Introduction
The parasite Entamoeba histolytica is distributed throughout the world, being most prevalent in tropical and subtropical areas (Haque et al. Reference Haque, Huston, Hughes, Houpt and Petri2003; Stanley, Reference Stanley2003; van Hal et al. Reference van Hal, Stark, Fotedar, Marriott, Ellis and Harkness2007; Pritt and Clark, Reference Pritt and Clark2008; Al-Areeqi et al. Reference Al-Areeqi, Sady, Al-Mekhlafi, Anuar, Al-Adhroey, Atroosh, Dawaki, Elyana, Nasr, Ithoi, Lau and Surin2017; Yimer et al. Reference Yimer, Zenebe, Mulu, Abera and Saugar2017). It is a leading parasitic burden in developing countries (Bercu et al. Reference Bercu, Petri and Behm2007; Peterson et al. Reference Peterson, Singh, Petri, Guerrant, Walker and Weller2011). In developed countries, amoebiasis primarily affects travellers to and migrants from E. histolytica endemic regions (Hailemariam et al. Reference Hailemariam, Kassu, Abebe, Abate, Damte, Mekonnen and Ota2004; Moran et al. Reference Moran, Ramos, Ramiro, Curiel, Gonzalez, Valadez, Gomez, Garcia, Melendro and Ximenez2005; Pritt and Clark, Reference Pritt and Clark2008; Mukherjee et al. Reference Mukherjee, Das, Bhattacharya, Nozaki and Ganguly2010) and is prevalent in certain population groups (Salit et al. Reference Salit, Khairnar, Gough and Pillai2009; Hung et al. Reference Hung, Chang and Ji2012).
There are no licensed vaccines for E. histolytica (Quach et al. Reference Quach, St-Pierre and Chadee2014). There are limited treatment options, the most common being metronidazole described >50 years ago (Gonzales et al. Reference Gonzales, Dans and Martinez2009), in combination with luminal agents such as iodoquinol and paromomycin (Marie and Petri, Reference Marie and Petri2013). Metronidazole is highly effective, however, it has toxic side-effects and E. histolytica can gain resistance to it (Kimura et al. Reference Kimura, Nakamura and Nawa2007; Debnath et al. Reference Debnath, Parsonage, Andrade, He, Cobo, Hirata, Chen, Garcia-Rivera, Orozco, Martinez, Gunatilleke, Barrios, Arkin, Poole, McKerrow and Reed2012). Auranofin, an approved drug for rheumatoid arthritis, is a promising therapeutic option for E. histolytica (Debnath et al. Reference Debnath, Parsonage, Andrade, He, Cobo, Hirata, Chen, Garcia-Rivera, Orozco, Martinez, Gunatilleke, Barrios, Arkin, Poole, McKerrow and Reed2012). The few, suboptimal, treatment options raise the very real possibility of resistance. Given its global reach and high incidence in certain population groups, it is important that new viable treatment options for E. histolytica disease be identified.
A better understanding of the intracellular pathways of E. histolytica would increase therapeutic options that target components specific to E. histolytica, including nuclear transport mechanisms (Aslam et al. Reference Aslam, Bhattacharya and Bhattacharya2012; Uribe et al. Reference Uribe, Almaraz Barrera Mde, Robles-Flores, Mendoza Hernandez, Gonzalez-Robles, Hernandez-Rivas, Guillen and Vargas2012). Interestingly, proteins involved in phagocytosis and trogocytosis are also present in the parasite nucleus (Aslam et al. Reference Aslam, Bhattacharya and Bhattacharya2012; Uribe et al. Reference Uribe, Almaraz Barrera Mde, Robles-Flores, Mendoza Hernandez, Gonzalez-Robles, Hernandez-Rivas, Guillen and Vargas2012; Ralston et al. Reference Ralston, Solga, Mackey-Lawrence, Somlata, Bhattacharya and Petri2014).
In this review, we provide a brief overview of E. histolytica life cycle, pathology and current knowledge of parasite nuclear transport mechanisms.
Life cycle
Entamoeba histolytica exists as an infectious cyst or an amoeboid trophozoite (Marie and Petri, Reference Marie and Petri2013; Ralston, Reference Ralston2015) (Fig. 1). It is transmitted via person to person contact or indirectly via inadequate sanitation or consumption of contaminated food and water (van Hal et al. Reference van Hal, Stark, Fotedar, Marriott, Ellis and Harkness2007). Infection begins with ingestion of cysts that move through the stomach, protected from the acidic environment by a chitin-containing cell wall. Excystation takes place in the small intestine and eight trophozoites emerge. Trophozoites are involved in development of lytic phagosomes (most common) or amoebiasis (less common) (Begum et al. Reference Begum, Quach and Chadee2015).

Fig. 1. The life cycle and pathogenic mechanisms of Entamoeba histolytica. (A) Infection occurs by ingestion of cysts (generally from fecally contaminated food or water). Excystation occurs in the ileum of the small intestine, releasing eight trophozoites. Trophozoites multiply by binary fission in the large intestine, colonizing it; mostly causing asymptomatic disease. Some trophozoites may invade the intestinal mucosa causing amoebic colitis, or enter the bloodstream, accessing liver and causing a liver abscess. Cyst formation is triggered by the dehydration of gut contents followed by excretion of cyst in feces. (B) Attachment to cells (live or apoptotic) or other particles is mediated by different amoebic cell surface molecules. (C) Larger or more deformable cells are more likely to be ingested by amoebic trogocytosis. (D) Smaller, less deformable or apoptotic cells are more likely to be ingested by phagocytosis. Signal transduction in the initiation of both processes includes amoebic kinases and calcium-binding proteins, all of which influence actin polymerization. Some calcium-binding proteins are found in the nucleus as well as cytoplasm; their nuclear transport mechanisms or nuclear functions are not known (indicated by question marks). Polymerized actin is present in the cytoplasm as F-actin and as shorter rods in the nucleus; their role is unclear.
