Introduction
Cystic echinococcosis (CE) is a worldwide zoonotic disease caused by the larval stage of the parasite Echinococcus granulosus sensu lato (s.l.) which provokes long-term infections in humans and animals, being a serious public health problem (Pavletic et al., Reference Pavletic, Larrieu, Guarnera, Casas, Irabedra, Ferreira, Sayes, Gavidia, Caldas, Lise, Maxwell, Arezo, Navarro, Vigilato, Cosivi, Espinal and Vilas2017). The life cycle of this parasite includes an intermediate host (domestic and wild ungulates and occasionally humans) and a definitive host (dogs and other canids) which have the adult worm in the intestine and release eggs with their feces. The eggs can be ingested accidentally by an intermediate host that can develop a hydatid cyst (Moro and Schantz, Reference Moro and Schantz2009).
Based on the World Health Organization Informal Working Group on Echinococcosis (WHO-IWGE) classification of cyst stages by imaging-based techniques, four treatment options have been suggested: percutaneous–aspiration–injection and re-aspiration (PAIR), surgery, anti-parasitic therapy with benzimidazoles (BZM) and a watch-and-wait approach for inactive cysts (Siles-Lucas et al., Reference Siles-Lucas, Casulli, Cirilli and Carmena2018). For PAIR and surgery options, pre- and post-treatment and peri-interventional treatment with anti-parasitic drugs are also used (Brunetti et al., Reference Brunetti, Kern and Vuitton2010).
Available anti-parasitic treatment against CE is mostly limited to the administration of BZM, mainly albendazole (ABZ) and mebendazole, which are the only anti-infective clinically efficient drugs to interrupt the larval growth of Echinococcus spp. More specifically, ABZ is the preferred option due to its high bioavailability and easy administration to patients (Wen et al., Reference Wen, Vuitton, Tuxun, Li, Vuitton, Zhang and McManus2019). ABZ oral administration is recommended at a dosage of 10–15 mg kg−1 day−1 divided in two doses and a continuous therapy is suggested. However, ABZ has undesirable side-effects such as abnormal liver function, leucopoenia, thrombocytopoenia and alopecia in 3–5% of patients and their effectiveness is about 50% (Pawlowski et al., Reference Pawlowski, Eckert, Vuitton, Ammann, Kern, Crai, Dar, De Rosa, Filice, Gottstein, Grimm, Macpherson, Sato, Todorov, Uchino, von Sinner, Wen, Eckert, Gemmell, Meslin and Pawlowski2001). A third of patients generally have been cured, 30–50% have developed a partial response while 20–40% of cases do not respond to treatment (Moro and Schantz, Reference Moro and Schantz2009). Based on the problems described, new treatment alternatives are urgently needed, including the identification, development and assessment of novel compound classes and drug targets (Siles-Lucas et al., Reference Siles-Lucas, Casulli, Cirilli and Carmena2018).
In the last few decades, there has been an increased interest in studying the anthelmintic activity of medicinal plants focusing on the research of clinically important parasites, including several species of trematodes, nematodes and cestodes (Tagboto and Townson, Reference Tagboto and Townson2001; Tandon et al., Reference Tandon, Yadav, Roy, Das, Srivastava and Kumar2011). Plant extracts or their components can be used as new options or in combination with actual anti-parasitic treatments.
In this context, many plant extracts have shown promising in vitro results on the viability of E. granulosus s.l. protoscoleces (Al-Abodi et al., Reference Al-Abodi, Al-Shadeedi, Al-Alo and Ghasemian2019; Tabatabaei et al., Reference Tabatabaei, Dehshahri, Taghi, Esfandiari, Sadjjadi, Ebrahimipour and Sadjjadi2019; Ali et al., Reference Ali, Khan, Khan, Adnan, Ali, Khan, Haleem, Rooman, Norin and Khan2020; Cheraghipour et al., Reference Cheraghipour, Beiranvand, Zivdari, Amiri, Masoori, Nourmohammadi, Maken Ali, Abbaszadeh, Moradpour and Marzban2021). However, only a few plant extracts have been tested on the E. granulosus s.l. murine model, showing a reduction in cyst development and, in some cases, a marked damage in the germinal layer of the hydatid cysts (Lv et al., Reference Lv, Jiang, Liao, Sun, Zhang and Peng2013; Moazeni et al., Reference Moazeni, Larki, Saharkhiz, Oryan, Ansary Lari and Mootabi Alavi2014; Haji Mohammadi et al., Reference Haji Mohammadi, Heidarpour and Borji2018, Reference Haji Mohammadi, Heidarpour and Borji2019; Luo et al., Reference Luo, Zhang, Liu, Yuan, Gao, Gao, Ke, Zhang, Shi, Ma, Zhang and Dong2018; Al-Abodi et al., Reference Al-Abodi, Al-Shadeedi, Al-Alo and Ghasemian2019; Labsi et al., Reference Labsi, Soufli, Khelifi, Amir and Touil-Boukoffa2019).
