Introduction
Nutrition during infancy plays a key role in the protection and development of health.Reference Cripps, Martin-Gronert and Ozanne 1 , Reference Michaelsen, Larnkjaer and Larsson 2 Increase in negative outputs, such as cardiovascular diseases, type-2 diabetes and obesity, is associated with low birth weight, low weight in the first year after birth, catch growth in childhood and changing body composition at an advanced age.Reference Barker, Gluckman and Godfrey 3 Accordingly, experimental studies have focused on adopting a ‘programming’ hypothesis in preterm and term infants. For these reasons, nutrition researchers have focused on the biological characteristics of breast milk, which is the main nutritional source of many newborns.Reference Savino, Benetti and Liguori 4
The adiponectin and leptin contents of breast milk vary widely.Reference Garcia, Duan and Brevaut-Malaty 5 These adipokines in breast milk decrease during the lactation period and do not change depending on the sex of the infant.Reference Bronsky, Karpisek and Bronska 6 – Reference Ng, Lee and Lam 8 Breast milk adiponectin has a negative relationship with growth (increased body fat) in the first few months after birth but is positively associated with growth (increase in length) in later periods.Reference Brunner, Schmid and Zang 9 , Reference Woo, Guerrero and Guo 10 Brunner et al.9 examined the correlation between the adiponectin content of breast milk and growth of infants and reported that the amount of adiponectin in breast milk had a negative correlation with the anthropometric values of infants in the first 4 months. Furthermore, there was a negative association between the adiponectin level of breast milk and the growth rate of infants.Reference Newburg, Woo and Morrow 11 In one study, the leptin content of breast milk in the first month showed a negative correlation with the body mass index (BMI) Z-score of term infants.Reference Fields and Demerath 12 Another study examined the effect of the leptin content of breast milk on the weight gain control of newborns. It was determined that there was a negative association between the leptin concentration of breast milk in the first month and BMI.
Although studies show some relationship between adipokine concentrations in breast milk and neonatal anthropometrics, the relationship between leptin and adiponectin and short-term growth in infants is unclear. The aim of this study was to evaluate the relationship between breast milk adipokine levels and short-term growth of the preterm and term infants.
Material and methods
Design
This research was conducted as a prospective, cohort and observational study. Ethics committee approval, 23.02.2016, 15/2016, was received from the Clinical Research Ethics Committee of the Republic of Turkey Ministry of Health Zekai Tahir Burak Women’s Health Training and Research Hospital (2011-KAEK-19). All participants provided written informed consent.
Setting
The participants of the study were the mothers and infants who consulted the Department of Pediatrics at the Faculty of Medicine, Gazi University in Ankara. The mothers were interviewed within 1 week after birth and were informed that breast milk samples would be taken when their children were 15–30 days old.
Sample
The mothers (aged between 18 and 35 years) of 31 preterm (median=35.3 weeks, range 31–36 weeks) and 34 term (median=38.7 weeks, range 37–42 weeks) infants were recruited from the Department of Pediatrics in the Faculty of Medicine, Gazi University in Ankara. The exclusion criteria included infants that had any congenital infection/malformations, chronic disease, intrauterine growth retardation and necrotizing enterocolitis; were born with multiple births; or had started complementary foods during the first month of lactation. Mothers who had any chronic disease and used medication during the study period were excluded. The inclusion criteria for infants were those fed exclusively breast milk in the first month and those fed predominantly breast milk in the following months.
Data collection
Currently, there is no single sampling protocol that is universally applicable for every constituent of potential interest in human milk. Standardizing milk collection to a particular time of day, preferably in the morning, is preferred for milk collection protocols. The composition of breast milk is affected by the gestational age of the infant,Reference Kociszewska-Najman, Borek-Dzieciol and Szpotanska-Sikorska 13 breastfeeding hourReference Karaağaoğlu and Eroğlu Samur 14 and beginning v. end of breastfeeding.Reference Karatas, Durmus Aydogdu, Dinleyici, Colak and Dogruel 15 Therefore, our milk collection protocol was as follows: breast milk samples were taken once during the 1.5–2 h of the hunger period of the mothers and 1.5 h after the latest breastfeeding (foremilk) by manual expression from one breast into 5-ml tubes containing ethylenediaminetetraacetic acid and proteinase inhibitor (aprotinin) between the 15th and 30th days in accordance with the infant’s calendar age (mature milk) between 8 am and 11 am.