Lytic phagosome
Trophozoites move into and colonize the large intestine (Faust and Guillen, Reference Faust and Guillen2012) and can survive for extended periods feeding on intestinal bacteria through phagocytosis (described below, Subsection ‘Phagocytosis’), forming a lytic phagosome where bacteria are lysed (Becker et al. Reference Becker, Cho, Guo, Fendig, Oosman, Whitehead, Cohn and Houpt2010). Trophozoites multiply by binary fission and some become encysted. Cysts can survive for months in the environment and reinfect another host (Eichinger, Reference Eichinger2001; Aguilar-Diaz et al. Reference Aguilar-Diaz, Carrero, Arguello-Garcia, Laclette and Morales-Montor2011). In some disease, states trophozoites are excreted but these cannot survive outside of the host (Haque et al. Reference Haque, Huston, Hughes, Houpt and Petri2003; Stanley, Reference Stanley2003; Pritt and Clark, Reference Pritt and Clark2008).
Amoebiasis
Trophozoites can invade the intestinal mucosa, where they feed on epithelial and red blood cells, causing amoebic colitis (Stanley, Reference Stanley2003; Pritt and Clark, Reference Pritt and Clark2008). Once the intestinal mucosal barrier has been breached, trophozoites can spread through the body causing a variety of systemic diseases.
Amoebic colitis
Clinical symptoms of amoebic colitis include cramping abdominal pain, weight loss, and watery or bloody diarrhoea for several weeks (Haque et al. Reference Haque, Huston, Hughes, Houpt and Petri2003; Stanley, Reference Stanley2003; Pritt and Clark, Reference Pritt and Clark2008). If the mucosal surface is invaded, blood is present in feces, and in rare cases, patients present with fever (Haque et al. Reference Haque, Huston, Hughes, Houpt and Petri2003; Stanley, Reference Stanley2003; Bercu et al. Reference Bercu, Petri and Behm2007; Pritt and Clark, Reference Pritt and Clark2008). Extensive fulminant necrotizing colitis is the most severe form of amoebic colitis and is often fatal.
Extra intestinal disease
A systemic infection of E. histolytica may occur months or years after the initial infection. The most common organ it invades is the liver, forming an amoebic liver abscess (Pritt and Clark, Reference Pritt and Clark2008). The patient can present with jaundice, fever (in 85–90% of cases), cough, rigor, cold sweats, enlarged liver and weight loss (Haque et al. Reference Haque, Huston, Hughes, Houpt and Petri2003; Pritt and Clark, Reference Pritt and Clark2008).
Entamoeba histolytica can also invade other organs and systems. Colonization of the pleuro-pulmonary site results in cough, chest pain and difficulty breathing. Rarely, E. histolytica spreads to the central nervous system and presents with headaches, vomiting and change in mental status. Other, very rare, sites of infection include the heart, renal system, genitourinary tract and the skin (Haque et al. Reference Haque, Huston, Hughes, Houpt and Petri2003; Stanley, Reference Stanley2003; Pritt and Clark, Reference Pritt and Clark2008).
Current treatment options
Asymptomatic intestinal colonization with E. histolytica is usually treated with luminal agents such as lodoquinol and paromomycin (Kimura et al. Reference Kimura, Nakamura and Nawa2007) while tissue invasive disease is treated with metronidazole. Metronidazole is a non-specific antibiotic used to target anaerobic bacteria and protozoa infections (Stanley, Reference Stanley2003) and can cause cardiovascular and gastrointestinal adverse effects (Kimura et al. Reference Kimura, Nakamura and Nawa2007). As the only mainstream treatment for invasive amoebiasis, there is rising concern that E. histolytica may become resistant to metronidazole (Debnath et al. Reference Debnath, Parsonage, Andrade, He, Cobo, Hirata, Chen, Garcia-Rivera, Orozco, Martinez, Gunatilleke, Barrios, Arkin, Poole, McKerrow and Reed2012). There are limited studies investigating alternatives to metronidazole, e.g. a trial of a herbal preparation in patients with amoebiasis (Shah et al. Reference Shah, Usmanghani, Akhtar, Akram, Asif and Hasan2016). Debnath et al. (Reference Debnath, Parsonage, Andrade, He, Cobo, Hirata, Chen, Garcia-Rivera, Orozco, Martinez, Gunatilleke, Barrios, Arkin, Poole, McKerrow and Reed2012) have found that auranofin (an approved drug used therapeutically for rheumatoid arthritis) is active against E. histolytica in culture raising the possibility of drug re-positioning. Auranofin targets the E. histolytica thioredoxin reductase, which prevents thioredoxin reduction and enhances the sensitivity of trophozoites to reactive oxygen-mediated killing (Debnath et al. Reference Debnath, Parsonage, Andrade, He, Cobo, Hirata, Chen, Garcia-Rivera, Orozco, Martinez, Gunatilleke, Barrios, Arkin, Poole, McKerrow and Reed2012). In rare cases and if treatment fails to treat invasive amoebiasis, surgical intervention is required (Stanley, Reference Stanley2003).
Further understanding of the internal functioning of E. histolytica may open new doors for therapeutic interventions that target components specific to E. histolytica.
Pathogenicity
The mechanisms of E. histolytica pathogenicity include adherence, cytotoxicity and phagocytosis/trogocytosis.
Adherence
Adherence is essential for E. histolytica pathogenic infection. Thinning of colonic mucin by the secreted cysteine proteases allows binding of trophozoites to the mucin layer via Gal/GalNAc lectin. Subsequently, trophozoites are able to adhere directly to the host epithelial cells (Begum et al. Reference Begum, Quach and Chadee2015; Singh et al. Reference Singh, Walia and Kanwar2016).
Cytotoxicity
Following adherence, E. histolytica can kill host cells and intestinal bacteria, via direct contact with the parasite and indirect exposure to secreted proteinases (Christy and Petri, Reference Christy and Petri2011). Recent data suggest that direct contact leading to trogocytosis/phagocytosis may be the primary cytotoxic mechanism (Ralston, Reference Ralston2015). While the necrotic pathway may predominate (Berninghausen and Leippe, Reference Berninghausen and Leippe1997) in some situations, E. histolytica often triggers apoptotic cell death at sites of invasion (Becker et al. Reference Becker, Cho, Guo, Fendig, Oosman, Whitehead, Cohn and Houpt2010).
