Introduction
Pallas (Reference Pallas1781) first described Macracanthorhynchus hirudinaceus from a pig as Taenia hirudinacea that later on became known as Echinorhynchus hirudinaceaus as the genus Echinorhynchus Müller, 1776 had been the nominal genus for this species. Travassos (Reference Travassos1917) established the genus Macracanthorhynchus and included Macracanthorhynchus hirudinaceus Pallas, Reference Pallas1781 as its type species from pigs which is also found in squirrels, chipmunks, dogs, peccaries, moles, hyaenas, and muskrats. Between 1781 and 1917, this species acquired a number of names in the helminthological literature including Taenia haeruca Pallas, 1776; T. hirudinacea Pallas, Reference Pallas1781; Echinorhynchus gigas (Bloch, 1782) Johnston, 1918; Echinorhynchus hirudinaceus (Pallas, Reference Pallas1781); Gigantorhynchus gigas Bloch, 1782; Gigantorhynchus hirudinaceus (Pallas, Reference Pallas1781) Hamann, 1892; and Hormorhynchus gigas (Bloch, 1782) Johnston, 1918 (see Amin, Reference Amin2013). Macracanthorhynchiasis is cosmopolitan in pigs and with related manifestations in other mammals and its epizootology is widely reported especially in pigs from Russia (Petrochenko, Reference Petrochenko1958) and subsequently described from wild boars in Iran (Sarkari et al., Reference Sarkari, Mansouri, Najjari, Derakhshanfar and Mowlavi2016).
Three other species of Macracanthothynchus have been subsequently described from other hosts. These are: (1) Macracanthothynchus catulinus Kostylew, 1927 from dogs and other carnivores such as foxes, badgers and wild cats in Central Asia and Turkestan (Petrochenko, Reference Petrochenko1958); (2) Macracanthothynchus erinacei Dollfus, Reference Dollfus1953 from the Algerian hedgehog and Atelerix algirus (Lereboullet) in Morocco which has never been reported since. Dollfus (Reference Dollfus1953), however, reported Oligacanthorhynchus erinacei (Rudolphi, 1793) Meyer, 1932 as a synonym of M. erinacei (p. 217)]; and (3) Macracanthothynchus ingens (von Linstow, 1879) Meyer, 1932 [syns. Echinorhynchus hirudinaceus ingens von Linstow, 1879; Prosthenorchis ingens (von Linstow, 1879) Travassos, 1917] from raccoons, skunks, mink, grey foxes, moles and occasionally dogs and humans, especially children, from the Nearctic region. Macracanthorhynchus major Machado Filho, 1963 from the collared peccary, Tayassu tajacu (Lin.) in Brazil is a synonym of Oligacanthorhynchus major (Machado Filho, 1963) Schmidt, 1972 (see Petrochenko, Reference Petrochenko1958; Golvan, Reference Golvan1994; Amin, Reference Amin2013).
Despite the long history of research and publications about M. hirudinaceus since its original description over 240 years ago, we could still find previously undescribed features, in addition to reporting chemical analysis of hooks and new molecular data for a European population for the first time, all of which are reported here in the present work.
Materials and methods
Collections
Three long specimens were collected from one wild boar, Sus scrofa Linn. in Radomyshl, Zhyromyr Oblast, Ukraine (50°29′41″N 29°14′00″E) on 20 December 2005. Two female specimens were used for microscopy, another whole specimen was used for scanning electron microscopy (SEM) and hook metal analysis (energy-dispersive X-ray analysis (EDXA)), and trunk parts were used for molecular analysis.
Methods for microscopy
Freshly collected acanthocephalans were extended in water until proboscides were everted and fixed in 70% ethanol for transport to our Institute of Parasitic Diseases in Arizona, USA, for processing and further studies. The anterior and posterior portions of the two specimens processed for microscopy were stained in Mayer's acid carmine, destained in 4% hydrochloric acid in 70% ethanol, dehydrated in ascending concentrations of ethanol reaching 100% (24 h each) and cleared in 100% xylene and then in 50% Canada balsam and 50% xylene (24 h each). Worm parts (proboscides and posterior ends) were then mounted in Canada balsam. Measurements are in micrometres, unless otherwise noted. Width measurements represent maximum width. Trunk length does not include proboscis, neck or bursa.
Voucher specimens were deposited in the University of Nebraska's State Museum's Harold W. Manter Laboratory (HWML) collection in Lincoln, Nebraska, USA.
SEM
The specimen that had been fixed and stored in 70% ethanol was processed for SEM following standard methods (Lee, Reference Lee1992). These included critical point drying in sample baskets and mounting on SEM sample mounts (stubs) using conductive double-sided carbon tape. Samples were coated with gold and palladium for 3 min using a Polaron #3500 sputter coater (Quorum (Q150 TES) www.quorumtech.com) establishing an approximate thickness of 20 nm. Samples were placed and observed in an FEI Helios Dual Beam Nanolab 600 (FEI, Hillsboro, Oregon) scanning electron microscope with digital images obtained in the Nanolab software system (FEI, Hillsboro, Oregon) and then transferred to a Universal Serial Bus (USB) for future reference. Samples were received under low vacuum conditions using 10 KV, spot size 2, 0.7 Torr using a GSE detector.
EDXA
Standard methods were used for preparation similar to the SEM procedure. Specimens were examined and positioned with the above SEM instrument which was equipped with a Phoenix energy-dispersive X-ray analyser (FEI, Hillsboro, Oregon). X-ray spot analysis and live scan analysis were performed at 16 Kv with a spot size of 5 and results were recorded on charts and stored with digital imaging software attached to a computer. The TEAM (Texture and Elemental Analytical Microscopy) software system (FEI, Hillsboro, Oregon) was used. Data were stored in a USB for future analysis. The data included weight percentage and atom percentage of the detected elements following correction factors.