Infant growth was followed by the researcher (B.K.) in the first, second and third months with measurements of length, body weight, head circumference, triceps and subscapular skinfold thicknesses (SSF) according to the measurement techniques. The length, body weight and head circumference of infants at birth were taken from hospital records.
Measurement
For the analysis of adiponectin and leptin adipokines, the enzyme-linked immunosorbent assay method was used with BioVendor–Laboratorni medicina a.s. (Modrice, Czech Republic) kits. Breast milk analyses were performed in accordance with the instructions of the kit manufacturer and with reference to Bronsky et al.Reference Bronsky, Karpisek and Bronska 6 , Reference Bronsky, Mitrova and Karpisek 16
The anthropometric measurements that are frequently used to assess growth in newborns are body weight, length, growth velocity, head circumference and skinfold thickness.Reference Pereira-da-Silva 17 The body weight, length and head circumference of the infants were measured according to the standard techniques. 18 Body weight in grams was measured using a portable electronic baby scale (Seca 727, Seca) that was accurate to within 10 g. Infant length in cm was measured using a portable infantometre (Seca 416, Seca) with the infant in the supine position on a flat and stable surface, such as a table. Head circumference in cm was measured with flexible non-stretchable tape around the largest area of the infant’s head or the distance around the back of the head with the tape measure held above the eyebrows and ears.
The skinfold thicknesses in mm of infants were taken using standard callipers (Holtain Ltd, Croswell, Crymych, UK) at the triceps and subscapular regions according to published measurement techniques.Reference Lohman, Roache and Reynaldo 19 Triceps skinfold thickness (TSF) was measured at the midpoint between the acromion and olecranon, and SSF was measured at the inferior angle of the scapula in the skinfold axis. Body mass index was calculated according to the formula BMI (kg/m2)=body weight (kg)/length (m2). 20
For the evaluation of the growth of preterm infants, researchers used the Fenton growth curve for head circumference and length and body weight according to gestational age and a calculation program that enables Fenton weight Z-score measurements.Reference Fenton and Kim 21 , Reference Fenton and Sauve 22 The Lubchenco classification was used for the classification according to weight-for-gestational age: below the 10th percentile ‘Small for Gestational Age – SGA’; 10th–90th percentile ‘Appropriate for Gestational Age – AGA’; over the 90th percentile ‘Large for Gestational Age – LGA’.Reference Lubchenco, Hansman, Dressler and Boyd 23
The World Health Organization (WHO) 2006 growth standards 24 , 25 and WHO Anthro (version 3.2.2, January 2011) program were used to calculate weight, length, head circumference, TSF and SSF Z-scores according to the gestational age of term infants. The measurements were classified according to the Z-score junctions. 20
Data analysis
The data obtained from this study were evaluated with SPSS 22.0 package software. Descriptive statistical variables [median, interquartile range (IQR)] were used to evaluate the data. To examine the differences between gestational weeks and preterm–term breast milk, a t-test or Mann–Whitney U test was used, depending on the normality of the distributions of the variables. A paired-samples test, repeated analysis of variance or Wilcoxon Saigned Rank test and Friedman test were used to evaluate the anthropometric measurements of infants according to the normality distribution. Linear regression analyses were used to test for an association among adiponectin, leptin, gender, maternal age, mode of delivery, gestational age, post-partum BMI, pre-pregnancy BMI, body weight at birth, head circumference at birth and body weight increments at 0–1 months, 1–2 months and 2–3 months. To identify correlations between parameters, Spearman’s correlation analysis was used, and the results were evaluated within a 95% confidence interval and at the P<0.01 and P<0.05 significance levels.
Results
The mean preterm infant body weight was 2120.5±552.56 g at birth, and the mean weight gain was 882.6±216.67 g/month during follow-up. Regarding the distribution of the body weight categorization according to gestational age in the fetal growth curve of preterm infants, 90.3% of preterm infants had an AGA at birth; however, this rate became 67.8% in the third month. In the third month, there was an increase in the SGA (16.1%) and LGA (16.1%) categories.