E. histolytica encoded amoebapores can form pores in lipid bilayers and are implicated in phagocytosis as they can mediate lysis of ingested content within phagosomes (Berninghausen and Leippe, Reference Berninghausen and Leippe1997; Ralston, Reference Ralston2015). Work with mammalian cell lines has shown that E. histolytica virulence complex proteins can initiate epithelial damage by interaction with tight junction proteins followed by their degradation (Betanzos et al. Reference Betanzos, Javier-Reyna, Garcia-Rivera, Banuelos, Gonzalez-Mariscal, Schnoor and Orozco2013); it is not clear if this is indeed the case in vivo. Amoebapores may also contribute to contact-mediated target cell cytotoxicity.
Trogocytosis
Recent research suggests that trogocytosis is a major E. histolytica cytotoxic mechanism for engulfing/eating live cells (Ralston et al. Reference Ralston, Solga, Mackey-Lawrence, Somlata, Bhattacharya and Petri2014). Parasite binds to live cells followed by actin rearrangements and biting off of small portions of the ingested host cell (Fig. 1C). The host cell eventually dies and the trophozoite dissociates from it (Ralston et al. Reference Ralston, Solga, Mackey-Lawrence, Somlata, Bhattacharya and Petri2014; Ralston, Reference Ralston2015).
Phagocytosis
Phagocytosis is essential for acquiring nutrients, invading host tissues and causing pathogenicity (Christy and Petri, Reference Christy and Petri2011). Phagocytosis is initiated when a particle binds to a cell surface receptor (Fig. 1D). This leads to rearrangement of parasite actin cytoskeleton providing the necessary force for phagosome formation (Christy and Petri, Reference Christy and Petri2011). Early phagosomes are surrounded by a rim of filamentous (F) actin that is gradually depolymerized as the phagosome matures. Phagosome maturation is controlled by amoebic homologues of Ras superfamily members, the Rab proteins (Saito-Nakano et al. Reference Saito-Nakano, Loftus, Hall and Nozaki2005; Avalos-Padilla et al. Reference Avalos-Padilla, Betanzos, Javier-Reyna, Garcia-Rivera, Chavez-Munguia, Lagunes-Guillen, Ortega and Orozco2015; Verma et al. Reference Verma, Saito-Nakano, Nozaki and Datta2015; Hanadate et al. Reference Hanadate, Saito-Nakano, Nakada-Tsukui and Nozaki2016; Verma et al. Reference Verma, Nozaki and Datta2016; Verma and Datta, Reference Verma and Datta2017), phosphatidylinositols and intracellular protein kinases (Somlata et al. Reference Somlata, Bhattacharya and Bhattacharya2011; Somlata et al. Reference Somlata, Kamanna, Agrahari, Babuta, Bhattacharya and Bhattacharya2012). Several E. histolytica encoded Rab proteins have been shown to have roles in different stages of phagocytosis; exactly how the various proteins work together or synergize to enable phagocytosis is still not clear. Similar to higher eukaryotes, endocytosis in E. histolytica appears to be driven by ESCRT (endosomal sorting complexes required for transport). EhVps4 is suggested to be involved in phagocytosis and virulence based on the localization of a tagged, overexpressed, EhVps4 around ingested erythrocytes (Lopez-Reyes et al. Reference Lopez-Reyes, Garcia-Rivera, Banuelos, Herranz, Vincent, Lopez-Camarillo, Marchat and Orozco2010). EhVps32 has been shown to bind to EhADH112 (ALIX related protein) through the Bro1 domain in the latter; the exact functional outcome of this interaction is not yet defined (Banuelos et al. Reference Banuelos, Garcia-Rivera, Lopez-Reyes, Mendoza, Gonzalez-Robles, Herranz, Vincent and Orozco2012) but may be related to multivesicular bodies formation. Actin-binding proteins (ABPs) are key controllers of phagocytosis and are involved in regulation of actin cytoskeleton dynamics at multiple levels. Somlata et al. (Reference Somlata, Bhattacharya and Bhattacharya2011) and Somlata et al. (Reference Somlata, Kamanna, Agrahari, Babuta, Bhattacharya and Bhattacharya2012) showed that the initiation of erythrophagocytosis in E. histolytica depends on a C2-containing domain kinase (EhC2PK), actin and calcium-binding proteins (EhCaBP1 and EhCaBP3).
The role of calcium-binding proteins in phagocytosis
Calcium (Ca2+) is essential for many eukaryotic processes. In E. histolytica Ca2+ is involved in lysis of epithelial cells and in the parasite's developmental stages (cysts or trophozoites). Calcium-dependent processes are mediated by calcium-binding proteins and E. histolytica produces several of these (EhCaBPs), only some of which have been functionally characterized (Bhattacharya et al. Reference Bhattacharya, Padhan, Jain and Bhattacharya2006).
(Aslam et al. Reference Aslam, Bhattacharya and Bhattacharya2012) showed that phagocytic cups and newly formed phagosomes contain EhCaBP3, actin and myosin 1B. EhCaBP3 directly binds actin, affecting its polymerization and bundling activity and, with myosin 1B, assists closure of phagocytic cups to form phagosomes. Overall, calcium binding was shown to be essential for phagosome initiation and formation.
At least two calcium-binding proteins in E. histolytica, EhCaBP3 and EhCaBP1, are ABPs implicated in phagocytic processes and are localized in both the nucleus and cytoplasm of the parasite (Sahoo et al. Reference Sahoo, Labruyere, Bhattacharya, Sen, Guillen and Bhattacharya2004).
Nuclear transport in eukaryotes
There is limited literature on nuclear–cytoplasmic transport pathways in E. histolytica, or indeed, any protozoan parasite. We do know that these pathways are well conserved across eukaryotes, with yeast, mouse and human pathways being best characterized (Wente and Rout, Reference Wente and Rout2010).
Nuclear transport
The eukaryotic genomic material is separated from the cytoplasm by the double membrane of the nuclear envelope (Rout and Aitchison, Reference Rout and Aitchison2001) that contains numerous nuclear pore complexes (NPCs) that allow selective passage of molecules (Rout and Aitchison, Reference Rout and Aitchison2001; Wente and Rout, Reference Wente and Rout2010).