Plants from the Stevia genus (Asteraceae) are a potential source of anti-protozoal and anti-microbial compounds (Ruiz-Ruiz et al., Reference Ruiz-Ruiz, Moguel-Ordoñez and Segura-Campos2017; Borgo et al., Reference Borgo, Laurella, Martini, Catalán and Sülsen2021). Recently, it has been shown that dichloromethane extracts of Stevia species presented a significant activity on Trypanosoma cruzi epimastigotes (Beer et al., Reference Beer, Frank, Elso, Bivona, Cerny, Giberti, Malchiodi, Martino, Alonso, Sülsen and Cazorla2016). On the other hand, several derivatives isolated from S. alpina showed antiparasitic activity against T. cruzi and Leishmania braziliensis making these promising candidates for the development of effective compounds for the treatment of Chagas disease and leishmaniasis (Sülsen et al., Reference Sülsen, Lizarraga, Elso, Cerny, Sanchez Alberti, Bivona, Malchiodi, Cazorla and Catalán2019; Elso et al., Reference Elso, Bivona, Sanchez Alberti, Cerny, Fabian, Morales, Catalán, Malchiodi, Cazorla and Sülsen2020).
In the current study, we demonstrated the in vitro efficacy of a Stevia multiaristata extract against protoscoleces and murine cyst of E. granulosus sensu stricto (s.s.). Moreover, we investigated the clinical efficacy of this extract in a murine model of CE.
Materials and methods
Plant material
The aerial parts of S. multiaristata Spreng. (Asteraceae) were collected in the province of Buenos Aires, Argentina, in February 2012. A voucher specimen was deposited at the Museo de Farmacobotánica, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires under the number BAF 742.
Preparation of S. multiaristata crude extract
For the preparation of the crude extract of S. multiaristata, the dried aerial parts were extracted by maceration twice with dichloromethane (10% w/v). The extract was filtered through filter paper (Schleicher & Schuell – Whatman, grade 0859, medium, smooth, 90 mm) and taken to dryness in a rotary evaporator.
Cell culture
The human hepatic cell line Huh7 was cultured in DMEM culture medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mm l-glutamine, 100 μg mL−1 streptomycin and 100 U mL−1 penicillin (DMEM-C) at 37°C under a 5% CO2 atmosphere. Cell viability at the beginning of each experiment was assessed by the trypan blue exclusion test.
Chemicals
For in vitro studies involving E. granulosus, the S. multiaristata extract was dissolved in dimethyl sulphoxide (DMSO) at a concentration of 35 mg mL−1 and added to the culture medium resulting in final concentrations of 100, 50, 10 and 5 μg mL−1. The maximum amount of DMSO added in the culture medium never exceeded 3 μL mL−1. For in vitro studies involving Huh7 cells, the S. multiaristata extract (10 mg) was sequentially resuspended in 20 μL of DMSO, 40 μL of ethanol 96% and finally, 40 μL of deionized water, giving a solution of 100 mg mL−1. Dilutions were carried out in complete DMEM-10% FBS. For in vivo treatments, ABZ (Pharmaceutical grade, Parafarm, Argentina) suspension (5.25 mg mL−1) was prepared by dispersion of pure ABZ in distilled and deionized water (pH = 7.0) with carboxymethylcellulose (CMC, Todo Droga, Córdoba, Argentina) (0.1% w/v, pH = 6.0). Stevia multiaristata extract was dissolved in olive oil with DMSO (0.5% v/v) at a drug concentration of 6.67 mg mL−1. Both suspensions were shaken for 5 h and sonicated for 1 h.
Parasite material, protoscoleces collection and cyst obtention
Liver and lung hydatid cysts were obtained from cattle slaughtered in an abattoir located in the Buenos Aires province, Argentina. Protoscoleces were removed aseptically from cysts as previously described (Elissondo et al., Reference Elissondo, Dopchiz, Ceballos, Alvarez, Sánchez Bruni, Lanusse and Denegri2006). Parasitic material was genotyped by sequencing a fragment of the gene coding for mitochondrial cytochrome c oxidase subunit 1 (CO1), as previously described (Cucher et al., Reference Cucher, Prada, Mourglia-Ettlin, Dematteis, Camicia, Asurmendi and Rosenzvit2011). Based on sequencing analysis, the G1 genotype was identified. To obtain murine cysts female CF-1 mice (body weight 25 ± 5 g) were infected by intraperitoneal inoculation with 1500 E. granulosus s.s. (G1 genotype) protoscoleces/animal, suspended in 0.5 mL of medium 199 (Mediatech, USA). At 6 months post inoculation, mice with experimental secondary CE were euthanized and necropsy was carried out immediately thereafter. At necropsy, the peritoneal cavity was opened and the hydatid cysts were carefully removed (Elissondo et al., Reference Elissondo, Ceballos, Alvarez, Sánchez Bruni, Lanusse and Denegri2009).