The mean body weight of term infants was 3375.4±405.93 g. The mean weight gain of term infants was 993.8±229.43 g/month. The percentage of term infants whose weight-for-age was normal (AGA) was 88.2%; their SGA was 11.8%. There were no LGA infants at birth. In the following months, the percentage of term infants with a normal weight was 76.6% and the percentage of overweight-obese term infants was 5.8% and the percentage of risk of underweight term infants was 17.6% in the third month.
The monthly increments in all anthropometric measurements (except for length) were found to be significant in both groups (P<0.05). Comparing preterm and term infants’ growth, all anthropometric measurements were found to be significantly higher in term infants during follow-up, whereas for increments in anthropometric measurements, only head circumference increment (cm/month) was significantly different (preterm: 2.4±0.52 cm/month, term: 1.9±0.44 cm/month, P<0.001).
The median (IQR) adipokine levels were higher in preterm infants [adiponectin=24.6 (14.3) and 22.9 (9.7) ng/ml for preterm infants and term infants, respectively, and leptin=2.0 (2.5) and 0.0 (2.3) ng/ml for preterm infants and term infants, respectively]; however, the difference between the groups was not statistically significant (P> 0.05).
According to the Lubchenco classification, there was not a significant difference among SGA, AGA and LGA preterm infants in terms of the median (IQR) adiponectin [23.8 (36.9), 24.9 (10.7) and 22.1 (0.0) ng/ml, respectively] and leptin [2.0 (2.3), 2.0 (2.5) and 2.0 (0.0) ng/ml, respectively] levels in the first month of life. However, according to the WHO Z-score classification for term infants in the first month of life, the median (IQR) adiponectin [33.1 (12.5) and 21.9 (7.5), respectively, P=0.003] levels were higher in underweight compared to normal weight infants, but there were no differences in leptin levels [0.0 (1.5) and 0.0 (2.4), respectively, P=0.222] (there were no overweight-obese infants).
The breast milk adiponectin levels were negatively correlated with length increment and positively correlated with BMI increment in second–third month for preterm infants (r s=−0.628 P=0.001; r s=0.553 P=0.006, respectively). However, the breast milk adiponectin levels were negatively associated in the first, second and third months with body weight, head circumference at birth, BMI in the first–third month and BMI increment at birth–first month in term infants (P<0.05). In addition, leptin was negatively associated with head circumference at birth (r s=−0.392, P=0.029) in preterm infants and the TSF increment in first–second month term infants (r s=−0.531, P=0.023) (Table 1).
Table 1 The relationship between anthropometric measurements of infants at birth and during follow-up and breast milk adiponectin and leptin levels
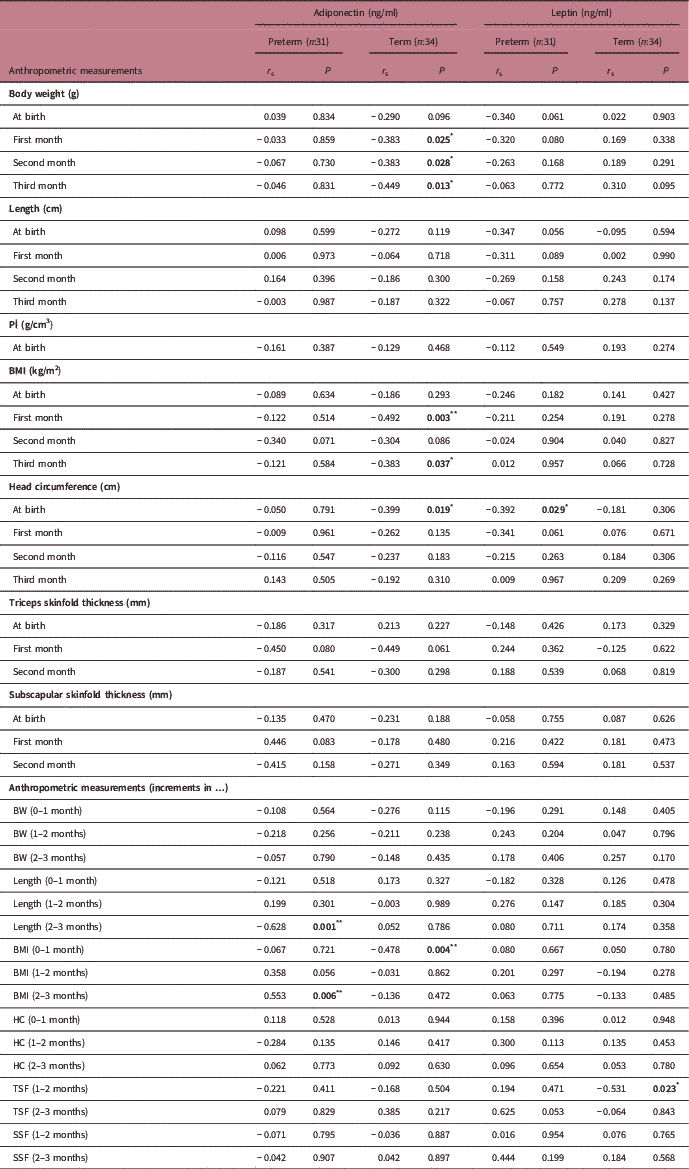
PI, Ponderal İndeks; BW, body weight; BMI, body mass index; HC, head circumference; SSF, subscapular skinfold thicknesses; TSF, riceps skinfold thickness.