Ion sectioning of hooks
A dual-beam SEM with a gallium (Ga) ion source (GIS) is used for the liquid-metal ion source (LMIS) part of the process. The hooks of the acanthocephalans were centred on the SEM stage and cross sectioned using a probe current between 0.2 nA and 2.1 nA according to the rate at which the area is cut. The time of cutting is based on the nature and sensitivity of the tissue. Following the initial cut, the sample also goes through a milling process to obtain a smooth surface. The cut was then analyzed with X-ray at the tip and middle of hooks at the edge and centre of hooks for chemical ions with an electron beam (Tungsten) to obtain an X-ray spectrum. Results were stored with the attached imaging software then transferred to a USB for future use. The intensity of the GIS was variable according to the nature of the material being cut.
Molecular methods
Total genomic DNA was extracted from two specimens of M. hirudinaceus preserved in ethanol 70% using Qiagen™ (Valencia, California, USA) DNeasy® Tissue Kit following the manufacturer's instructions. Partial nuclear small subunit ribosomal DNA (18S rDNA) gene was amplified (50 μl total volume) using ExcelTaqTM SMOBIO® PCR Master Mix (Taiwan). Primer pairs and amplification conditions used were as described in Amin et al. (Reference Amin, Heckmann, Dallarés, Constenla and Ha2019a). A negative and positive control were included in polymerase chain reaction (PCR) runs in order to detect any potential contamination and to have a reliable sample to compare with, respectively. PCR amplicons were sequenced directly for both strands using the same PCR primers.
Obtained sequences were edited using ContigExpress implemented in the software Vector NTI Advance® v10.3.0 and submitted to GenBank under accession number OL305846. The sequence was aligned with available sequences for the family Oligacanthorhynchidae using Muscle as implemented in MEGA v6 (Tamura et al., Reference Tamura, Stecher, Peterson, Filipski and Kumar2013) for comparative sequence analysis. With this purpose, pairwise genetic distance matrices were calculated using the ‘p-distance’ and ‘No. of differences’ models implemented in MEGA v6.
Results
Morphological observations of our Ukrainian specimens (figs. 1–12)
We provide morphological description of the proboscis and hooks of the available female specimens of M. hirudinaceus collected from a wild boar in Ukraine and compare with similar structures described by other investigators (table 1). Moreover, we present additional features of M. hirudinaceus emphasizing the proboscis, proboscis hooks and hook roots using SEM (figs. 1, 2, 4 and 7–12) and light microscopy (figs. 3, 5 and 6). The specimens studied microscopically from S. scrofa in Ukraine were similar to those described by other observers depicted only by line drawings but varied in the shape and detail of proboscis, hooks and hook roots.
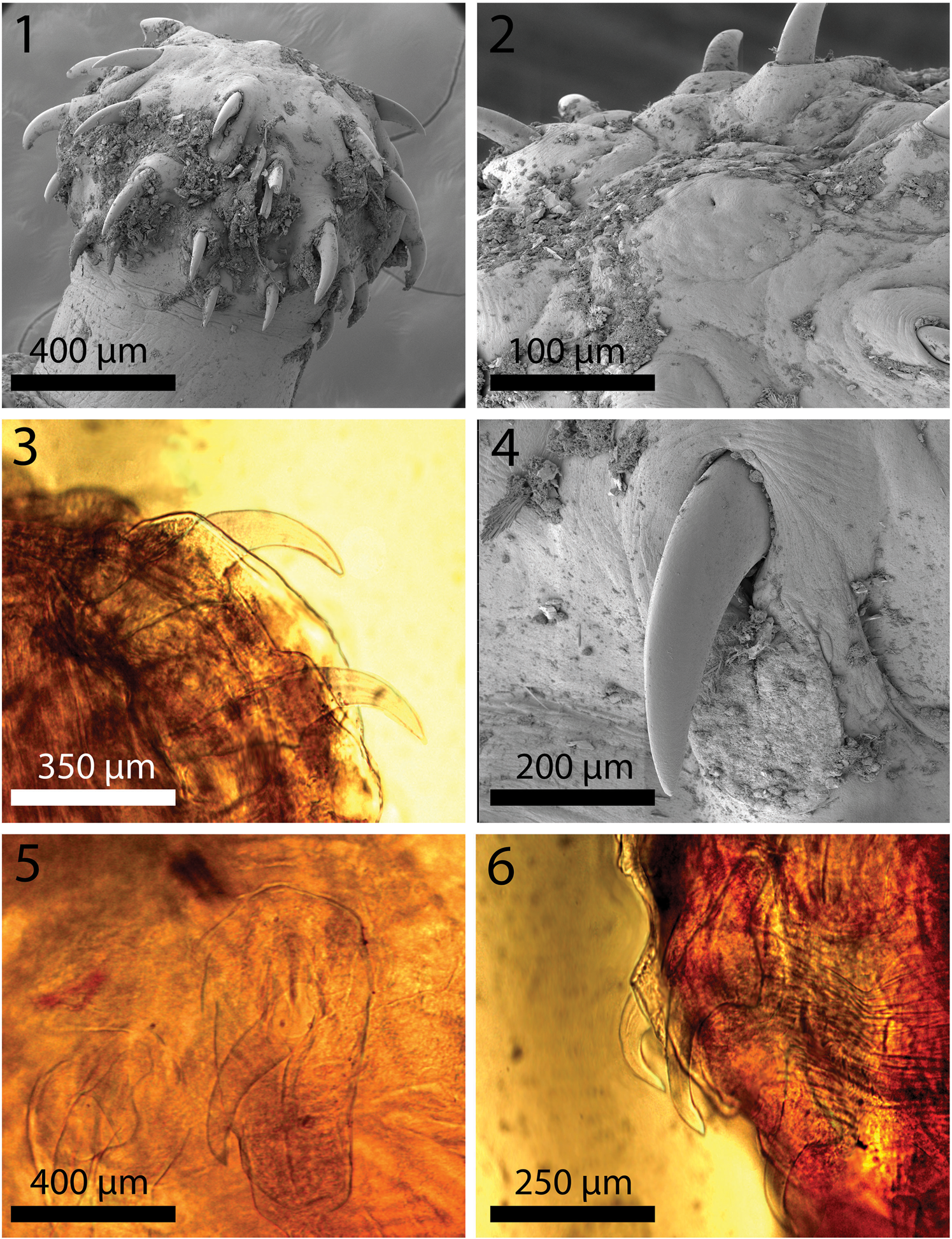
Fig. 1–6. Scanning electron microscopy and light microscopy images of the proboscis and hooks of Macracanthorhynchus hirudinaceus from wild boar, Sus scrofa Linn. in Radomyshl, Zhyromyr Oblast, Ukraine. 1. A lateral view of a proboscis showing the general organization of hooks of variable sizes anterio-posteriorly. 2. An apical view of a proboscis showing the dome-shaped orifice surrounding the apical sensory pore. 3. Anterior and second hooks and their characteristic roots. 4. The external shape and curvature of a large hook showing its smooth surface embedded in an elevated boat-like cuticular distention. 5. A microscope perspective of two middle hooks similar to that in fig. 4 showing more detail of the roots and an outline of the outer cuticular distension. 6. A microscope image of a posterior-most hook and the one just anterior to it with anteriorly manubriated root similar to those shown in figs. 3 and 4.