In vitro assays
Protoscolicidal activity
Viable and free protoscoleces (2000 per Leighton tube) were cultured in 6 mL of culture medium 199 at 37°C without changes in medium during the entire experiment as previously described (Elissondo et al., Reference Elissondo, Dopchiz, Ceballos, Alvarez, Sánchez Bruni, Lanusse and Denegri2006). Stevia multiaristata extract was added and the final achieved concentrations were 100, 50, 10 and 5 μg mL−1. Control protoscoleces were incubated in culture medium with DMSO (3 μL mL−1). Cultures were performed in triplicate and the experiment was repeated three times. To determine the appearance of morphological alterations, culture tubes were followed microscopically every day. Viability assessment using the methylene blue exclusion test was performed every day until day 6 and then, every 3 days. Samples of protoscoleces from each of the treatment groups and the control were periodically taken for ultrastructural studies by scanning electron microscopy (SEM).
Cysticidal activity
Groups of 10 cysts were placed in Leighton tubes containing 6 mL of medium 199. Stevia multiaristata extract was added to the medium resulting in final concentrations of 100, 50, 10 and 5 μg mL−1. Cysts incubated with the culture medium containing DMSO (3 μL mL−1) were used as control. Culture tubes were maintained at 37°C without changes in medium during the entire experiment (Elissondo et al., Reference Elissondo, Ceballos, Dopchiz, Andresiuk, Alvarez, Bruni, Lanusse and Denegri2007). Cultures were performed in triplicate and the experiment was repeated two times. Culture tubes were followed macro and microscopically every day. The criteria for cyst viability assessment included the loss of turgidity and the collapse of the germinal layer (Fabbri et al., Reference Fabbri, Maggiore, Pensel, Denegri, Gende and Elissondo2016).
In vitro cytotoxicity
Huh7 cells were harvested with trypsin 0.25% EDTA. Cell pellets were resuspended after centrifugation in dye mix [100 μg mL−1 acridine orange (AO), 100 μg mL−1 ethidium bromide (EB) in phosphate-buffered saline (PBS)] and visualized by fluorescence microscopy (Olympus BX51, America Inc.). Huh7 cells were cultured in the presence of the S. multiaristata extract (5–250 μg mL−1) or DMEM-C or solvent as controls, during 48 and 96 h. At least 200 cells were counted for each dose and cells were discriminated according to its stain pattern. Cells with homogeneous AO staining without EB stain were considered alive and cells with homogeneous EB staining were considered death (Cavaliere et al., Reference Cavaliere, Papademetrio, Lombardo, Costantino, Blanco and Álvarez2014). All experiments were performed in triplicate.
Selectivity index
Stevia multiaristata extract selectivity index (SI) was calculated at 96 h as the relation between CC50 (50% cytotoxicity concentration) on Huh7 cells and the EC50 (half maximal effective concentration) value determined by germinal layer collapse of E. granulosus s.s. cysts.
In vivo assay
Experimental animals and infection
Female CF-1 mice (body weight 25 ± 5 g) were intraperitoneally infected with 1500 E. granulosus s.s. protoscoleces/animal, suspended in 0.5 mL of medium 199.
Clinical efficacy study
At 6 months post-infection, female CF-1 mice (n = 30) were allocated into the following experimental groups (10 animals/group): (1) control group, (2) ABZ group, animals treated with ABZ suspension (25 mg kg−1) every 24 h for 30 days and (3) S. multiaristata group, animals treated with the extract (50 mg kg−1) every 24 h for 20 days. Treatments were performed by intragastric administration. At the end of the treatment period, all mice remained alive and they were euthanized. Necropsy was carried out immediately thereafter, the peritoneal cavity was opened and the hydatid cysts were carefully removed. The weight of the cysts collected from each animal was recorded using an analytical balance. Samples of cysts from each group were taken and fixed for histopathological analysis and SEM.
