 6-hydroxydopamine lesions result in significant PPI reduction (Bubser & Koch, Reference Bubser and Koch1994; Ellenbroek et al. Reference Ellenbroek, Budde and Cools1996; Zavitsanou et al. Reference Zavitsanou, Cranney and Richardson1999). Conversely, increased PFC DA activity after local apomorphine infusions also disrupts PPI (Broersen et al. Reference Broersen, Feldon and Weiner1999; Lacroix et al. Reference Lacroix, Spinelli, White and Feldon2000).
Our results also strengthen the model of an interaction between prefrontal DA levels and PPI according to an inverted U-shaped curve (Bitsios et al. Reference Bitsios, Giakoumaki and Frangou2005). Our findings support our previous suggestion that the PFC influences PPI levels and, by inference, the early stages of attentional processing (Bitsios et al. Reference Bitsios, Giakoumaki, Theou and Frangou2006; Giakoumaki et al. Reference Giakoumaki, Bitsios and Frangou2006). The COMT is also expressed in other brain regions involved in PPI modulation, particularly the hippocampus (Swerdlow et al. 2001 a; Matsumoto et al. Reference Matsumoto, Weickert, Akil, Lipska, Hyde, Herman, Kleinman and Weinberger2003), which may have influenced our results. By extending the influence of the COMT genotype on PPI in healthy subjects, the present study contributes to the understanding of the genetic architecture of the PPI endophenotype.
Gender differences in the effects of COMT polymorphism on human PPI are possible because no effect of COMT polymorphism on PPI was found in a recent study with 96 German females (Montag et al. Reference Montag, Hartmann, Merz, Burk and Reuter2008). Unfortunately, however, that study is not directly comparable to the present one because of the different stimulus parameters used in startle elicitation. We used standard stimulation with a 40-ms, 115-dB pulse with nearly instantaneous rise time (<1 ms) and two 20-ms prepulses, 5-dB and 15-dB above background, whereas Montag et al. (Reference Montag, Hartmann, Merz, Burk and Reuter2008) used a weaker pulse (106-dB, 35-ms, 5-ms rise time) and one prepulse, 19 dB above background. For a given prepulse, PPI increases with weaker pulses (Csomor et al. Reference Csomor, Yee, Quednowa, Stadler, Feldon and Vollenweider2006), and therefore it is conceivable that small between-group PPI differences may be overriden by a ceiling effect when weak pulses are used. More importantly, prepulses closer to background noise, such as those used in the present study, increase the susceptibility of PPI to modulation by manipulations of dopaminergic transmission, for example PPI disruption by the DA agonist apomorphine (Davis et al. Reference Davis, Mansbach, Swerdlow, Campeau, Braff and Geyer1990) or attention (Gewirtz & Davis, Reference Gewirtz and Davis1995).
To our knowledge, this is the first demonstration in humans of a DA gene-specific influence on PPI levels that may explain observed individual differences. Future studies examining the effect of the COMT Val158Met polymorphism on pharmacological manipulations of the PPI following administration of DA agonists or COMT inhibitors, or the effects on PPI of other COMT polymorphisms that convey differences in enzyme activity (Diatchenko et al. Reference Diatchenko, Slade, Nackley, Bhalang, Sigurdsson, Belfer, Goldman, Xu, Shabalina, Shagin, Max, Makarov and Maixner2005; Nackley et al. Reference Nackley, Shabalina, Tchivileva, Satterfield, Korchynskyi, Makarov, Maixner and Diatchenko2006; Roussos et al. in press), could further enhance our understanding of the relationship between COMT, PPI and cognition.
Acknowledgements
We thank Professor C. Savakis for his encouragement and support and Dr S. Oehler for assistance with RFLP analysis. This project was supported by the University of Crete Research Funds Account (E.L.K.E. 1348). P. Roussos holds a ‘Manasaki’ scholarship and S. G. Giakoumaki was supported by a ‘Propondis Foundation’ post-doctorate fellowship.
Declaration of Interest
None.







