Introduction
The beetle Cerambyx welensii Küster (=C. velutinus Brullé) (Coleoptera: Cerambycidae: Cerambycinae) is among the largest longhorn species in the Palaearctic fauna (Bense, Reference Bense1995). Its host plants include species in the genus Quercus, although this wood-boring beetle has been also occasionally reported from other broadleaf trees (Bense, Reference Bense1995; Vives, Reference Vives2000). Populations of C. welensii are most abundant in the southern part of its European distribution, particularly in dehesa ecosystems, a singular type of Mediterranean open woodland similar to savannah landscape, typically populated by holm oak (Quercus ilex L.) and cork oak (Quercus suber L.) (Montero et al., Reference Montero, San Miguel, Cañellas, Jiménez-Díaz and Lamo de Espinosa1998). Like other wood-boring longhorns developing on oaks, C. welensii was thought to primarily utilize old, decayed and diseased trees, forming part of the highly diverse assemblage of saproxylic insects. The activity of this functional group of species – often in combination with wood-degrading saprophyte microorganisms – is considered essential in ecological and biodiversity terms, particularly for being primary producers of arboreal cavities and shelters exploited as niches for an array of animals, including invertebrate, reptile, birds and mammals (Grove, Reference Grove2002; Buse et al., Reference Buse, Ranius and Assmann2008).
The impact of C. welensii has changed drastically in last decades. This insect is now found causing injuries to healthy and young trees, threatening the high resilience and stability of the dehesa ecosystem (Martín et al., Reference Martín, Cabezas, Buyolo and Patón2005; López-Pantoja et al., Reference López-Pantoja, Domínguez Nevado and Sánchez-Osorio2008; Carrasco, Reference Carrasco2009; Torres-Vila et al., Reference Torres-Vila, Sánchez-González, Ponce-Escudero, Martín-Vertedor and Ferrero-García2012a, Reference Torres-Vila, Sánchez-González, Merino-Martínez, Ponce-Escudero, Conejo-Rodríguez, Martín-Vertedor and Ferrero-García2013). A large study on 6000 trees across Extremadura (Southwestern Spain) showed that nearly 40% of oak trees had longhorn galleries and 10% had larval activity (Naveiro et al., Reference Naveiro, Pulido, Del Pozo, Morcuende, González and Muñoz1999). Tunnelling activity of larvae into cambium and xylem can alter sap flow, trigger wilting, die-back, leaf fall, vigour loss and tree decay. Larvae bore increasingly wider and longer galleries into sapwood and heartwood along the tree trunk and main branches causing huge physiological, mechanical and structural damage, and even tree death (Torres-Vila et al., Reference Torres-Vila, Sánchez-González, Ponce-Escudero, Martín-Vertedor and Ferrero-García2012a, Sallé et al., Reference Sallé, Nageleisen and Lieutier2014). In addition, larvae favour the spread of oak pathogens such as the charcoal disease by creating entryways to the inner tissues (Martín et al., Reference Martín, Cabezas, Buyolo and Patón2005). Therefore, C. welensii is considered a major inciting factor involved in oak decline in the Iberian Peninsula (López-Pantoja et al., Reference López-Pantoja, Domínguez Nevado and Sánchez-Osorio2008; Carrasco, Reference Carrasco2009; Torres-Vila et al., Reference Torres-Vila, Sánchez-González, Ponce-Escudero, Martín-Vertedor and Ferrero-García2012a; Morales-Rodríguez et al., Reference Morales-Rodríguez, Sánchez-González, Conejo-Rodríguez and Torres-Vila2015), and acts similarly to that reported for other wood-boring insects (Führer, Reference Führer, McManus and Liebhold1998; Thomas et al., Reference Thomas, Blank and Hartmann2002; Evans et al., Reference Evans, Moraal, Pajares, Lieutier, Day, Battisti, Grégoire and Evans2004; Sallé et al., Reference Sallé, Nageleisen and Lieutier2014).