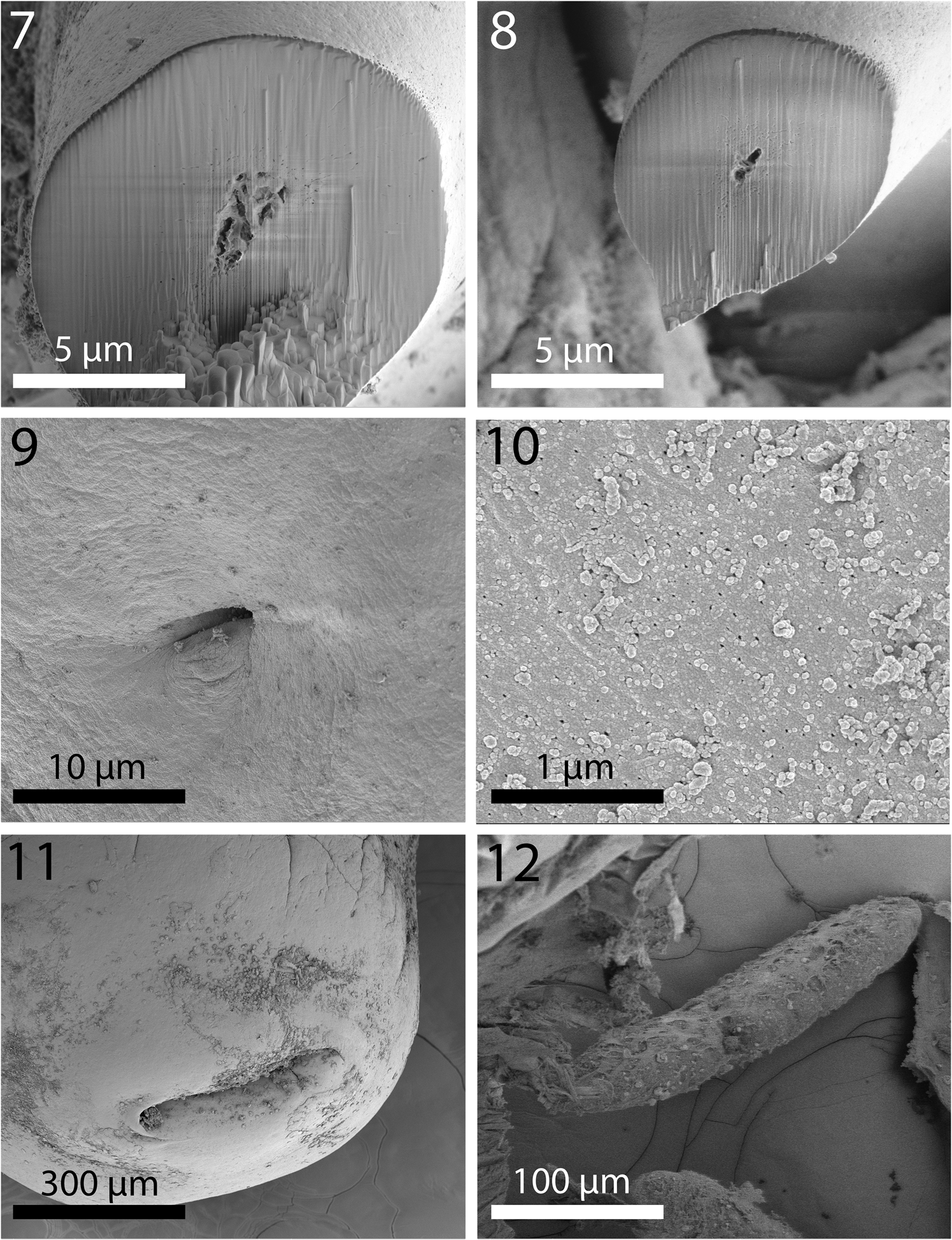
Fig. 7–12. Scanning electron microscopy images of Macracanthorhynchus hirudinaceus from wild boar, Sus scrofa Linn. in Radomyshl, Zhyromyr Oblast, Ukraine. 7. Perfectly spherical gallium cut section of a hook near its terminal end. 8. A gallium cut section of a smaller hook near its basal end with prominent ventral expansion. Note the solid core and thin cortical layer in both sections. This pattern is similar to that found in Nephridiacanths major (Bremser, 1811 in Westrump, 1821) Golvan, Reference Golvan1962; another Oligacanthorhynchid acanthocephalan parasite of hedgehogs (Amin et al. Reference Amin, Sharifdini, Heckmann and Zarean2020a, b, c). 9. Sensory pore on the neck of a specimen. 10. The cuticular surface of a specimen showing the size and distribution of micropores. 11. The thick-lipped orifice of the terminal and laterally extended female gonopore. 12. A larger well-developed ovarian ball.