Blood biochemical assays
At the end of the clinical efficacy study, animals were anaesthetized with a mixture of ketamine (100 mg kg−1) and xylazine (10 mg kg−1), and blood samples were collected by cardiac puncture. Plasma was separated by centrifugation at 2000 g for 15 min and stored at −20°C until further analysis. To evaluate liver function, plasma levels of the enzymes alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), and glutamate-pyruvate transaminase (GPT) were measured using commercial kits from Wiener Lab (Rosario, Argentina). The activity of ALP was determined using the protocol described by Bessey et al. (Reference Bessey, Lowry and Brock1946). Plasma GGT activity was measured using the modified Szasz method (IFCC) (Szasz, Reference Szasz1969; Shaw et al., Reference Shaw, Stromme, London and Theodosen1983). For the estimation of GPT activity, the optimized UV method (IFCC) was followed (Bergmeyer et al., Reference Bergmeyer, Bowers, Horder and Moss1976). The enzymatic activities were expressed in units/litre (U/L).
Histopathological analysis
Echinococcus granulosus cysts were washed twice with PBS and fixed in 10% neutral-buffered formalin at 4°C for 72 h. Then, the fixed samples were dehydrated in an ascending series of ethanol, embedded in paraffin, cut into 5 μm sections and stained with haematoxylin and eosin for microscopic examination.
Electron microscopy
Samples of protoscoleces taken from the in vitro studies and samples of metacestodes taken from in vivo studies were processed for SEM following the protocol described by Elissondo et al. (Reference Elissondo, Ceballos, Dopchiz, Andresiuk, Alvarez, Bruni, Lanusse and Denegri2007).
Statistical analysis
All statistical analyses were conducted within the R environment (R Core Team, 2021). For all tests P values less than 0.05 were considered statistically significant. For the in vitro incubation of protoscoleces with the S. multiaristata extract, a generalized linear model with a quasi-binomial distribution was fitted with the proportion of viability as a response variable and treatments and time in days as explanatory variables. To determine whether time–treatment interactions had to be included in the model, we used the ‘ANOVA’ command from the ‘car’ package (Fox and Weisberg, Reference Fox and Weisberg2019). Differences among the S. multiaristata extract concentrations and control were assessed by pairwise contrasts of the interaction means using the ‘emmeans’ package (Lenth, Reference Lenth2021).
For the in vitro incubation of cysts and hepatic cells with S. multiaristata extract, differences between treatments and exposure times were tested by fitting an analysis of variance model with extract concentrations and exposure time as explanatory variables, and the percentage of cysts with loss of turgidity and germinal layer collapse, and cell death as response variables for each case. Then a Tukey's HSD test was applied for pairwise contrasts. Additionally, the EC50 of the extract was calculated using the ‘ec50estimator’ package in R program (Alves, Reference Alves2020).
For the in vivo experiments, differences between treated groups in the weight of the cysts and the plasma levels of ALP, GGT and GPT enzymes were all assessed by using the Kruskal–Wallis test followed by Dunn's multiple comparisons test. The weights of the cysts for each treatment are reported as median and interquartile range (IQR).
Ethic statement and experimental animals
Animal procedures and management protocols were approved by the Institutional Animal Care and Use Committee (RD No. 211/2018) of the Faculty of Exact and Natural Sciences, National University of Mar del Plata, Argentina and carried out in accordance with the revised form of The Guide for the Care and Use of Laboratory Animals (National Research Council US, Reference National Research Council US2011). Unnecessary animal suffering was avoided throughout the study. Animals were housed in a temperature-controlled (22 ± 1°C), light-cycled (12 h light/dark cycle) room. Food and water were given ad libitum.
Results
In vitro incubation of protoscoleces with S. multiaristata extract
Protoscoleces survival after incubation with S. multiaristata extract is shown in Fig. 1. Viability in the control group was 90.9 ± 3.6 after 12 days of incubation. Viability decreased quickly with 100 and 50 μg mL−1 of S. multiaristata extract, reaching 0% on days 7 and 8, respectively. The concentrations of 10 and 5 μg mL−1 reduced the viability to approximately 50% between days 7 and 8; at the end of the experiment, approximately above 10% of the protoscoleces treated with these concentrations remained viable.

Fig. 1. Estimates of Echinococcus granulosus s.s. protoscoleces survival after in vitro exposure to the Stevia multiaristata extract. The lines and ribbons indicate the predicted fits and 95% confidence intervals from a generalized linear mixed-effects model.