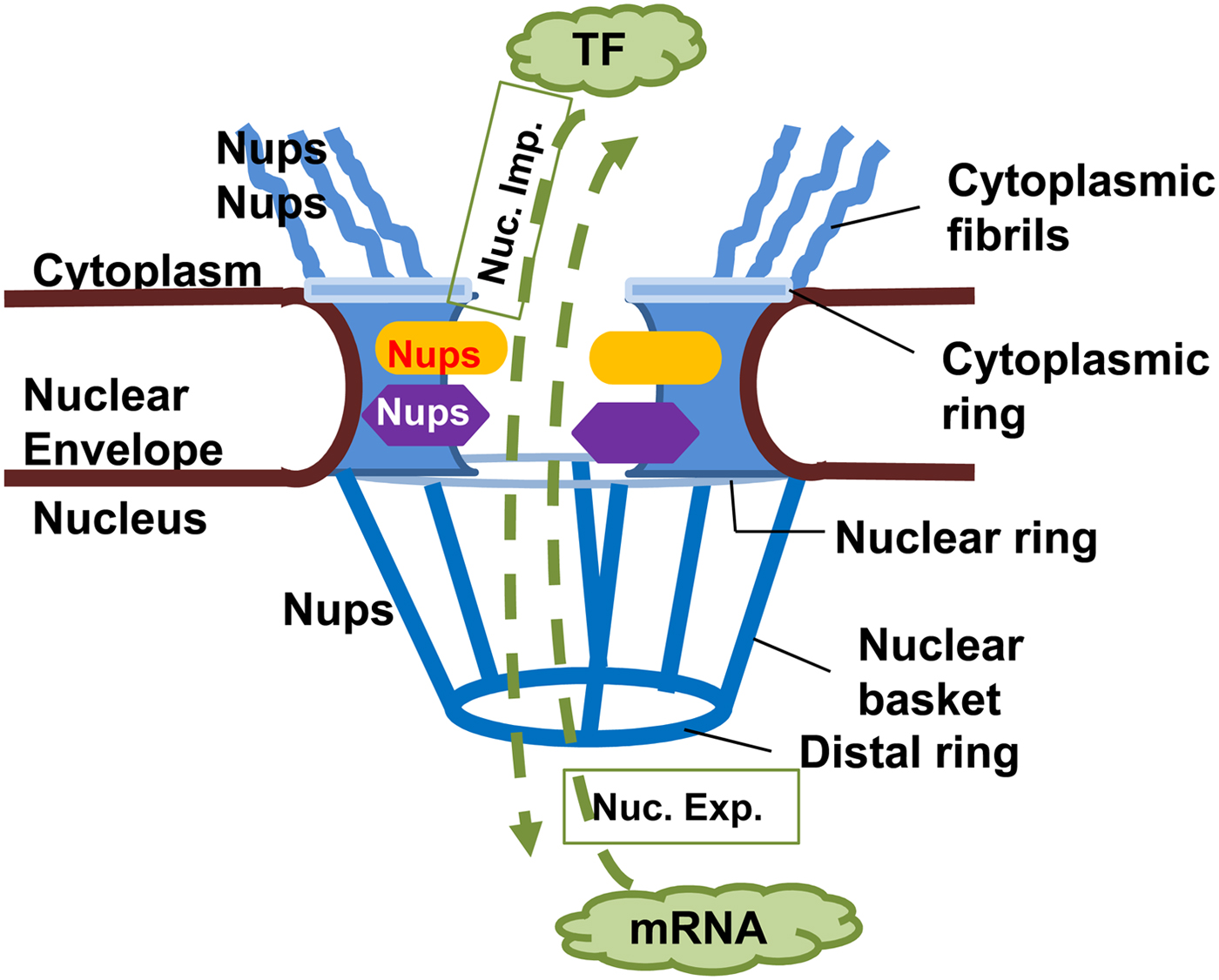
NPCs are large complex structures containing multiple copies of nucleoporins (Nups), forming a hollow central core, a nuclear basket, a luminal ring and cytoplasmic filaments (Fig. 2) (Rout and Aitchison, Reference Rout and Aitchison2001; Wente and Rout, Reference Wente and Rout2010). The central core Nups have numerous phenylalanine and glycine repeats (FG Nups) and mediate nucleocytoplasmic transport via interaction with transport components (Rout and Aitchison, Reference Rout and Aitchison2001; Wente and Rout, Reference Wente and Rout2010). Cytoplasmic filaments provide an initial docking site for active nuclear import. Nups or Nup-orthologues have been described in several protozoans, but only some of them have been shown to be bona fide NPC components. The delineation of Nups in the protozoan Trypanosoma brucei that localized to the nuclear envelope suggests a common origin from a complex NPC followed by extensive divergent evolution (Rout and Field, Reference Rout and Field2001; Degrasse and Devos, Reference Degrasse and Devos2010).