Table 1. Morphometric comparisons of selected characters among geographical populations of Macracathorhynchus hirudinaceus from Sus scrofa.

a Petrochenko (Reference Petrochenko1958) reported 20 mm long females with eggs.
b Figure 48 of Travassos (Reference Travassos1917) shows the third hook to be considerably larger than the second.
c Trunk measurements in mm; all other measurements in micrometres. Numbers in boldface type are extremes of low or high measurements.
Description
Two females measured 110 and 120 mm long and 5 and 6 mm wide anteriorly, gradually tapering posteriorly. Proboscis (fig. 1) cylindrical, globular, broader anteriorly, 750–950 (850) long and 933–1000 (966) wide anteriorly, and apically flat with central apical organ pore in a dome-like orifice (fig. 2). Hooks in five or six spiral rows each with five or six hooks appearing as five types of hooks; position of the sixth hook uncertain. Hook and root measurements almost identical in both worms and measure as follows: Apical hook about 361 long by 109 wide at base (fig. 3). Second hook longer than first hook, 383 long but with slightly narrower blade and equal width at base (fig. 3). Third hook, the largest, 438 long by 143 wide at base (fig. 4). Fourth hook more slender and smaller, 336 long by 141 wide at base (fig. 6). Posterior-most hook smallest, 232 long by 101 wide. All hooks embedded in raised boat-like cuticular orifices larger than the hooks themselves (figs. 4 and 5). Hook roots stout, bifurcated, cylindrical, angled anteriorly, broadest and with ventral knob (stubby first root) at junction with hooks. Roots of same five types of hooks in same order measure. First: 339 long by 175 wide at knob. Second smaller: 295 long by 164 wide. Third: unavailable. Fourth: is smaller: 250 by 133. Fifth smallest: unavailable. Neck with two sensory pores. Lemnisci twisted, with six and seven giant nuclei each. Gallium cut sections of hooks similar in all five hook types with very dense homogeneous core and very thin cortical layer. Hook sections near the tip perfectly cylindrical (fig. 7) but with characteristic ventral triangular expansion near base (fig. 8). One neck sensory pore in fig. 9 and micropores in fig. 10. Female gonopore terminal with thick-lipped orifice and laterally extended (fig. 11). Eggs elongate–ovoid with uneven and irregularly sculptured surface texture (fig. 12), 94–135 (107) long by 72–94 (80) wide. Young ovarian balls ovoid becoming more elongate when well developed, 208–416 (310) long by 73–114 (95) wide. Cortical layer of eggs with very high levels of phosphorous compared to other metals (table 2).
Table 2. Chemical composition of a gallium cut hook of Macracanthorynchus hirudanaceus specimensa.

a Listed in percentage by weight. Common protoplasmic elements (carbon, nitrogen and oxygen) and processing elements (gallium, gold and lead) are omitted from table.
b All are cross-section cuts. No longitudinal cuts due to size of hooks.
c Numbers in boldface type of hook tip edge were used to generate the spectrum in fig. 13.
All above features depicted in figs. 1–12 and table 2 are new and have not been previously or adequately depicted.
Taxonomic summary of the Ukrainian material
Host: Wild boar, S. scrofa Linn. (Mammalia)
Locality: Radomyshl, Zhyromyr Oblast, Ukraine (50°29′41″N 29°14′00″E)
Site of infection: Intestine.
Specimens: HWML. Collection no. 216364 (one slide of the posterior, portion of two females)
GenBank ID: OL305846.
Micropores
Micropores of various diameters and distribution characteristic of M. hirudinaceus evenly covered the whole trunk of specimens (fig. 10). These observations are consistent with our findings, commonly observed, in other species of acanthocephalans.
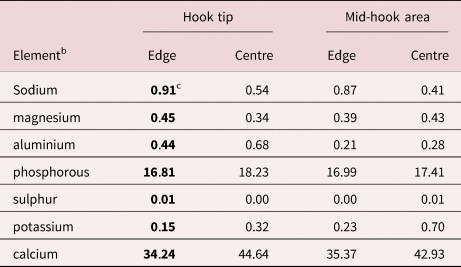
EDXA
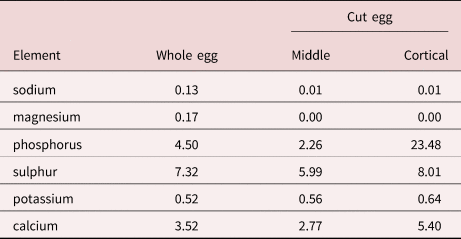
The results of the EDXA study of the hooks and eggs are given in tables 2 and 3 and fig. 13. The elements involved in the hardening of the hooks (calcium and phosphorus) are prominent in the scans with high content at the centre of hook tips and middle hooks compared to hook edge (table 2, fig. 13). Sulphur, which usually contributes to the hardening of hooks, was practically absent from the hooks of M. hirudanaceus. It (sulphur) was, however, abundant in whole eggs and in both in middle and cortical egg layers. Calcium and phosphorous were also present in eggs at moderate levels but phosphorous peaked at 23.48% in the cortical layer in stark contrast to its levels elsewhere in eggs (table 3).

Fig. 13. Energy dispersive X-ray spectrum of the tip cut edge of a gallium cut hook of a Macracanthorhynchus hirudinaceus specimen showing high levels of calcium and phosphorous. The X-ray data are the elemental analysis of the hook edge (boldface type figures in table 2). Insert: scanning electron microscopy of a cross gallium cut section of the anterior part of a hook.
Table 3. Chemical composition of an egg of Macracanthorhychus hirudanaceus specimen whole cut with a gallium beama.
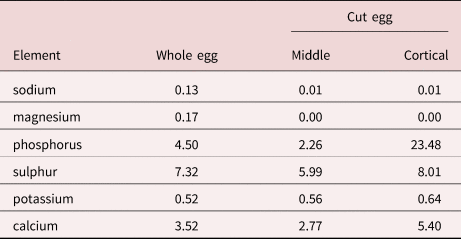
a Listed in percentage by weight.