Figures 2 and 3 show representative images of protoscoleces treated with different concentrations of S. multiaristata extract. Tegumental alterations were consistent with the results of the viability test. Control protoscoleces remained unaltered throughout the experimental period and no changes in structure and ultrastructure were observed (Figs 2A and 3A). After 6 days post incubation, protoscoleces treated with the lowest concentrations (5 and 10 μg mL−1) showed rostellar disorganization and alterations in the tegument (Fig. 2B and C). At day 3 post incubation, protoscoleces treated with 50 or 100 μg mL−1 presented total loss of hooks and blebs in the tegument (Fig. 2D and F). Soma contraction could be detected in the first day of incubation with 100 μg mL−1 (Figs 2E and 3E). Studies by SEM revealed that the ultrastructure of protoscoleces treated during 3 days with 50 or 100 μg mL−1 were altered with tegumental alterations, loss of microtriches of the scolex region, rostellar disorganization and soma contraction (Fig. 3D and F). Protoscoleces incubated during 6 days with the lowest concentrations showed milder ultrastructural alterations (Fig. 3B and C).
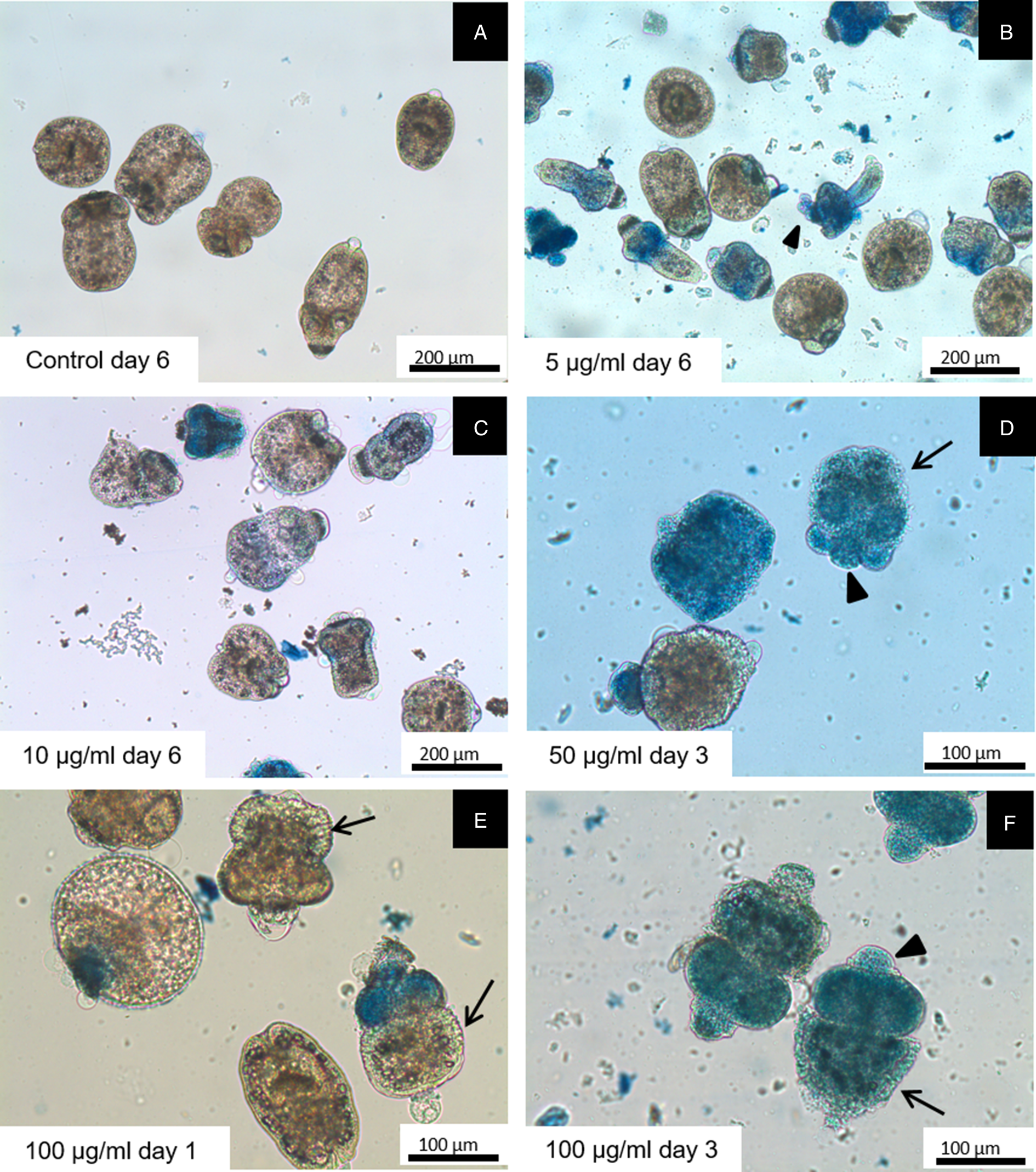
Fig. 2. Light microscopy of E. granulosus s.s. protoscoleces incubated in vitro with different concentrations of S. multiaristata extract and stained with methylene blue. Blue protoscoleces are not viable. Observe the contracted soma (arrows) and rostellar disorganization (arrowheads).