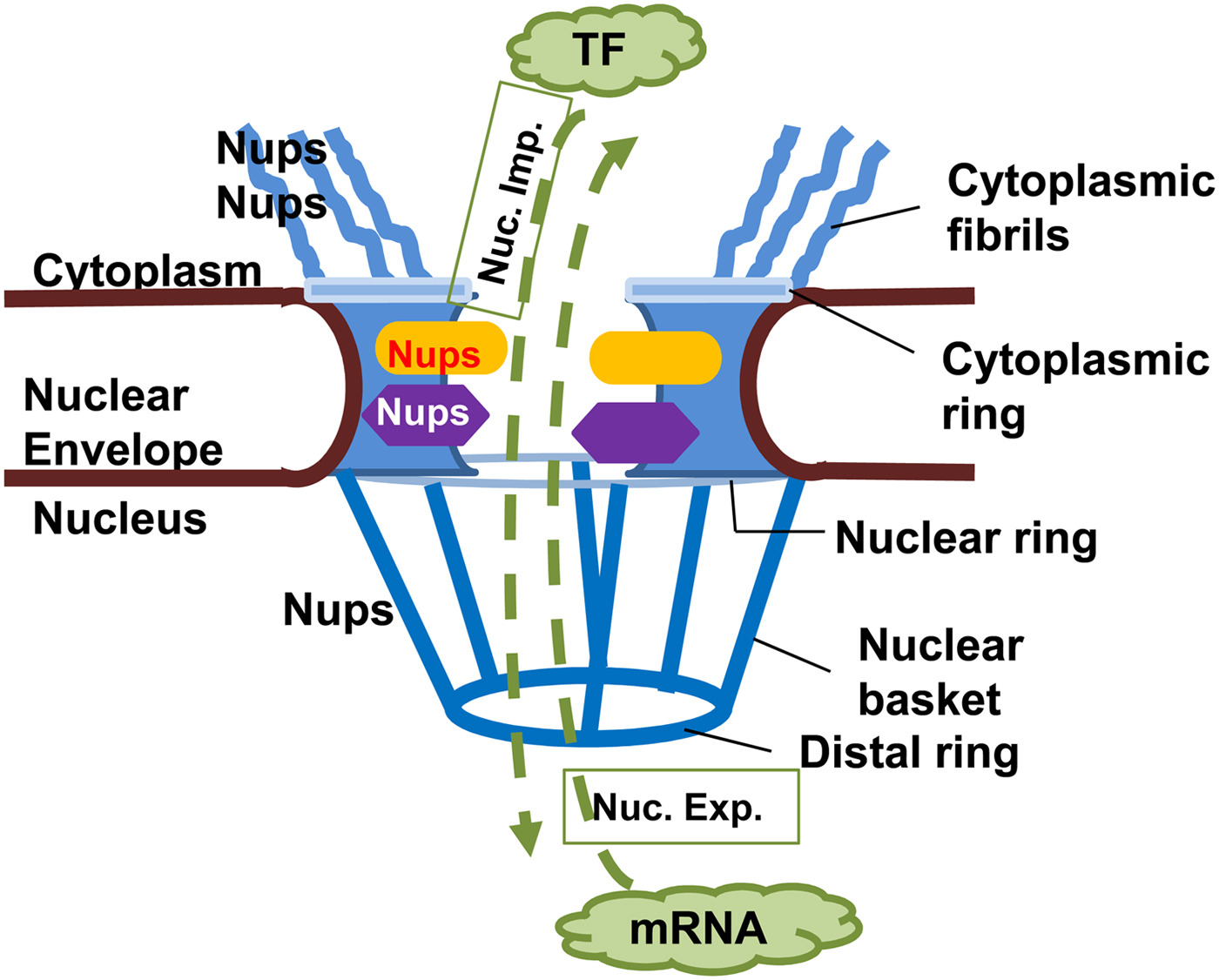
Fig. 2. Nuclear pore complex in Eukaryotes. The nuclear pore complex (NPC) is composed of a central channel, cytoplasmic fibrils, and a nuclear basket situated within a nuclear envelope. Nucleoporins (Nups) form the structure of the NPC, facilitating transport of macromolecules through it in both directions. Proteins, e.g. transcription factors (TF) must move from cytoplasm to the nucleus (nuclear import, Nuc. Imp.), while newly transcribed mRNA must move from the nucleus to the cytoplasm (nuclear export, Nuc. Exp.) to be translated into proteins.
Karyopherins (importins or exportins) bind to their cargoes by recognition of specific nuclear localization signals (NLSs) or nuclear export signals (NESs, Fig. 3) (Rout and Aitchison, Reference Rout and Aitchison2001; Wente and Rout, Reference Wente and Rout2010). The unidirectional nature of protein import and export through NPCs results from the participation of the small GTPase Ran that exists in different conformations when bound to GTP or GDP (Rout and Aitchison, Reference Rout and Aitchison2001; Wente and Rout, Reference Wente and Rout2010); nuclear Ran is largely GTP bound and cytoplasmic Ran is not. Ran is highly conserved across eukaryotes, highlighting its key function in eukaryotic biology (Feldherr et al. Reference Feldherr, Akin, Littlewood and Stewart2002). Importins (IMPs) and Exportins (EXPs) share an α-superhelical structure and their ability to interact with Ran (Wente and Rout, Reference Wente and Rout2010). IMPs and EXPs have a cargo-binding domain, an NPC-binding domain(s) and an amino-terminal Ran-binding domain; numerous isoforms of IMPα, IMPβ and EXPs are known in human, mouse and yeast cells. To date, the number of karyopherins in protozoa is not known, and no studies have examined karyopherins experimentally in E. histolytica.

Fig. 3. Nuclear Import and Export of proteins. (i) Nuclear import usually initiates by the recognition of NLS containing cargo by importins (IMP), either IMPβ alone or via IMPα/β complex. The cargo-importin complex is transported into the nucleus where IMPβ is displaced by its binding to RanGTP and the cargo is released. (ii) Nuclear export usually initiates by the recognition of NES containing cargo by exportin (EXP) in its RanGTP bound state. The cargo-EXP-Ran complex is transported into the cytoplasm where hydrolysis of RanGTP to RanGDP (via the action of RanGAP1/RanBP1) leads to dissociation of the complex and release of cargo. RanGDP is recycled into the nucleus by its specific transporter NTF2, where it is changed to RanGTP via the action of RCC1. In the context of excess calcium (Ca2+), e.g. due to release from intracellular stores, the IMP/EXP/Ran mediated transport may be inhibited. In this case, (iii) Calmodulin (CaM) can mediate nuclear import, while (iv) Calreticulin (CaN) can mediate nuclear export of cargo proteins that carry appropriate binding motifs (CmBM, CnBM).
Nuclear import
The nuclear import cycle [Fig. 3(i)] is mediated by direct binding of IMPβ to cargo molecules or indirect binding via an adaptor protein (IMPα) (Wente and Rout, Reference Wente and Rout2010). In the nucleus, RanGTP binds to the IMPβ complex which induces cargo displacement and IMPβ–RanGTP is transported back into the cytoplasm. Ran GTPase-activating protein (RanGAP) releases Ran from IMPβ through hydrolysis of RanGTP to GDP. The cycle starts over again when RanGDP is transported from the cytoplasm into the nucleus by nuclear transport factor 2 (NTF2). In the nucleus the exchange factor regulator of chromosome condensation 1 (RCC1) allows RanGDP to be exchanged to its GTP-bound form. Unlike Ran, functional RCC1 and RanGAP have not been identified in all protozoans analysed, raising the possibility of an evolutionarily divergent protozoa-specific Ran cycle (Frankel and Knoll, Reference Frankel and Knoll2009).