Molecular results
One new partial 18S rDNA sequence (482 nt length) was generated from one adult specimen of M. hirudinaceus. The amplified region corresponded to the approximate middle region of the small subunit ribosomal ribonucleic acid gene, which is ~1800 nt in length (see García-Varela et al., Reference García-Varela, Masper, Crespo and Hernández-Orts2021). Comparative sequence analysis revealed that this sequence differs by 1 nt (0.21%), consisting in a nucleotide substitution, from the three M. hirudinaceus sequences obtained by Kamimura et al. (Reference Kamimura, Yonemitsu, Maeda, Sakaguchi, Setsuda, Varcasi and Sato2018) from specimens infecting wild boar in Japan (LC350000-2), the only 18S rDNA sequences for this species currently available in GenBank. The other species of this genus for which genetic data are available, M. ingens, is represented by two 18S rDNA sequences in GenBank. One of them (KU160506) is a short sequence of ca. 400 bp in length, identical to the present newly-generated sequence. Despite not having been officially published, this sequence was included in the comparative analysis due to the generalized lack of sequences for this genus and family. The other 18S rDNA sequence available for M. ingens (AF001844) differs by 2 nt (0.41%), both nucleotide substitutions, from the present new sequence. Regarding comparisons with sequences from other genera of the family Oligacanthorhynchidae, 1 nt (0.21%) difference, a nucleotide substitution, was noted with Nephridiacanthus major (MN612079), 2 nt (0.42%), both nucleotide substitutions, with Oligacanthorhynchus tortuosa (AF064817) and 3 nt (0.62%), one nucleotide substitution and two nucleotide indels (insertion/deletion), with Oncicola sp. (AF064818). In these three cases, only one sequence was available for comparison. All the described nucleotide differences were distributed in a fairly homogeneous way along the length of the 18S rDNA newly generated sequence.
Amplification of more variable genes, such as cytochrome c oxidase I (cox1) was, unfortunately, not possible in the present study due to limitations related to sample condition.
Discussion
General discussion
Specimens of M. hirudanaceus are found in pigs as well as in a few other species of mammals throughout the world and they appeared to exhibit considerable morphological variation in size of certain taxonomically important structures apparently related to the geographical distribution and host species. Van Cleave (Reference Van Cleave1953) provided the best description of the status of M. hirudanaceus. He stated (pages 109 and 110) that ‘It is frequently true that some of the best-known species are poorly and incompletely described in the literature. This is due, at least in part, to the fact that forms which are regarded as common are presumed to be so easily recognized that no one considers it worth-while to offer a detailed description’. Very few of the descriptions of M. hirudanaceus give measurements for the proboscis and many of the details are at variance in the writings of different authors (table 1). Meyer (Reference Meyer1932) quotes from Travassos (Reference Travassos1917) the statement that the proboscis of this species is 1.0 mm long and 0.5 mm maximum width. It seems probable that these two measurements were transposed. Kaiser (Reference Kaiser1891: 9) mentions the small proboscis which scarcely reaches the diameter of 1.0 mm. His statement is in keeping with observations of the present writers who have found that in this species the proboscis is very commonly about 0.9 mm in maximum diameter and only about 0.6 mm long.
Standard descriptions are originally based on the accounts of Travassos (Reference Travassos1917), then followed by Meyer (Reference Meyer1932–1933), Petrochenko (Reference Petrochenko1958), Hoklova (Reference Hoklova1986) and Lisitsyna (Reference Lisitsyna2019) (table 1). Limited accounts of selected characters such as trunk or proboscis hook sizes have been published in the context of surveys or wildlife investigations. Practically all investigators used the same line drawings first depicted by earlier observers. For instance, the lateral row of hooks and the egg depicted in Travassos (Reference Travassos1917, figs. 48 & 46), and the male and female trunk and the whole proboscis of Meyer (Reference Meyer1932–1933, figs. 232 & 233) were consistently copied by subsequent authors including Petrochenko (Reference Petrochenko1958) and Hoklova (Reference Hoklova1986). We provide the only SEM images of the proboscis and hooks of M. hirudinaceus except for those of Miller & Dunagan (Reference Miller and Dunagan1971) who presented shrunken and distorted images of a proboscis and a hook because of fixing in alcohol–formalin–acetic acid and processing in ethylene glycol tetraacetic acid wash. We also provide the first light microscope images of hooks and roots.
Present investigation
It is surprising to see the scarcity of these elementary measures of taxonomic importance in the infrequent descriptive accounts of such a common species that has been in the literature for about 230 years since its original description by Pallas (Reference Pallas1781) and its pivotal description by Travassos (Reference Travassos1917) over a 100 years ago. We discuss in some detail morphological variations in the size and shape of trunk, proboscis, proboscis hooks, hook roots and eggs of M. hirudinaceus in various geographical populations of S. scrofa (table 1). The variability in morphometric parameters of all populations of M. hirudinaceus noted above often but not always fell within the range of variation of that species. Morphometrics in all these cases stand at variance from specimens of our Ukraine material. Our selected accounts based on availability (table 1) show that our specimens from the Ukraine had the smallest trunk (110–120 mm long), longest and thickest hooks with the third hook being the largest, largest eggs and a proboscis wider than long (fig. 1).
The number of giant lemniscal nuclei was seven compared to the reported five to seven. Altogether, our Ukraine population appears to be morphometrically distinguished from other populations studied elsewhere.