Fig. 3. SEM of E. granulosus s.s. protoscoleces incubated in vitro with different concentrations of the S. multiaristata extract.
In vitro incubation of cysts with S. multiaristata extract
Control cysts appeared macroscopically turgid with no observable collapse of the germinal layer throughout the in vitro experiment (Fig. 4Aa). In contrast, loss of turgidity was detected in 95 ± 3.4% of cysts incubated during 2 days with 100 μg mL−1 of S. multiaristata extract (Fig. 4Ab and Ba). After 4 days post incubation, the collapse of the germinal layer was observed in 32 ± 8.7% and 60 ± 9.3% of cysts treated with 50 and 100 μg mL−1 (Fig. 4Ac and Bb) of the S. multiaristata extract, respectively. Stevia multiaristata extract showed a dose and time dependent effect against cysts (Fig. 4B, Fig. S1).

Fig. 4. (A) Light microscopy of E. granulosus s.s. cysts incubated in vitro with 100 μg mL−1 of the S. multiaristata extract. Observe the loss of turgidity (arrowhead) and collapse of the germinal layer (arrow). (B) Effect of S. multiaristata extract on E. granulosus s.s. cysts after 2, 4 and 12 days of in vitro exposure to different concentrations. The criteria used to evaluate the cysticidal effect were: (a) loss of turgidity and (b) germinal layer collapse. Each bar represents the mean percentage ± s.d. (standard deviation). Similar letters indicate no differences (P < 0.05).
The EC50 value of the S. multiaristata extract against E. granulosus s.s. cysts was 69.6 μg mL−1.
In vitro cytotoxicity on a hepatic cell line
The percentage of viable cells after treatment with S. multiaristata extract at concentrations between 5 and 100 μg mL−1 was higher than 69.5%, at both times evaluated. Only at a dose of 250 μg mL−1 the extract induced a significant percentage of cell death in relation to the control (P < 0.0001), with values of 77.5 ± 4.8% and 99.0 ± 0.6% cell death after 48 and 96 h, respectively (Fig. 5). Moreover, there is no time-dependence in the response of Huh7 cells in the presence of the S. multiaristata extract. The CC50 values for 48 and 96 h were 207.7 and 132.2 μg mL−1, respectively. The SI for E. granulosus s.s. cysts was 1.9 (96 h).

Fig. 5. Effect of different concentrations of S. multiaristata extract on Huh7 cells after 48 and 96 h of in vitro exposure. The graph shows the percentage of cell death determined as % cells PI+/total cell. ‘Control’ corresponds to cells cultured with 10% DMEM, ‘SV’ corresponds to cells culture in the presence of the solvent used to resuspend the S. multiaristata extract. Each bar represents the mean percentage ± s.d. Three independent experiments were performed. Different letters indicate significant differences (P < 0.0001).
Clinical efficacy study
The behaviour and appearance of the animals were normal throughout the entire experimental period. Furthermore, no statistical differences were found in ALP, GGT and GPT activities between control and treated mice (P > 0.05).
Hydatid cysts developed in all the infected animals involved in the clinical efficacy study. Table 1 summarizes the cyst weights (median and IQR) recorded after treatments of the different experimental groups involved in the study. Although the median weight of cysts recovered from ABZ-treated mice was lower than that observed in the control group, no significant differences were found (P > 0.05). In contrast, S. multiaristata treatment caused a significant decrease in the weight of the cysts compared with the control group (P < 0.05).
Table 1. Clinical efficacy study

Median weight (g) and IQR of the Echinococcus granulosus cyst recovered from experimentally infected mice from the unmedicated and treated groups.
* Statistically significant differences with the control group (P < 0.05).
An intact consecutive germinal layer and laminated layer were observed in the control group (Fig. 6A). Histopathological analysis showed no differences between control and treated mice (Fig. 6B and C).

Fig. 6. Histopathological images of hydatid cysts recovered from infected mice. Cuts were stained with haematoxylin–eosin: (A) control; (B) ABZ (25 mg kg−1) and (C) S. multiaristata extract (50 mg kg−1). LL, laminated layer; GL, germinal layer.
Studies by SEM of metacestodes from the control group revealed the typical features of E. granulosus cysts, with an intact germinal layer composed of a multitude of different cell types (Fig. 7A). Cysts recovered after treatment with ABZ 25 mg kg−1 showed a germinal layer with reduction in the number of cells and damaged cells (Fig. 7B). On the other hand, cysts recovered after treatment with the S. multiaristata extract 50 mg kg−1 displayed a severe impairment of the germinal layer and a significant decrease in the number of cells (Fig. 7C).