Nuclear export
Nuclear export [Fig. 3(ii)] is similar to nuclear import, in the opposite direction. Several EXPs have been described (Guttler and Gorlich, Reference Guttler and Gorlich2011), with the best characterized being CRM1. CRM1 recognizes proteins with a leucine-rich NES and in complex with RanGTP, translocates the complex to the cytoplasm through the NPC (Rout and Aitchison, Reference Rout and Aitchison2001; Wente and Rout, Reference Wente and Rout2010). In the cytoplasm, RanGTP is hydrolysed to cause dissociation of the CRM1–RanGTP–cargo complex. The unbound CRM1 and RanGDP are free to recycle back into the nucleus, to repeat the cycle. Limited data in protozoa suggest a minimalistic nuclear export system with CRM1 being the main EXP for all nuclear export (Fukuzawa et al. Reference Fukuzawa, Abe and Williams2003; Cuevas et al. Reference Cuevas, Frasch and D'Orso2005; Mitra et al. Reference Mitra, Gupta, Sahar, Pandey, Dangi, Reddy, Chauhan and Gaur2016).
Calcium-dependent nuclear transport
Apart from the well-characterized nuclear transport pathways described above, there is at least one well-conserved IMP/EXP-independent transport pathway. Calmodulin-mediated nuclear import of proteins [Fig. 3(iii)] and calreticulin-mediated nuclear export [Fig. 3(iv)] are regulated by intracellular calcium mobilization. They are active under conditions of high intracellular calcium that inhibit IMP/EXP-Ran-mediated pathway (Wagstaff and Jans, Reference Wagstaff and Jans2009). Change in calcium levels may affect all nuclear transport within a cell as suggested by the observed conformational change in NPC on the release of calcium from cellular stores (Mooren et al. Reference Mooren, Erickson, Moore-Nichols and Dunn2004; Erickson et al. Reference Erickson, Mooren, Moore, Krogmeier and Dunn2006). Calcium-dependent signalling is well conserved across eukaryotes (Plattner and Verkhratsky, Reference Plattner and Verkhratsky2015), but calmodulin-dependent nuclear transport has not been investigated in protozoa to date.
Nuclear transport in E. histolytica
Limited literature suggests the existence of nuclear transport pathways in E. histolytica similar to those in higher eukaryotes, e.g. the canonical NLS of the SV40 large T antigen mediates nuclear localization in E. histolytica (Pemberton and Paschal, Reference Pemberton and Paschal2005). A few NLS and NES containing E. histolytica proteins have been described, including EhNCABP166 (Uribe et al. Reference Uribe, Almaraz Barrera Mde, Robles-Flores, Mendoza Hernandez, Gonzalez-Robles, Hernandez-Rivas, Guillen and Vargas2012) an ABP with a role in pathogenicity. The in silico predicted NLSs were able to mediate nuclear localization of a fusion partner that is normally cytoplasmic, confirming that they are functional in E. histolytica. Additionally, the predicted C-terminal NES was able to mediate cytoplasmic localization of a normally nuclear fusion partner. EhCaBP3, another ABP with a defined role in phagocytosis, is also localized to nucleus and cytoplasm of the parasite, but its transport mechanisms have not been delineated (Rout et al. Reference Rout, Padhan, Barnwal, Bhattacharya and Chary2011).
In silico analysis of the E. histolytica genome has predicted the presence of Ran (Uribe et al. Reference Uribe, Almaraz Barrera Mde, Robles-Flores, Mendoza Hernandez, Gonzalez-Robles, Hernandez-Rivas, Guillen and Vargas2012) as well as several IMP/EXP orthologues (Aurrecoechea et al. Reference Aurrecoechea, Barreto, Brestelli, Brunk, Caler, Fischer, Gajria, Gao, Gingle, Grant, Harb, Heiges, Iodice, Kissinger, Kraemer, Li, Nayak, Pennington, Pinney, Pitts, Roos, Srinivasamoorthy, Stoeckert, Treatman and Wang2011); however, to date, no studies have confirmed their functionality.
Entamoeba histolytica has a robust calcium-dependent signalling mechanism, with effective communication from surface receptor engagement to induction of specific gene expression (Cruz-Vera et al. Reference Cruz-Vera, Clara, Hernandez-Kelly, Alfredo Mendez, Perez-Salazar and Ortega2003). The identification of several E. histolytica calcium-binding proteins, including at least two that are defined as calmodulin-like, may suggest the existence of an active calcium-dependent nuclear transport pathway (Bhattacharya et al. Reference Bhattacharya, Padhan, Jain and Bhattacharya2006). Indeed, a recent study has shown that EhCaBP6 is a nuclear–cytoplasmic shuttling protein with calcium-dependent nuclear transport (Verma et al. Reference Verma, Murmu, Gourinath, Bhattacharya and Chary2017). Importantly, the study shows the existence of calcium-dependent nuclear transport in E. histolytica. This is consistent with the observation that increase in intracellular calcium induces changes in mRNA level of several E. histolytica genes, probably through a change in promoter occupancy (Debnath et al. Reference Debnath, Akbar, Mazumder, Kumar and Das2005; Moreno et al. Reference Moreno, Linford, Gilchrist and Petri2010). That calcium signalling cascades are well developed in E. histolytica is also evidenced by the identification of serco-endoplasmic reticulum and plasma membrane Ca2+-ATPases (Martinez-Higuera et al. Reference Martinez-Higuera, Salas-Casas, Calixto-Galvez, Chavez-Munguia, Perez-Ishiwara, Ximenez and Rodriguez2013).
The recent data on nucleocytoplasmic shuttling proteins in E. histolytica, in the context of existing literature on eukaryotic nuclear transport mechanisms, lead us to hypothesize that E. histolytica has nuclear transport mechanisms similar to higher eukaryotes, which may or may not be as extensive. When considered with the importance of calcium-mediated cytoskeleton-binding-dependent phagocytic mechanisms in E. histolytica pathogenesis, this leads us to further hypothesize that targeting the nuclear transport pathways will lead to inhibition or abrogation of E. histolytica pathogenicity. However, these hypotheses remain to be tested experimentally.
Despite recent literature on the subject, there are many questions that remain unanswered with respect to the nuclear transport mechanism within E. histolytica. For example, does E. histolytica have complex nuclear pores like higher eukaryotes with a similar array of nucleoporins? What known eukaryotic nuclear transport pathways are active in E. histolytica? There is only one study showing the presence of calcium-dependent nuclear transport (Verma et al. Reference Verma, Murmu, Gourinath, Bhattacharya and Chary2017). Are there NTFs and/or pathways specific to E. histolytica?