Spearman’s correlation *P<0.05, **P<0.01.
There was a negative correlation between the weight-for-length Z-score and adiponectin in term infants only in the first month (r=−0.375, P=0.029). However, there was not any significant correlation between the Z-score and adipokines for 3 months in preterm infants.
When factors that could affect body weight increments (in 0–1 months, 1–2 months and 2–3 months, separately) were evaluated with linear regression analyses, only the 0–1 months model was found important (R 2=0.314, P=0.016; R 2=0.237, P=0.137; R 2=0.192, P=0.441; respectively). While gestational age, adiponectin and leptin were not related, maternal age and pre-pregnancy BMI had an effect on the body weight increment in 0–1 months (P<0.05) (Table 2).
Table 2 Linear regression analysis for body weight increment prediction
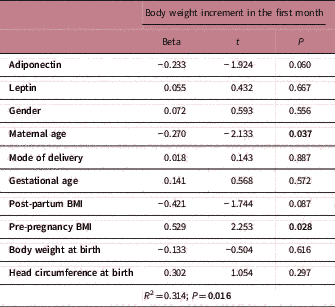
BMI, body mass index.
Discussion
The weight gain of preterm infants in the first month was insufficient; however, positive linear growth was observed in the following months according to the 3-month follow-up recommendations for preterm infants proposed by the High Risk Infant Observation Guide of the Republic of Turkey Ministry of Health (2014). 26 Breast milk volume can decrease with a preterm birth, disease of the mother or infant, separation of the mother from the infant, anxiety, fatigue and emotional stress.Reference Bundak and Neyzi 27 , Reference Zuppa, Sindico and Orchi 28 An insufficient weight gain of preterm infants in the first month can be due to the above-stated reasons.
The anthropometric measurements of term infants during follow-up in our study were found to be the same as found in the literature.Reference Bundak and Neyzi 27 , Reference İnce, Kondolot and Yalçın 29 , Reference Köksal 30 According to the WHO Child Growth Standards, infants’ growth was found to be within the normal Z-score range. 31
Preterm infants have a similar growth rate as foetuses and are expected to have a growth rate similar to healthy term infants after 40 weeks;Reference Kleinman and Greer 32 however, our study showed that only the head circumference values of preterm and term infants differed among the other growth rate criteria in the first 3 months. Furthermore, in our study, there were no statistically significant differences in breast milk adipokines between the groups. In the literature, breast milk is stated to display a wide range of leptin (0.16–105 ng/ml) and adiponectin (1.25–90 ng/ml) content.Reference Garcia, Duan and Brevaut-Malaty 5 It has been stated that the breast milk of the mothers of term infants contains more leptin than that of preterm infant mothers,Reference Bronsky, Karpisek and Bronska 6 , Reference Ng, Lee and Lam 8 , Reference Mehta and Petrova 33 while the breast milk of the mothers of preterm infants contains more adiponectin,Reference Mehta and Petrova 33 but show variability due to ethnicity.Reference Martin, Woo and Geraghty 7 Our results may have been due to the fact that there was not a substantial difference between the median values of preterm and term infants at the gestational birth week. Also, compared to other similar studies, ethnicity may a reason for differences in the results.