Fig. 7. SEM of E. granulosus metacestodes recovered from infected mice treated with ABZ or S. multiaristata extract during the clinical efficacy study. Treatments were administered orally at the doses of 25 mg kg−1 of ABZ and 50 mg kg−1 of S. multiaristata extract every 24 h. (A) Metacestode from the control group. Observe the germinal layer (GL) with different types of cells. (B) Germinal layer of metacestode from mice treated with ABZ suspension showing loss of cells. (C) Altered metacestode recovered from S. multiaristata extract treated mice. Note the extensive areas without cells and the presence of cellular debris.
Discussion
Phytotherapy has a high potential in the fight against neglected diseases for which prevention and cure are not sufficiently available. As stated by the World Health Organization, up to 80% of the population in developing countries depends on the use of traditional medicine and medicinal herbs for primary health care (WHO, 2013).
In traditional medicine whole plants or mixtures of plants are used rather than isolated compounds. There is evidence that crude plant extracts often have greater in vitro and/or in vivo anti-parasitic activity than isolated constituents at an equivalent dose (Rasoanaivo et al., Reference Rasoanaivo, Wright, Willcox and Gilbert2011). One hypothesis that could explain this is the synergistic interaction or multi-factorial effects between compounds present in herbal extracts (Duke and Bogenschutz-Godwin, Reference Duke, Bogenschutz-Godwin, Kaufmann, Cseke, Warbler, Duke and Brielmann1999; Gilbert and Alves, Reference Gilbert and Alves2003).
Natural compounds have been used as medicines against infectious diseases caused by fungi, bacteria, viruses and parasites (Cos et al., Reference Cos, Vlietinck, Berghe and Maes2006). In the last few decades, a large number of plant extracts and compounds with anthelmintic activity against E. granulosus s.l. were reported. However, in most of these studies, only in vitro experiments were carried out (Ali et al., Reference Ali, Khan, Khan, Adnan, Ali, Khan, Haleem, Rooman, Norin and Khan2020).
In Argentina, the genus Stevia is represented by more than 30 species and several varieties growing in the northern and central areas of the country (Zuloaga et al., Reference Zuloaga, Morrone and Belgrano2008); however, the majority have not been investigated so far. Stevia multiaristata Spreng. is a native species which has shown antiprotozoal activity against T. cruzi (Beer et al., Reference Beer, Frank, Elso, Bivona, Cerny, Giberti, Malchiodi, Martino, Alonso, Sülsen and Cazorla2016).
The in vitro efficacy of S. multiaristata extract against E. granulosus s.s. protoscoleces and murine cysts was demonstrated. In both cases, the effect was time and dose dependent. Stevia multiaristata extract caused a marked reduction on protoscoleces viability which was consistent with the alterations observed such as contraction of soma region, the presence of blebs in the tegument, rostellar disorganization and loss of hooks and microtriches. Cysts treated with the S. multiaristata extract showed a rapid loss of turgidity and germinal layer collapse. The ultrastructural alterations were similar to those observed in E. granulosus s.s. protoscoleces and cysts incubated in vitro with other drugs and natural products as thymol, carvacrol and essential oils of oregano and thyme (Elissondo et al., Reference Elissondo, Pensel and Denegri2012; Pensel et al., Reference Pensel, Maggiore, Gende, Eguaras, Denegri and Elissondo2014; Fabbri et al., Reference Fabbri, Maggiore, Pensel, Denegri, Gende and Elissondo2016).
Even though the S. multiaristata extract showed a low SI, neither of the experimental concentrations (5–100 μg mL−1) caused a cytotoxic effect, indicating that this compound has the capacity to inhibit the parasite growth without displaying a significant toxicity on the host's cells at the assayed concentrations.
In the current study, the effect of S. multiaristata extract was also evaluated in an in vivo murine model of CE. To the present, there are only a few plant extracts that were investigated for preventive or therapeutic activities against E. granulosus s.l. in the search for new alternative treatment for CE (Ali et al., Reference Ali, Khan, Khan, Adnan, Ali, Khan, Haleem, Rooman, Norin and Khan2020). Some of these studies include the use of the methanolic extract of Allium sativum, the aqueous extract of Huaier, the aqueous extract of Punica granatum peel, the aqueous extract of Sophora moorcroftiana seeds and the methanolic extract of Zataria multiflora. All the extracts caused a decrease in the weight of the cysts recovered from treated mice compared with the control groups (Lv et al., Reference Lv, Jiang, Liao, Sun, Zhang and Peng2013; Moazeni et al., Reference Moazeni, Larki, Saharkhiz, Oryan, Ansary Lari and Mootabi Alavi2014; Labsi et al., Reference Labsi, Khelifi, Mezioug, Soufli and Touil-Boukoffa2016; Haji Mohammadi et al., Reference Haji Mohammadi, Heidarpour and Borji2018; Luo et al., Reference Luo, Zhang, Liu, Yuan, Gao, Gao, Ke, Zhang, Shi, Ma, Zhang and Dong2018). Moreover, and in accordance with our results, the presence of ultrastructural alterations in the germinal layer was reported (Moazeni et al., Reference Moazeni, Larki, Saharkhiz, Oryan, Ansary Lari and Mootabi Alavi2014; Luo et al., Reference Luo, Zhang, Liu, Yuan, Gao, Gao, Ke, Zhang, Shi, Ma, Zhang and Dong2018).