In silico predicted NTFs in E. histolytica
To determine whether karyopherin alpha, beta and Ran were present within the E. histolytica genome, we used sequences of the previously characterized karyopherin alpha (KPNA), beta (KPNB) and Ran proteins from higher eukaryotes, unicellular organisms, free-living and parasitic protozoans (Table 1) to explore the genome/proteome of E. histolytica. Genes for all three proteins were identified in the published E. histolytica genome; however, there were clear differences in the predicted proteins.
Table 1. List of sequences downloaded from the web

On searching the Amoeba database, only one putative importin α and Ran transcript (and hence protein) were identified and five transcripts for importin β. For the latter, we used the transcript with the highest homology with human and mouse importin β1.
Phylogenetic trees were constructed for KPNA, KPNB and Ran sequences (Fig. 4A, C and D). Entamoeba histolytica KPNA (Fig. 4A) did not cluster with any other sequence (group A). KPNA sequences from L eishmania braziliensis, Trypanosoma cruzi and N aegleria gruberi clustered together (group B) as did sequences from Dictyostelium discoideum, Toxoplasma gondii and C. parvum (group C). As expected, KPNA sequences from Homo sapiens, Mus musculus and Saccharomyces cerevisiae clustered together (group D). The eukaryotic KPNA is composed of an importin-β-binding (IBB) domain (KPNB is also termed importin-β) and armadillo (ARM) repeats (Fig. 4B). As expected, the IBB and ARM domains were almost identical in H. sapiens, M. musculus and S. cerevisiae proteins, with each protein having one IBB and three ARM repeats. Interestingly, E. histolytica protein lacks an IBB domain and has only two ARM repeats. This raises the possibility of a KPNA or KPNB only nuclear import mechanism in E. histolytica or an as yet unknown mode of interaction between KPNA and KPNB.

Fig. 4. Nuclear transport components in Entamoeba histolytica: in silico studyProtein sequences of KPNA1 (A), KPNB1 (C) and Ran (D) from human, mouse, yeast and selected protozoan species were entered into Phylogeny.fr and advance analysis input data box. The bootstrap tool was set to 400 iterations and the output depicted as a rooted tree. The red circle represents the ultimate hypothetical common ancestor of all the species sampled. The trees are composed of branches representing the lineages changing over time and nodes (blue circles) representing the putative ancestors for the sample species. The boxes shown in dashed orange lines represent groups (A–D) which define clusters of species whose sequences are more similar to each other within a group than to species in other groups. Numbers at nodes are bootstrap values, calculated in PhyML (Phylogeny.fr); these provide a statistical measure of significance and are represented as a percentage of 400 iterations. (B) The conserved domain database (CDD) on the NCBI website was used to investigate the presence of functional domains within KPNA1 for Homo sapiens, Mus musculus and Saccharomyces cerevisiae, and then compare them with predicted KPNA1 of E. histolytica, a comparative figure is shown. IBB, importin-β-binding domain; ARM, armadillo repeats.
Similar to KPNA, E. histolytica KPNB did not cluster with KPNB from any other species (Fig. 4C, group A), as did D. discoideum (group B). S. cerevisiae and T. gondii clustered together (group C) as did T. cruzi, L. braziliensis, N. gruberi, H. sapiens and M. musculus (group D). Eukaryotic KPNB contains an Importin-beta N-terminal (IBN) domain and Huntingtin elongation factor 3 (HEAT) repeats. Unlike the well-studied KPNBs from humans, mice and yeast, KPNB from E. histolytica does not have IBN or HEAT repeats. Together with the bioinformatic analysis for KPNA, these limited analyses suggest that nuclear import pathways in E. histolytica evolved on a separate branch from the free-living amoebas, yeast and higher eukaryotes and a support a novel functional mechanism of KPNA and KPNB homologues.
The Ran sequences (Fig. 4D) clustered into three groups. Unlike the KPNA and KPNB trees, Ran from E. histolytica clustered with N. gruberi and D. discoideum (free-living amoebas) (group A). H. sapiens and M. musculus, as expected, clustered together (group B), while the remaining four species (C. parvum, T. gondii, L. donovani and S. cerevisiae) clustered together in group C. Interestingly, the cluster of E. histolytica with N. gruberi (free-living amoebas) suggests that Ran in these two organisms has evolved from the same putative ancestor; however, this relationship may not be significant (low confidence, bootstrap = 35%).
Per cent identity, expectation values (evalue) and bit scores were calculated for E. histolytica KPNA, KPNB and Ran sequences relative to human and mouse proteins (Table 2) in NCBI-BLAST. E. histolytica KPNA and KPNB sequences have low per cent identity when aligned with human or mouse sequences that are below the accepted 30% threshold required to infer homologues. However, this is clearly offset by the highly significant evalues (evalue 10 ×10 −10) and bit alignment scores (score of ⩾50 is considered significant). Together, the above data suggest the presence of homologues of human and mouse KPNA and KPNB proteins in E. histolytica. The putative Ran protein of E. histolytica is clearly a homologue of the human and mouse proteins, as evidenced by the high per cent identities, evalues and bit scores.
Table 2. Homology of Entamoeba histolytica KPNA, KPNB and Ran sequences with their human and mouse counterparts

a Per cent identity, threshold = 30%.
b Expectation value, threshold ⩽10 × 10−10.
c Alignment score, threshold ⩾50.
Nuclear transport in other protozoans
Research findings in other single-celled protozoans may have implications for the nuclear transport system in E. histolytica. Two of the best-studied protozoans in this context are D. discoideum and T. gondii.
Nuclear transport system in D. discoideum
Dictyostelium discoideum is a free-living amoeba that like Entamoeba, is classified in the amoebozoa supergroup.
Investigation of an intact D. discoideum nucleus using cryo-electron tomography, showed an NPC with cytoplasmic, spoke and nuclear rings (see Fig. 2) (Beck et al. Reference Beck, Lucic, Forster, Baumeister and Medalia2007). Snapshots of fluorescent cargo moving through the NPC showed that cytoplasmic filaments probably provide the initial docking sites for complexes that move through the central NPC channel. The nuclear basket region was less important for the transport process. Nuclear export of D. discoideum signal transduction and transcription protein (Dd-STATa) is regulated by phosphorylation, similar to that of several proteins in higher eukaryotes (Ginger et al. Reference Ginger, Dalton, Ryves, Fukuzawa, Williams and Harwood2000).