The American female specimens reported by Van Cleave (Reference Van Cleave1953) reached a maximum length of 650 mm. Van Cleave (Reference Van Cleave1953) reported proboscides longer than wide (table 1) but his line drawings of four proboscides (his figures 78–81, page 167) depicted proboscides markedly wider than long, similar to the Ukrainian specimen: ours (fig. 1, table 1) and Lisitsyna's (Reference Lisitsyna2019, fig. 9a, table 1). Lisitsyna's (Reference Lisitsyna2019) line drawings of hooks (fig. 9b1-5) showed hook no. 3 to be considerably smaller than hook no. 4, not reflecting her text measurements of hooks progressively decreasing in size posteriorly (table 1). We provide the only data on size of hook roots that appeared comparable to that of hook blades. Petrochenko (Reference Petrochenko1958) made an occasional reference to 20 mm long females with eggs. Travassos (Reference Travassos1917) reported proboscides considerably longer (1000) than wide (500) and second proboscis hook from anterior to be markedly longer (432) than third hook (398) despite showing a considerably larger third hook (his fig. 48). Hook measurements of the Russian specimens (Hoklova, Reference Hoklova1986) and those reported by Lisitsyna (Reference Lisitsyna2019) were identical. The length of hooks of the other Russian specimens reported by Petrochenko (Reference Petrochenko1958) was similar to the lower range of hooks reported by Hoklova (Reference Hoklova1986) and Lisitsyna (Reference Lisitsyna2019).
Other accounts
Some characters of taxonomic significance appearing in occasional publications on M. hirudinaceus from S. scrofa in other parts of the world shed more light on the full range of morphological variations in this acanthocephalan. Bizhga et al. (Reference Bizhga, Laçi and Gjoni2013) reported 100–350 mm long by 3–9 mm wide females from Albania with eggs measuring 90–110 by 50–65. In Tangshan, Xuesi et al. (Reference Xuesi, Fu and Shuangmin1982) recovered many larger 300–653 (409.6) mm long by 3–11 (6.5) mm wide females and 50–120 (85.6) mm long by 2–6 (3.8) mm wide males. The proboscis of the Tangshan specimens was 980 ± 290 long in females and 860 ± 430 long in males; no width measurements were given. In Thailand, Sakulpiroj & Chanthan reported two 100 mm and 350 mm long worms with eggs measuring 67–110 long by 40–65 wide. Macracanthorhynchus hirudinaceus was first reported from Assam, India by Bhattacharya (Reference Bhattacharya2003). His male specimens were 40–75 long by 3.5 wide and females were 225–1100 long by 8.7–10.0 wide. The proboscis was wider than long in males (626 long by 875 wide) and in females (875 long by 1125 wide), similar to our specimens from Ukraine and with six spiral rows of six hooks each. Eggs measured 80–90 long but according to the scale of his fig. 3, the egg would be 214 long by 119 wide (Bhattacharya, Reference Bhattacharya2003). which is an obvious error. Additional Indian specimens near Delhi, Western Uttar Pradesh, West Bengal, Raimona (Assam), and others from Burma, and Sri Lanka, were comparatively large and with small hooks (Naidu, Reference Naidu2012). Males were 50–90 mm long by 6–8 mm wide and females reached 650 mm long by 7–8 mm wide and the proboscis was slightly longer than wide measuring 600–1000 long by 50–92 wide and with six spiral rows of six hooks each and with the largest hook reaching 320 and the smallest basal hook reaching 260; the eggs were 92–110 long by 50 wide (Naidu, Reference Naidu2012). The eggs from specimens from Buenos Aires, Argentina averaged 108 long (erroneously listed as 10.8) by 51.6 wide (Ciocco et al., Reference Ciocco, Carpinetti, Rojas, Castresana and Notarnicola2019). In southwestern Iran, Sarkari et al. (Reference Sarkari, Mansouri, Najjari, Derakhshanfar and Mowlavi2016) reported 52–89 (68) long by 2–3 (2.8) wide males and 56–351 (205) long by 3–6 (4.6) wide females, and proboscis length of 580–830 (730) in males and 660–950 (820) in females; width measurements were not given. Proboscis hooks were in five to seven rows in males and five to six rows in females each with six to seven and six to nine hooks per row, respectively. The hook formula in these specimens clearly presented a serious departure from that known for this species; no indication was given on whether these were spiral rows. Hook length was very small in both males (130–212) and females (125–225); no indication was given of which hooks are these (Sarkari et al., Reference Sarkari, Mansouri, Najjari, Derakhshanfar and Mowlavi2016).
Comparative morphological findings
While the above taxonomic data may not reflect a definitive pattern of morphological variability of geographical populations, they are, however comparable to related relationships that have been observed in other acanthocephalans. For example, populations of Mediorhynchus papillosus Van Cleave, 1916 have a wide distribution in North and South America, Eastern Europe, Asia and many former Soviet republics, east to China, and Taiwan. Morphometric comparisons of specimens from Maryland, Colorado, Taiwan, Trans-Baikal, Lower Yenesei River, Volga, Ukraine and Bulgaria exhibited distinctive morphological variability in certain key characters ‘with specimens from Colorado and Taiwan being at the opposite ends of the variability spectrum’ (Amin & Dailey, Reference Amin and Dailey1998). Populations of M. hirudinaceus may have a disjunct distribution similar to that demonstrated for M. papillosus that was attributed to ‘geographical restriction of intermediate (and adult hosts) in the apparent absence or scarcity of paratenic/reservoir hosts. Disjunct populations would promote distinct geographical diversity with consistent morphological difference from each other’ (Amin & Dailey, Reference Amin and Dailey1998). New species would have been created from disjunct populations if it was not for their presence along the geographical west–east gradient. This proposition was not considered viable in the case of M. papillosus.