The median lethal dose (LD50) of S. multiaristata extract has not been described yet. On the other hand, Kujur et al. (Reference Kujur, Singh, Ram, Yadava, Singh, Kumari and Roy2010) reported a nontoxic effect of the leaves of S. rebaudiana in mice and suggested that the LD50 would be greater than 5 g kg−1 body weight in mice. This value is much greater than the dose used in the current study. No adverse side-effects on mice were observed during the entire treatment period. Moreover, we found no differences in the enzymes ALP, GGT and GPT activities between control and treated mice suggesting no hepatotoxic effect.
Oral administration of 50 mg kg−1 of S. multiaristata extract during 20 days in infected mice has a therapeutic effect on the hydatid cyst causing a significant reduction of the parasite weight. We found that the S. multiaristata extract caused both an in vitro effect on protoscoleces and cysts and also an in vivo therapeutic effect.
Stevia genus is characterized by the presence of sesquiterpene lactones, diterpenoids and flavonoids, among other phytochemical groups (Borgo et al., Reference Borgo, Laurella, Martini, Catalán and Sülsen2021). Anti-parasitic activity has been described for many compounds belonging to these groups (Sanchez Alberti et al., Reference Sanchez Alberti, Cerny, Bivona, Cazorla, Sülsen and Martino2018; Boniface and Ferreira, Reference Boniface and Ferreira2019; Sessa et al., Reference Sessa, Mengarda, Simplicio, Antar, Lago and de Moraes2020; Sülsen, Reference Sülsen2021). Sesquiterpene lactones are one of the major phytochemical groups of compounds present in the genus Stevia. In relation to their mechanism of action it has been reported that they exert their biological activities throughout the inhibition of proliferation by the induction of apoptosis when they were applied on cancer cells (Rasul et al., Reference Rasul, Khan, Ali, Li and Li2013; Moujir et al., Reference Moujir, Callies, Sousa, Sharopov and Seca2020). Regarding diterpenes with anti-parasitic activity, it has been shown that they inhibit nitric oxide production and inducible nitric oxide synthase (iNOS) expression by blocking the activation of STAT1, IRF3 and NF-κB on macrophages (Byoung, Reference Byoung2012). Flavonoids constitute another relevant phytochemical group found in the genus Stevia and the mode of action of these compounds may be multifaceted. It has been suggested that they can counter drug resistance, inhibit important enzymes or proteins or induce apoptosis of the parasites, among others (Mead and McNair, Reference Mead and McNair2006). The results of the in vitro and in vivo anti-Echinococcus activity of S. multiaristata could indicate the presence of bioactive compounds of these types in the organic extract. Phytochemical analysis and fractionation of the extract will be undertaken in order to isolate the active molecules.
In conclusion, we demonstrated high protoscolicidal and cysticidal effects, and significant reduction in the weight of the cysts in experimentally infected mice following treatment with the S. multiaristata extract.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182021002109
Acknowledgements
The authors thank Alejandra Goya, Sonia Ortega and Carolina Kelly (SENASA, Argentina). This study is an activity within the Research Network Natural Products against Neglected Diseases (ResNetNPND) (http://www.resnetnpnd.org).
Author contributions
AC and CE conceived and designed the study; EO, PD, GD, GB, BM and VS produced and provided plant extract; PA provided parasite material; AC, JF, PP and LF carried out the experiments and analysed and interpreted the data; NF performed statistical analyses; ZP carried out histopathological analyses; EN and GG performed biochemical analyses; AC, CE, JF, PP and VS wrote the article.
Financial support
This study was financially supported by the PICT 2015 No. 0717 and PICT 2019 No. 1123 (Agencia Nacional de Promoción Científica y Tecnológica, Argentina), PIP 11220150100158CO (CONICET, Argentina), EXA 975/20 (Universidad Nacional de Mar del Plata, Argentina), UBACYT 20020170100316BA (Universidad de Buenos Aires, Argentina).
Conflict of interest
None.