The increase in oak decay in Spain is often attributed to the mismanagement and overuse of dehesa woodlands in recent decades. Abusive pruning, improper decorking, poor forestry practices (lack of reforestation and protection of seedlings/saplings), livestock overgrazing and trampling, reduced use of soil-conditioning crops, low natural regeneration and ultimately progressive ageing are major factors predisposing to oak decay (Carrasco, Reference Carrasco2009; Torres-Vila et al., Reference Torres-Vila, Sánchez-González, Ponce-Escudero, Martín-Vertedor and Ferrero-García2012a). Among them, inappropriate pruning and decorking practices, providing suitable egg-laying sites and promoting early larval survival, have likely contributed to a population increase of C. welensii making it an oak pest. Additionally, oak decay due to wood-borers and other insects is likely to be intensified under the current climate change scenario. There is increasing evidence that the impact of secondary oak pests may increase with warming and drought, especially in Southern Europe (Allen et al., Reference Allen, Macalady, Chenchouni, Bachelet, McDowell, Vennetier, Kitzberger, Rigling, Breshears, Hogg, Gonzalez, Fensham, Zhang, Castro, Demidova, Lim, Allard, Running, Semerci and Cobb2010; Sallé et al., Reference Sallé, Nageleisen and Lieutier2014) and C. welensii could be an example of this. In any case, C. welensii is currently considered an emerging pest and a serious threat to dehesa woodlands, and there is a need to control it, at least in the short term (Carrasco, Reference Carrasco2009; Torres-Vila et al., Reference Torres-Vila, Sánchez-González, Ponce-Escudero, Martín-Vertedor and Ferrero-García2012a, Reference Torres-Vila, Sánchez-González, Merino-Martínez, Ponce-Escudero, Conejo-Rodríguez, Martín-Vertedor and Ferrero-García2013).
Adults of C. welensii are large and striking beetles whose occurrence in the wild does not go unnoticed to amateur and professional entomologists. Hence, C. welensii records are frequent in national and local faunas and its geographic distribution is well known (Bense, Reference Bense1995; Vives, Reference Vives2000; González-Peña et al., Reference González-Peña, Vives Noguera and de Sousa Zuzarte2007) even if occasional misidentifications or misuses by confusion with Cerambyx cerdo L. have occurred (Del Moral et al., Reference Del Moral, Gallego, Nuñez and Chica1989; Sallé et al., Reference Sallé, Nageleisen and Lieutier2014). The large size, robustness and taxonomic value of the chitinous structures of Cerambyx have even enabled the study of adult remains in aspects such as the fossil record (Harding & Plant, Reference Harding and Plant1978) and the raptor diets (Fattorini et al., Reference Fattorini, Manganaro, Piattella and Salvati1999).
The knowledge of the distribution of C. welensii contrasts sharply with the poor understanding of its reproduction and mating system. These aspects of its biology have received little attention in this longhorn as in most cerambycids (Hanks, Reference Hanks1999). Several factors could have limited field and laboratory research, including adult crepuscular habits, absence of long-distance sex pheromones, long adult diapausing stage, extended life cycle and particularly the difficulty of laboratory rearing (Hanks, Reference Hanks1999). Moreover, due to the seemingly reduced impact of C. welensii in the past, this species has been generally regarded as a secondary pest (or not a pest at all), so that the scientific literature dealing with its biology is very scarce (Sallé et al., Reference Sallé, Nageleisen and Lieutier2014). As a result of the new status quo of C. welensii, recent research has shed some light on important aspects of its ecology, including flight behaviour, dispersal potential, adult lifespan, population density, semiochemical attraction, mass trapping and natural enemies (López-Pantoja et al., Reference López-Pantoja, Domínguez Nevado and Sánchez-Osorio2008; Torres-Vila et al., Reference Torres-Vila, Sánchez-González, Ponce-Escudero, Martín-Vertedor and Ferrero-García2012a, Reference Torres-Vila, Sánchez-González, Merino-Martínez, Ponce-Escudero, Conejo-Rodríguez, Martín-Vertedor and Ferrero-García2013; Sánchez-Osorio et al., Reference Sánchez-Osorio, López-Pantoja, Paramio, Lencina, Gallego and Domínguez2015; Morales-Rodríguez et al., Reference Morales-Rodríguez, Sánchez-González, Conejo-Rodríguez and Torres-Vila2015).
The prevalence and impact of C. welensii in dehesa open woodlands is largely dependent on its reproductive output and fitness, aspects that remain to be investigated The knowledge of C. welensii fecundity is a critical goal, not only to understanding population dynamics and potential damage to host trees, but also to improve pest control strategies. In fact, means of managing bark and borer insects associated with oaks are almost non-existent (Evans et al., Reference Evans, Moraal, Pajares, Lieutier, Day, Battisti, Grégoire and Evans2004). Predicting the pattern of abundance of bark and boring beetles would be highly desirable to prevent oak decline in Europe (Führer, Reference Führer, McManus and Liebhold1998). Consequently, this study deals with assessing potential reproductive output, daily reproductive patterns, number of matings and related biological traits in both sexes of C. welensii under optimal laboratory conditions.