EDXA
We have used elemental analysis (EDXA) of hooks after gallium cuts (LMIS) and histopathology in other published studies, for example, Amin & Heckmann, Reference Amin and Heckmann2017; Heckmann et al., Reference Heckmann, Amin and Standing2007, Reference Heckmann, Amin, Radwan, Standing, Eggett and El Naggar2012; Standing & Heckmann, Reference Standing and Heckmann2014. The hardened hooks are effective in attaching to host tissue (fig. 13) and mineralization enhances the structure of the hook. The high levels of phosphorus and calcium at the edge and centre of hook tips and middle hooks are instrumental to its hardening. While sulphur content usually within the outer layer of the hook aids in forming the calcium phosphate apatite similar to the enamel of the mammalian tooth. In relation to hooks of other acanthocephalan species studied (Amin & Heckmann, Reference Amin and Heckmann2017; Amin et al., Reference Amin, Heckmann and Zargar2017, Reference Amin, Heckmann and Ha2018; Ha et al., Reference Ha, Amin, Ngo and Heckmann2018), hooks of M. hirudinaceus appear to lack this element (table 2) as they did in hooks of Nephridiacanthus major (Bremser, 1811 in Westrumb, 1821) Golvan, 1962 from the central Asian and Middle Eastern long-eared hedgehog, Hemiechinus auritus (Gmelin, 1770) and the Eastern European hedgehog, Erinaceus concolor Martin 1838 (Erinaceidae, 1838) from Iran (table 3, page 8 in Amin et al., Reference Amin, Sharifdini, Heckmann and Zarean2020a). This pattern appears to be specific for hooks of M. hirudinaceus. The EDXA pattern appears to be species-specific, working as fingerprints, and is shown to have significant diagnostic value in acanthocephalan systematics. For instance, the edge and centre of hook tip and hook middle of the closely related N. major (Bremser, 1811 in Westrumb, 1821) had higher levels of sulphur, calcium and phosphorus (table 3 of Amin et al. Reference Amin, Sharifdini, Heckmann and Zarean2020a) than in M. hirudinaceus. Moniliformis cryptosaudi Amin, Heckmann, Sharifdini, Albayati, 2019 from Iraq is morphologically identical to Moniliformis saudi Amin, Heckmann, Mohammed, Evans, 2016 from Saudi Arabia, and it was erected based primarily on its distinctly different EDXA pattern (Amin et al., Reference Amin, Heckmann, Sharifdini and Albayati2019b) as a cryptic species.
Acanthocephalan eggs are rarely investigated for metal elements. Metals in the eggs of M. hirudinaceus included a high level of phosphorous (23.48% by weight) in the cortical layer only, a basic element in the hardening of the egg shell as well as moderate levels of sulphur (5.99–8.01%) in all egg layers (table 3), especially the cortical layer. Sulphur aids in the mineralization of hardened structures forming the calcium phosphate apatite similar to the enamel of the mammalian tooth and hence its usual presence at high levels within the outer layer of hooks. The four outer eggshell layers E1–E4 surrounding the acanthor of M. hirudinaceus were shown to contain glycoproteins (Peters et al., Reference Peters, Taraschewski and Latka1991). Glycoproteins are structural molecules forming collagen and some include phosphorus in phosphoserine (an ester of serine and phosphoric acid) in the process of P-glycosylation (Murray et al., Reference Murray, Granner and Rodwell2006) which may explain the high phosphorous content of 23.48% in the cortical eggshell layer only (table 3). The outermost eggshell E1, derived from the fertilization membrane, contained neither chitin nor keratin filaments. Chitin occurs only in E4 and keratin in E2 and E3 (Peters et al., Reference Peters, Taraschewski and Latka1991). Chitin is an amino sugar protein generating hard outer shells or armour such as the exoskeleton of arthropods, and keratin is a protein that forms such structures as human hair and nails or horns. Chitin is a polysaccharide containing nitrogen and is combined with calcium carbonate to produce stronger compounds (Gilbert, Reference Gilbert2009). Keratin is a fibrous insoluble structural protein (scleroprotein) containing large amounts of the sulphur-containing amino acid cysteine forming disulphide bridges that confer additional strength and rigidity (Wang, Reference Wang2016). The calcium in chitin and the sulphur in keratin reflect their significant levels in our results (table 3). Eggs of M. hirudinaceus from the United States did not differ from eggs obtained in China (Peters et al., Reference Peters, Taraschewski and Latka1991).
Results of X-ray scans of eggs are available in one other terrestrial species of acanthocephalans, Centrorhynchus globocaudatus (Zeder, 1800) Lühe, 1911 (Centrorhynchidae) reported in birds of prey worldwide (Amin et al., Reference Amin, Heckmann, Dallarés, Constenla and Rubini2020b). Our specimens were obtained from Falco tinnunculus Linn. (Falconidae) and Buteo buteo Linn. (Accipitridae) in northern Italy. Eggs of C. globocaudatus had highest levels of sulphur in the cortical (12.94%) and core areas (8.04%) and a high level of phosphorus (9.01%) in the middle (table 6, fig. 32 in Amin et al., Reference Amin, Heckmann, Dallarés, Constenla and Rubini2020b). These findings agree to a large extent with our results for eggs of M. hirudinaceus and the interpretation of metal distribution based on the distribution of glycoproteins, chitin and keratin in the various eggshell layers applies to both species. Note especially fig. 18 of the gallium cut section of an egg of C. Globocaudatus (in Amin et al., Reference Amin, Heckmann, Dallarés, Constenla and Rubini2020b) for eggshell layers.
Our methodology for the detection of the chemical profile of hooks and eggs in the Acanthocephala has also been used in other parasitic groups including the Monogenea (Rubtsova et al., Reference Rubtsova, Heckmann, Smit, Luus-Powell, Halajian and Roux2018, Reference Rubtsova and Heckmann2019), and Cestoda (Rubtsova and Heckmann, Reference Rubtsova and Heckmann2020).