In this study, negative correlations were found between the adiponectin concentration of breast milk and some anthropometric measurements of preterm–term infants. On the other hand, in preterm infants, the adiponectin concentration had a positive correlation with BMI increment in the second–third months. Currently, there is strong evidence that adiponectin has an effect on the growth of newborns.Reference Fields and Demerath 12 , Reference Kon, Shilina and Gmoshinskaya 34 Likewise, Brunner et al.9 reported that breast milk adiponectin has a negative correlation with the anthropometric values of infants in the first 4 months. At the same time, we found that breast milk adiponectin was significantly higher in term infants who were in the underweight group in the first month. Similarly, in a study evaluating the correlation between breast milk adiponectin and weight-for-age, weight-for-length and length-for-age Z-scores of the newborns 3 months, the breast milk adiponectin concentration was negatively associated with weight-for-age and weight-for-length Z-scores.Reference Newburg, Woo and Morrow 11 In our study, although similar results were shown in term infants, the results were contradictory in preterm infants.
In our study, there was no significant association between the anthropometric measurements and breast milk leptin levels during the first 3 months in preterm infants. On the other hand, there was a negative correlation between leptin and the TSF increment in the first–second months in term infants. The breast milk leptin concentration is positively correlated with the plasma leptin levels of newborns, which is related to the growth of infants.Reference Ucar, Kirel and Bor 35 In addition, leptin has a negative effect on the body weight of the infant because it decreases energy intake and increases energy consumption.Reference Doneray, Orbak and Yildiz 36 , Reference Schuster, Hechler and Gebauer 37 However, our results suggest that leptin may not have significant effects on short-term growth.
The effects of milk adiponectin and leptin are not limited to the period in which the infant was fed with breast milk. Impacts of these adipokines may prevail in the following months.Reference Woo, Guerrero and Guo 10 For example, being fed breast milk containing high levels of adiponectin may prevent overweight/obesity risk during childhood because weight gain in the first 6 months of life is likely attributed to an increase in lean body mass.Reference Brunner, Schmid and Zang 9 In a study examining the effect of the leptin content of breast milk on the weight gain of newborns, there was a negative association between the leptin concentration of breast milk in the first month and BMI at the 18th and 24th months.Reference Miralles, Sanchez, Palou and Pico 38 Another study examined the correlation between the growth of infants until 2 years of age and the leptin concentration of a breast milk sample taken from the mothers of infants fed with breast milk partly or completely in the sixth week after birth. It was found that the breast milk leptin content was not associated with anthropometric measurements until 2 years of age.Reference Brunner, Schmid and Zang 9 Therefore, the breast milk adiponectin–leptin effects on growth may be different in the long term.
In a regression model, maternal age and pre-pregnancy BMI were related to body weight in the first month. Our results are consistent with other studies that suggest that maternal ageReference Hayward, Greenwood and Sibley 39 and maternal pre-pregnancy BMI have a direct relationship on infant growth.Reference Yu, Han and Zhu 40
Limitations
The limitations of this study are the fact adiponectin and leptin are only two bioactives in the milk that influence growth, that the body composition was not measured to evaluate growth, that the breast milk intake (by test weighing) for infants was not measured, that the doses of adiponectin and leptin (e.g., calculating infant breast milk intake and a specific adipokine ‘dose’ for each infant) were not measured and that only the milk adipokine concentration was evaluated. Infants may have consumed insufficient amounts of milk, thus affecting growth. Additional limitations include that infants were fed exclusively breast milk in the first month and that due to the small sample size, the regression model of growth may not explain the covariate effects fully. These factors should be taken into consideration in future research.
Conclusions
In conclusion, our results suggest that adiponectin, but not leptin, is significantly related to short-term infant growth. Our results may differ had extremely preterm infants (<28 weeks gestation) been included. We also confirm the previous reports that maternal age and pre- pregnancy BMI are related to infants growth. In future studies, it would be beneficial to carry out longer term longitudinal studies to evaluate the growth impacts of these adipokines on breastfed infants.
Acknowledgments
The authors are grateful to all the mothers who are participating in this study.
Financial Support
B.K. was supported by a research grant from ÖYP. ÖYP is the abbreviation for the Faculty Development Programme (Öğretim Üyesi Yetiştirme Programı), established to train academicians.
Conflict of Interest
None.