Materials and methods
Study species
C. welensii is univoltine flying from late May to early August. Adults are large (25–60 mm long) with a blackish-brown body and show sexual dimorphism. Females are slightly larger than males, but antennae are longer in males (twice as long as the body) than in females (just extend to the elytral apex). Adults feed mainly on sap and tree exudates while larvae are xylophagous. Daily activity of adults (feeding, flight, male fights for mates, mating and egg-laying) takes place mainly at dusk and early evening. Mated females wander over the host tree probing the bark with the ovipositor and lay eggs into suitable bark crevices and pruning wounds. After hatching, neonate larvae bore directly into the inner bark and initiate feeding. Larval development usually lasts 2–3 years. Pupation occurs in late summer within a pupal cell in the sapwood. Adults emerge from pupae in the autumn and overwinter protected within the pupal cell in a prereproductive status until the following year (late spring to early summer) (Vives, Reference Vives2000; Torres-Vila et al., Reference Torres-Vila, Sánchez-González, Ponce-Escudero, Martín-Vertedor and Ferrero-García2012a).
Insect origin and adult preparation for tests
Insects used in tests were collected in dehesa open woodlands at about 50 locations across Extremadura (southwestern Spain) during three consecutive years (2012–2014). Collections were made taking advantage of a parallel study dealing with the specific distribution and assemblage of large saproxylic cerambycids in Quercus species (mostly holm oak, Q. ilex L. and cork oak, Q. suber L.). Virgin adults were obtained in three ways: (1) collecting overwintering adults during October–April by cutting host trees with a chainsaw (mainly recently fallen branches) and carefully opening the logs using metal wedges; (2) rearing the mature larvae collected to adulthood on an agar-based artificial diet (Morales-Rodríguez et al., Reference Morales-Rodríguez, Sánchez-González, Conejo-Rodríguez and Torres-Vila2015); and (3) rearing larvae to adulthood from eggs obtained from field-collected ovipositing females on the same diet in the laboratory, although this method was not very profitable as artificial rearing is difficult in this species. The proportion of adults obtained by each of the three methods was about 80, 18 and 2%, respectively. Larvae were individually reared at room temperature (22–28°C and 50–70 relative humidity (r.h.)) in aerated 140 ml plastic containers. Pupae were held in rolls of laboratory blotting paper from Albet® (15–20 mm in diameter, 21 cm long), which were sealed on both ends with staples and arranged horizontally in plastic trays. Paper roll diameter was adjusted simulating a pupal cell, so that pupae were neither too loose nor too tight. In this way, the adults properly extended the wings and elytra. After emergence (September–October) diapausing adults were kept in the dark in a refrigerator (6–10°C) to overwinter.
In early June all adults were slowly warmed to 25°C to avoid thermal shock and allowed to complete sexual maturation before starting experiments (about 1–2 weeks later). Adults were considered to be sexually mature when they expelled the meconium and began feeding (see below). That day was assumed to be the day of emergence from the host tree and was scored as ‘day one’ to determine adult longevity. Adults were then sexed, measured (body length) and individually marked with identifying numbers by sex. A fine layer of waterproof white correction fluid (Tipp-ex®, BIC, France) was applied on each elytron (a rectangle of about 6 × 12 mm2) and after drying the reference number was written twice with a black permanent fine marker (Lumocolor®, Staedtler, Germany). In some cases, numbers were written directly on the elytra using a fine white permanent-ink marker (Paint Marker®, Pentel, Japan). In males, a small white spot was also painted on the pronotum to readily distinguish both sexes under the reduced lighting of the artificial dusk used in the experiments (see below).
Laboratory tests: general procedures
Laboratory tests were conducted in the summer coinciding with the presence of active adults in the field. We used 16l cardboard cages with a transparent cover as mating and oviposition chambers. A single cage was assigned to each female throughout her life to avoid handling errors. Males shared cages with females during tests and were kept in well-aerated 240 ml clear plastic containers during inactivity periods. Caged males and females were regularly sprayed with water and fed ad libitum on a saturated sugar–water paste simulating host tree exudates. Wood disks were prepared as an egg-laying substrate for females. Freshly cut cork oak branches 70–80 mm in diameter were sliced with a circular saw to produce disks 20 mm thick that were frozen until use. Cork layer was detached in one piece from cambium with a penknife and was put back and held in place with a rubber band. Decorking of disks greatly facilitated daily inspections and egg removal (see below). A wood disk bearing the female reference number was used per cage (being replaced by a new as necessary).