Micropores
Micropores are present throughout epidermal surface of the trunk of M. hirudinaceus like those reported in all other species of the Acanthocephala that we have investigated. They are associated with internal crypts and vary in diameter and distribution in different trunk regions corresponding with differential absorption of nutrients. We have documented this phenomenon in 16 other species of acanthocephalans (Heckmann et al., Reference Heckmann, Amin and El Naggar2013) and a few more since. The functional aspects of micropores in a few other acanthocephalan species including Rhadinorhynchus ornatus Van Cleave, 1918, Polymorphus minutus (Goeze, 1782) Lühe, 1911, Moniliformis moniliformis (Bremser, 1811) Travassos (1915), M. hirudinaceus, and Sclerocollum rubrimaris Schmidt and Paperna, 1978 were reviewed earlier by Amin et al. (Reference Amin, Heckmann, Radwan, Mantuano and Alcivar2009). Wright & Lumsden (Reference Wright and Lumsden1969) and Byram & Fisher (Reference Byram and Fisher1973) reported that the peripheral canals of the micropores are continuous with canalicular crypts. These crypts appear to ‘constitute a huge increase in external surface area … implicated in nutrient up take.’ Whitfield (Reference Whitfield1979) estimated a 44-fold increase at a surface density of 15 invaginations per 1 μm2 of M. moniliformis (Bremser, 1811) Travassos, 1915 tegumental surface. The micropores and the peripheral canal connections to the canaliculi of the inner layer of the tegument were demonstrated by transmission electron micrographs in Corynosoma strumosum (Rudolphi, 1802) Lühe, 1904 from the Caspian seal Pusa caspica (Gmelin) in the Caspian Sea (figs. 19, 20 of Amin et al., Reference Amin, Heckmann, Halajian and El-Naggar2011) and in Neoechinorhynchus personatus Tkach, Sarabeev, Shvetsova, 2014 from Mugil cephalus Linn. in Tunisia (figs. 26, 29, 30 in Amin et al., Reference Amin, Sharifdini, Heckmann, Rubtsova and Chine2020c).
Molecular findings in perspective
In the present contribution, this work presents the first 18S rDNA published sequence for this worldwide-distributed acanthocephalan obtained from specimens from Europe. Despite its short length, which can easily explain the low number of nucleotide differences observed with respect to most other species of the family, we believe it represents a valuable contribution to the genetic data available for this species, considering the scarcity of sequences, not only for M. hirudinaceus, but also for its genus and family.
As in the morphological description, it is also surprising to note the scarcity of genetic data for a such widely distributed acanthocephalan. This is often observed in this group of parasites. For instance, a similar situation occurs with Echinorhynchus gadi Zoega in Müller, 1776 and Acanthocephalus clavula (Dujardin, 1845), two widely distributed acanthocephalans that can infect a wide range of hosts for which, contrary to expectations, very few genetic data are actually available. There are, however, examples of widespread acanthocephalans for which a rather detailed genetic characterization has proved successful to elucidate important ecological and evolutionary features. For instance, populations of Pomphorhynchus laevis (Zoega in Müller, 1776) Porta, 1908, which is a widely distributed species throughout Britain, Ireland and other regions from Europe, have intraspecific variability in mitochondrial DNA (0.7%) in populations of from the same host (Salmo trutta Linn.) from Ireland, England and Scotland, while it increased up to 0.35% between populations of different hosts (Leuciscus cephalus Linn. and Cottus gobio Linn.) (O'Mahony et al., Reference O'Mahony, Bradley, Kennedy and Holland2004), and even up to 2.3–3.3% in populations from one host (Squalius cephalus Linn.) from the River Sava basin and its tributary the Sutla River in Croatia (Vardić-Smrzlićet al. Reference Vardić Smrzlić, Valić, Kapetanović, Filipović, Gjurčević and Teskeredžić2015). However, populations of Pomphorhynchus bulbocolli Linkins in Van Cleave, 1919, also a widely distributed species but across the Nearctic region, seems to have very low genetic divergence values (García-Varela et al., Reference García-Varela, Mendoza-Garfias, Choudhury and Pérez-Ponce de León2017). Other widely distributed paleoacantochepalan such as Corynosoma australe Johnston, 1937 show very low intraspecific genetic divergence among populations (1 to 1.7%), even though the haplotype network divides two clearly separated clusters from different localities (from Northern and Southern Hemispheres) and suggests secondary and independent colonization events to other mammal hosts (García-Varela et al., Reference García-Varela, Masper, Crespo and Hernández-Orts2021). Whereas Echinorhynchus gymnocyprii Liu, Wang, Yang, 1981 populations from Qinghai-Tibetan Plateau (China) show rich genetic diversity inferred from four subclades corresponding to different geographical locations and water systems (Lei et al., Reference Lei, Cai, Li, Fu, Sun, Ma, Li and Zhang2020). Considering eoacanthocephalan species, Pinacho-Pinacho et al. (Reference Pinacho-Pinacho, García-Varela, Sereno-Uribe and Pérez-Ponce de León2018) detected ten additional linages from samples of species of Neoechinorhynchus Stiles and Hassall, 1905 from the Americas, highlighting the need for molecular analyses to detect cryptic species. Recently, Rosas-Valdez et al. (Reference Rosas-Valdeza, Morrone, Pinacho-Pinacho, Domínguez-Domínguez and García-Varela2020) also detected genetic diversification of the genus Floridosentis Ward, 1953 from mullets from the Americas, suggesting the important role of the currents in the Atlantic Ocean, Pacific Ocean and Gulf of Mexico, in combination with the biology of the definitive hosts, in the distribution of species.
Following the examples provided above, there is no doubt that further studies providing sequences of small and large rDNA subunits, and specially of fast-evolving regions such as cox1 and the internal transcribed spacer, will be of large interest in order to start to understand the genetic diversity, correlations between morphotypes and genotypes, transmission patterns or biogeography, among others, which are of special interest in such a widespread and potentially zoonotic species as M. hirudinaceus.
Acknowledgements
We thank Madison Laurence, Bean Museum, Brigham Young University (BYU), Provo, Utah and Nataliya Rubtsova, Institute of Parasitic Diseases, Scottsdale, Arizona for expert help in the preparation and organization of plates and figures, and Michael Standing, Electron Optics Laboratory (BYU), for his technical help and expertise
Financial support
This project was supported by the Department of Biology, Brigham Young University, and by an Institutional Grant from the Institute of Parasitic Diseases, Scottsdale, Arizona.
Conflicts of interest
None.