Nuclear transport system in T. gondii
Toxoplasma gondii is the causative agent of toxoplasmosis and used as a model for analysis because it is amenable to genetic manipulation, and can be studied its natural host (Frankel et al. Reference Frankel, Mordue and Knoll2007; Frankel and Knoll, Reference Frankel and Knoll2008; Frankel and Knoll, Reference Frankel and Knoll2009).
A virulence gene in T. gondii is a divergent homologue of RCC1, named TgRCC1. An atypical T. gondii Ran orthologue (TgRan) was also identified, which localized in the nucleus and cytoplasm, whereas in higher eukaryotes Ran is localized predominantly in the nucleus (Frankel and Knoll, Reference Frankel and Knoll2008). However, TgRan shared functional similarities with eukaryotic Ran: binding of GTP, GTPase activity and interaction with RCC1. The absence of RCC1 in both T. gondii and higher eukaryotes results in Ran being excluded from the nucleus.
The findings from T. gondii suggest that RanGTP and associated proteins in protozoans function similarly to higher eukaryotes.
Nuclear transport as a therapeutic target
Several inhibitors of nuclear import and export have been developed as anti-cancer therapeutic strategy (Bi et al. Reference Bi, Jones, Abbasi, Lee, Stultz, Hursh and Mortin2005; Parikh et al. Reference Parikh, Cang, Sekhri and Liu2014; Mahipal and Malafa, Reference Mahipal and Malafa2016; Ha et al. Reference Ha, Jeong, Oh, Rhee and Ham2017), with at least two (Selinexor and SL-801) currently being in human clinical trials (NIH, 2017). Basic discovery research over the last two decades has shown that disruption of nuclear transport is a key aspect of several medically important cancers (Mahipal and Malafa, Reference Mahipal and Malafa2016). Once the specific NTFs had been identified, several high-throughput screening and in silico approaches were used to identify and design small molecule inhibitors. Majority of the identified inhibitors are still in the discovery and animal testing stage, with a few that have progressed to human trials. Inhibitors of nuclear export are the best studied of these, and at least two are in human trials for various cancers (Senapedis et al. Reference Senapedis, Baloglu and Landesman2014). One such inhibitor has been approved for use in veterinary practice for canine cancers (London et al. Reference London, Bernabe, Barnard, Kisseberth, Borgatti, Henson, Wilson, Jensen, Ito, Modiano, Bear, Pennell, Saint-Martin, McCauley, Kauffman and Shacham2014). Nuclear transport inhibitors with anti-cancer potential have recently been reviewed in depth (Mahipal and Malafa, Reference Mahipal and Malafa2016). A number of small molecule inhibitors of nuclear transport are currently under study by various groups for anti-viral efficacy [e.g. (Shechter et al. Reference Shechter, Thomas, Lundberg, Pinkham, Lin, Wagstaff, Debono, Kehn-Hall and Jans2017; Wang et al. Reference Wang, Yang, Smith, Forwood and Jans2017)], however, there are none in clinical trials as yet.
The main challenge to the use of inhibitors of nuclear transport as anti-amoebic drugs is the cytotoxic effects on host cells due to nuclear transport pathways being well conserved across eukaryotes (O'Reilly et al. Reference O'Reilly, Dacks and Field2011; Serpeloni et al. Reference Serpeloni, Vidal, Goldenberg, Avila and Hoffmann2011). Thus, Leptomycin B, a highly effective inhibitor of CRM1 is not clinically viable due to high toxicity. This has been addressed to some extent by the recent development of synthetic CRM1 inhibitors with reduced toxicity and in vivo efficacy [Caly et al. (Reference Caly, Ghildyal and Jans2015) and references therein]. Another strategy has been to target a specific interaction rather than a function of the transport factors, e.g. inhibition of the interaction of a dengue virus non-structural protein with IMPα/β protects against severe disease in vivo (Fraser et al. Reference Fraser, Rawlinson, Wang, Jans and Wagstaff2014). In the context of current knowledge of nuclear transport pathways in protozoan parasites, and the accumulating evidence that any mechanisms present are likely to be closely related to mechanisms in higher eukaryotes albeit of less complexity, the latter option of targeting specific interactions probably presents the best chances of developing successful anti-amoebic therapeutic strategies. Targeting interactions of the calmodulin-like proteins of E. histolytica (EhCaBP3, EhCaBP6, Eh-α-actinin2) may be feasible. Given their low homology with human calmodulin, targeting these proteins and their functions should result in low toxicity (Bhattacharya et al. Reference Bhattacharya, Padhan, Jain and Bhattacharya2006).
Summary
Entamoeba histolytica, the causative agent of human amoebiasis, is a protozoan parasite capable of invading the colonic mucosa and reaching extra-intestinal organs. Understanding its invasion mechanisms requires knowledge of how it interacts with external cell components and how it kills cells (trogocytosis/phagocytosis). Recent research suggests that optimal phagocytosis requires signalling events from the cell surface to the nucleus, and the induction of several factors that are transported to the plasma membrane. There is limited research elucidating the existence of nuclear transport pathways in E. histolytica. Bioinformatics and indirect evidence suggest the existence of at least some (rudimentary?) form of Ran-dependent and calcium-dependent nuclear transport pathways. To date, there is no information (direct or indirect) regarding the structure of the NPC in E. histolytica, and no Nups have been identified. Given that nuclear to cell surface signalling appears to be involved in trogocytosis/phagocytosis of E. histolytica, this is a significant gap in our understanding of the parasite's pathological mechanisms. A deeper understanding of the molecular mechanisms underlying E. histolytica pathology is needed to identify new, viable drug targets. The recent development of small molecule inhibitors of nuclear transport for treatment of other disorders provides an opportunity to re-position these inhibitors for the treatment of invasive amoebiasis but this requires considerable research effort to define the nuclear transport pathways in E. histolytica. Re-positioning of drugs with known bioavailability and safety for human use offers a shorter pathway to the clinic for new indications compared with the traditional protocols for drug discovery and translation that can take decades.