All tests were performed in a controlled environmental room at 25 ± 1°C, 60 ± 10% r.h. and a L(14 + 2):D8 photoperiod, simulating typical summer conditions in the study area (July). The first 14 photophase hours were at a 1000 lux luminosity and the last 2 h at 25 lux simulating dusk. The sexual activity of adults was continuously observed at dusk, and observations were continued during the dark phase when necessary using a small red light LED lantern to avoid disturbing adults. Under the conditions described, adults fed, mated and oviposited normally.
Female tests
An array of variables were recorded to characterize the reproductive output of C. welensii females (n = 45), including fecundity (total eggs), fertility (per cent of eggs that hatched), preoviposition period (the elapsed time between mating and first oviposition), oviposition period (the time between first and last oviposition), postoviposition period (the time between last oviposition and female death), longevity, daily fecundity and egg size. Some variables were just studied in a random subset of females (see below).
The effect of number of matings (polyandry) on fecundity was assessed by allowing virgin females to mate singly or multiply, from one to more than 20 times. Females were scored in seven classes according to their lifetime mating number (1, 2, 3, 4–5, 6–10, 11–20 and 20< matings/female). Two marked males were caged with each 3–4-day-old virgin female, 15–30 min before the onset of artificial dusk. If mating occurred, the unmated male was removed quickly to avoid male fights leaving the pair-bonded adults in the cage. The mated male was removed the next morning when the mating was completed. If mating did not occur within the dusk period, the two males were removed leaving the female isolated in her cage to prevent unobserved matings overnight. New males were routinely added/removed every 2–5 days following the same protocol until the predetermined number of matings was achieved for each female. Matings (both pair-bonding and male intromissions) were always verified at dusk (fig. 1A, B).

Fig. 1. (A) A conflict between two C. welensii males for a female on the cork oak disk used as egg-laying substrate, (B) mating at dusk of C. welensii during male intromission and (C) a recently laid egg of C. welensii with the micropylar region visible on the top.
Wood disks were daily inspected after removing the cork layer and eggs counted to assess daily fecundity (fig. 1C). The small room between the cambium and cork layer showed to be extremely attractive for ovipositing females as most eggs were found in this location (>95%). Eggs were carefully detached, stored by date in well-aerated 30 ml plastic vials and incubated at 25°C in the environmental chamber. Per cent fertility was assessed after hatching. Eggs damaged when they were removed from wood disks were excluded from fertility estimates. Some unhatched eggs in which a dead larvae was clearly visible were considered fertile (<5%). Mated but unfertilized females (100% unhatched eggs) were excluded from the data analysis. Incubation time (the elapsed time between egg laying an eclosion) was also determined in a random sample of 523 eggs obtained from 33 females over the first 3 weeks of the oviposition period.
Mean egg size was estimated for each tested female over 3–4 weeks from five eggs randomly chosen per oviposition day, or from all eggs if fewer eggs were available in a given day (total sample n = 1600 eggs). Eggs were measured using a Nikon DS-U1 digital camera connected to a Leica S6D stereomicroscope. Resulting images were analysed with Eclipse Net 1.20 software to determine egg dimensions: length l, width w and thickness t. Egg volume (V, mm3) was calculated as an ellipsoid according to the formula V = π/6 (l · w · t). The correlations between egg size (estimated as either length or volume) and neonate size (head width), and between head width and mandible size (length from condyle to apex) was determined in a sample of 50 eggs from 29 females. We used as estimator of mandible size the average length of both mandibles as the left one tended to be longer (see results).
Male tests
The reproductive output of C. welensii males (n = 78) was assessed in terms of number of matings (polygyny) and longevity. Both variables were obtained from the set of males that were allowed to mate with the tested females. In addition, the effect of number of matings on male longevity was also investigated following an experimental approach. We compared two randomly chosen male groups (10–12 males per group) that were allowed to mate at either a high (one mating every 2–3 days) or low (one mating per week) mating rate. Each male was caged with two females 15–30 min before dusk, the unmated female was removed after pair-bonding and the mated female was removed the next morning when mating was completed. If mating did not occur at dusk, the two females were removed to prevent unobserved matings overnight. New females were routinely added/removed according to the required mating rate and this protocol was repeated throughout the male's lifetime.
Data analysis
All analysed variables were tested for a departure from normality prior to statistical test computation using probability plots. Only per cent fertility was arcsine transformed. Linear regression analysis was used to test the correlation between some reproductive variables, both in females (lifetime fecundity, daily fecundity, fertility, longevity, oviposition period, number of matings, egg size, neonate size and female length) and males (longevity, number of matings and male length). Nested and Model I analysis of variance (ANOVAs) (either one- or multiple-way) were computed for comparison of means and to explore the interaction between some of the studied traits. A nested ANOVA was computed to assess the effect of egg size (nested to female as random factor) on egg hatching. We exploited a subset of 22 females in which a sufficient sample of hatched (chorions) and unhatched eggs was available. We measured five eggs per egg-laying day or all eggs if fewer eggs were available as usual (n = 1117 eggs). The effects of female and oviposition week on incubation time were explored through a two-way ANOVA, in which both oviposition week (two classes: eggs laid either on the first week or on the second-to-third week) and female itself were considered fixed factors. Analysis of covariance (ANCOVA) was used to examine the effect of female mating number (fixed factor) on fecundity using female size as covariate (Sokal & Rohlf, Reference Sokal and Rohlf1995). ANCOVA assumptions were verified prior to analysis (see results). All analyses were performed with Systat (2000) software.
Results
Females
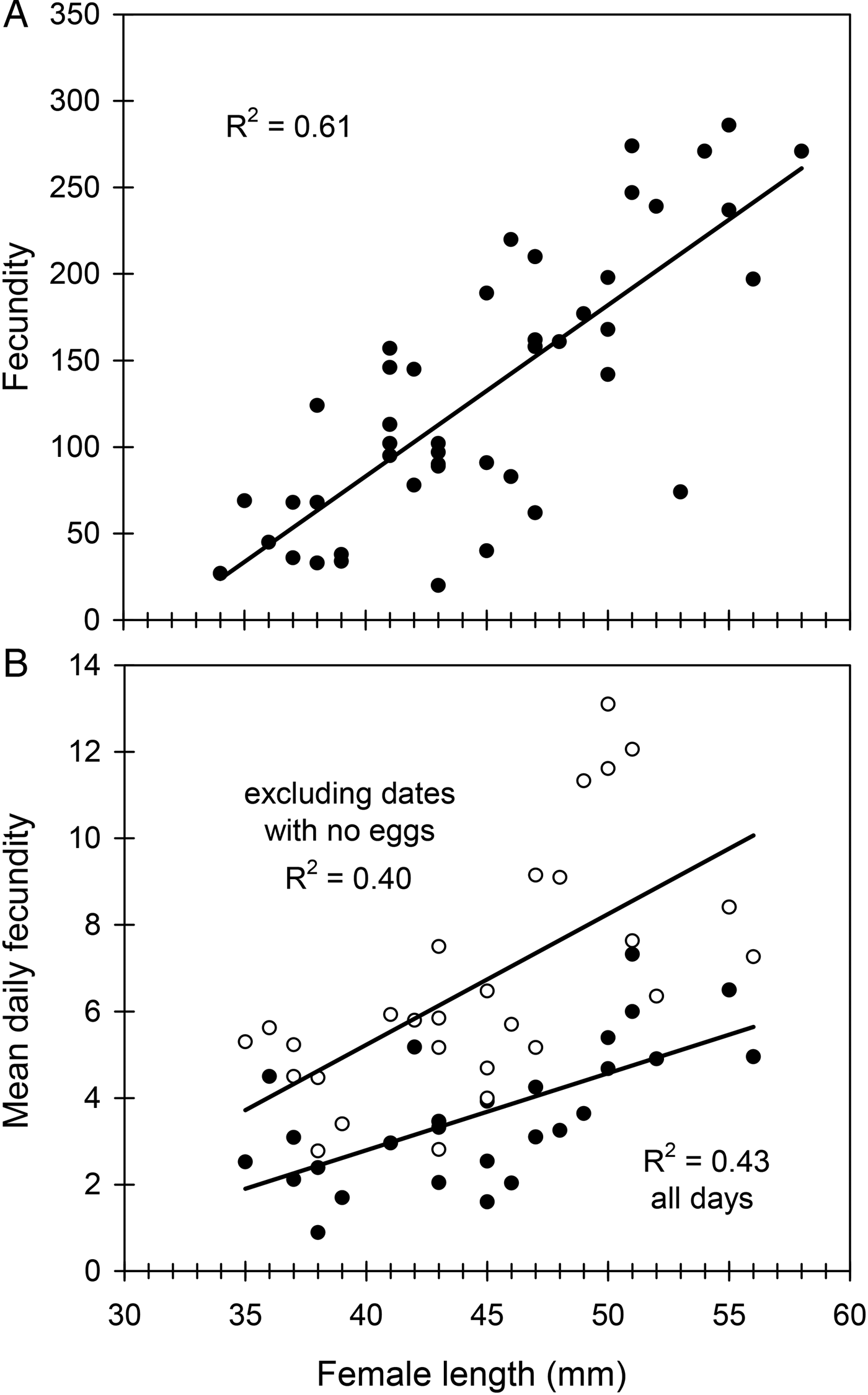
Female length (mean ± SE) was 44.9 ± 0.9 mm (range 34–58) in the studied sample (n = 45). Female fecundity was 132 ± 12 eggs (range 20–286) and per cent fertility was 70 ± 1 % (range 60–78). Female longevity was 70 ± 3 days (range 22–117). The preoviposition period was 2 ± 0.2 days (range 1–7), the oviposition period 44 ± 3 days (range 10–84) and the postoviposition period averaged 19 ± 3 days (range 1–71). Fecundity was positively correlated with female length (fig. 2A) and with female longevity (R 2 = 0.13, F 1,43 = 6.47, P = 0.015). However, the linear relationship was stronger between fecundity and oviposition period (R 2 = 0.34, F 1,43 = 22.12, P < 0.001). Larger females did not live longer (R 2 < 0.001, F 1,43 = 0.0008, P = 0.98) but showed longer oviposition periods (R 2 = 0.12, F 1,43 = 5.82, P < 0.05). Per cent fertility was not related to either fecundity (R 2 = 0.05, F 1,43 = 2.14, P = 0.15) or female length (R 2 = 0.01, F 1,43 = 0.33, P = 0.57).

Fig. 2. The effect of female size (female length) on lifetime fecundity (A) and mean daily fecundity (B) in C. welensii. In the lower graph two regression lines are plotted, either including all egg-laying days (full circles) or excluding those days in which no eggs were laid (open circles). Regression equations and statistics were: (A) y = 9.88x − 312.22, F 1,43 = 66.67, P < 0.001; (B) y = 0.30x − 6.85, F 1,26 = 17.24, P < 0.001 (excluding dates with no eggs), and y = 0.18x − 4.33, F 1,26 = 19.81, P < 0.001 (all days).
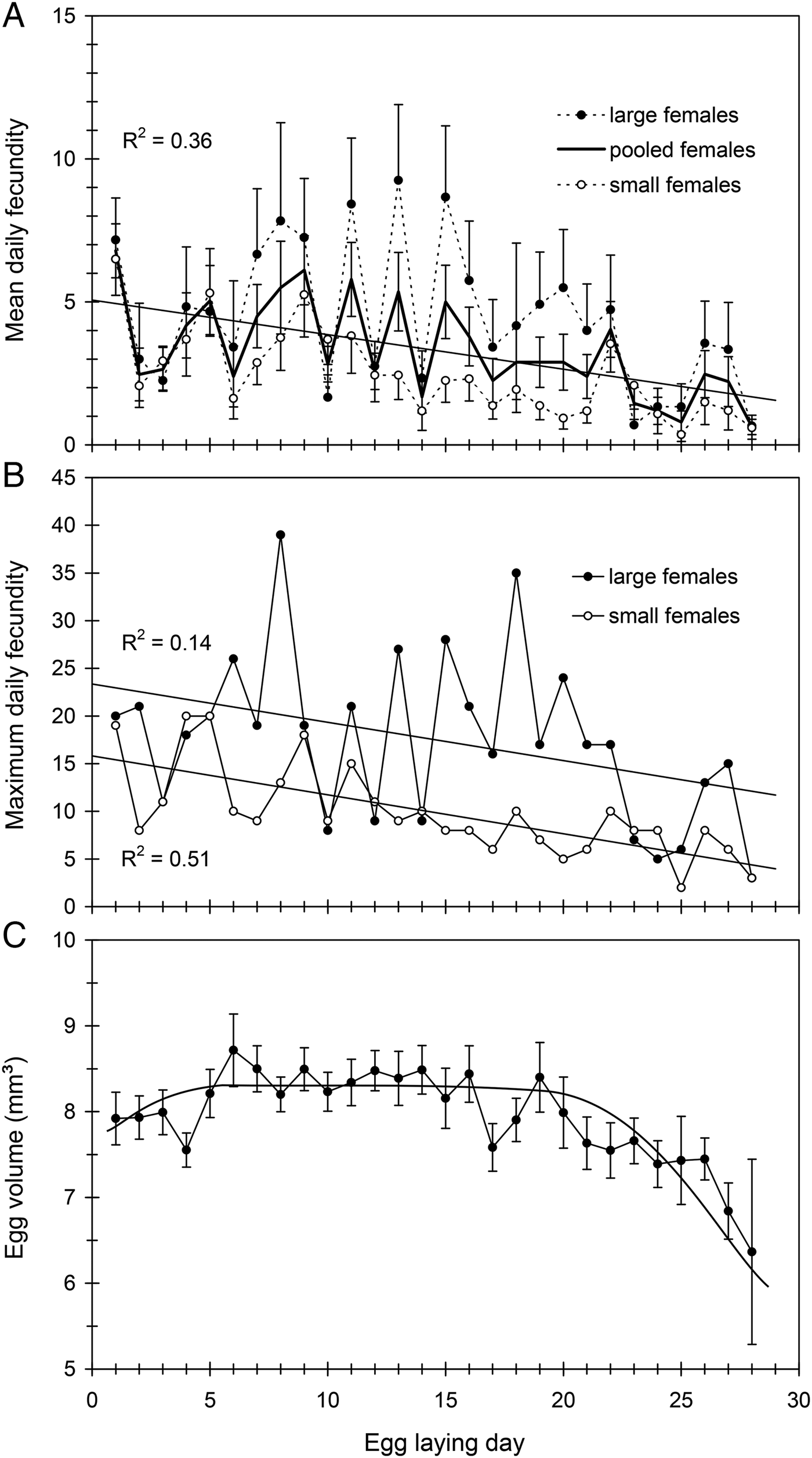
Mean daily fecundity (±SE) averaged 3.0 ± 0.2 eggs/day and ranged from about 0.8–5.8 eggs/day, so that there was considerable fluctuation even between consecutive days (fig. 3A). There was a slight, but significant, decrease in daily oviposition over the female's oviposition period (fig. 3A). Maximum daily fecundity ranged widely depending on female size and egg laying day, with some large females reaching values of up to 30–40 eggs/day (fig. 3B). Mean daily fecundity of each female positively correlated with her body size, results being similar when days with no eggs laid were excluded (fig. 2B). Consequently, large females had higher reproductive output than small females over the whole oviposition period (fig. 3A, B).

Fig. 3. (A) Mean daily fecundity (by female size and pooled), (B) maximum daily fecundity (by female size) and (C) egg size (egg volume) variation over the oviposition period in C. welensii. In the upper graphs, females were scored in two body size classes (large and small, above and below mean female length: 44.9 mm, see text). Vertical lines represent the SE of the mean.
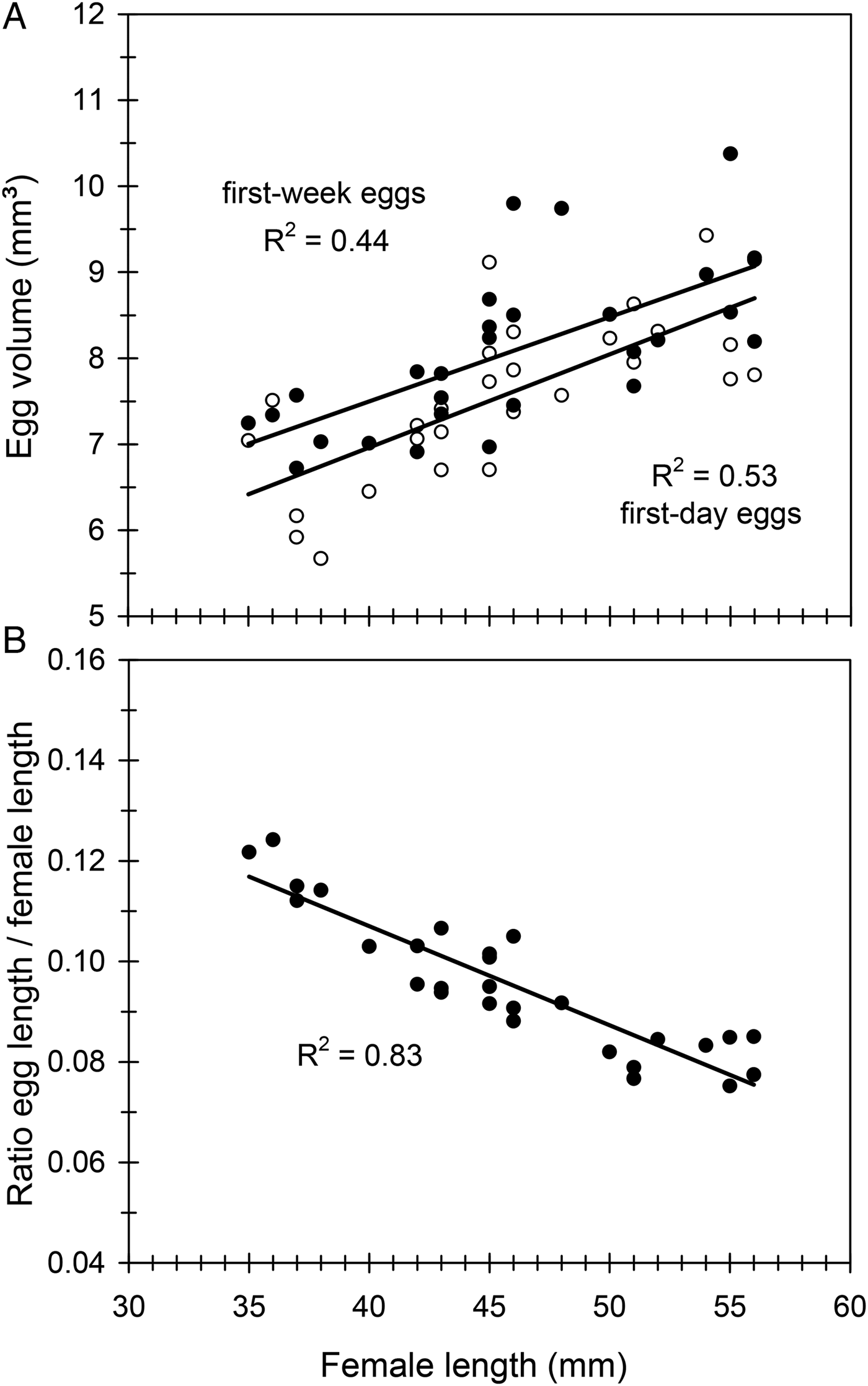
Mean (±SE) egg dimensions, length, width and thickness were l = 4.24 ± 0.01 mm (range 2.7–5.6), w = 2.25 ± 0.01 mm (range 1.5–3.1) and t = 1.64 ± 0.01 mm (range 0.8–2.3) (n = 1600 eggs). Therefore, egg volume averaged 8.14 ± 0.04 mm3 (range 3.8–16.1). Egg size increased very slightly during the first egg-laying week, remained relatively constant for much of the oviposition period and showed a substantial drop in the fourth week (fig. 3C). Egg size was positively correlated with female length when the first-day eggs were considered, and a similar relationship was obtained when considering the first-week eggs (fig. 4A). However, the relative size of eggs (the ratio egg length/female length) was negatively correlated with female length (fig. 4B), so that smaller females produced proportionally larger eggs. A nested ANOVA showed differences in egg size among females (F 21,22 = 12.93, P < 0.001) but the size of hatched and unhatched eggs was not significantly different (F 22,1073 = 1.11, P = 0.33) showing that hatchability did not depend on egg size.

Fig. 4. The effect of female size (female length) on egg size (egg volume) (A) and the effect of female length on the ratio egg length/female length (B) in C. welensii. In the upper graph two regression lines are plotted, either considering the first-day eggs (open circles) or the first-week eggs (full circles) (see text). Regression equations and statistics were: (A) y = 0.10x + 3.56, F 1,26 = 20.43, P < 0.001 (first-week eggs), and y = 0.11x + 2.62, F 1,26 = 29.29, P < 0.001 (first-day eggs); (B) y = –0.002x + 0.186, F 1,26 = 124.02, P < 0.001.
Incubation time was 13.9 ± 0.1 days (range 7–22, n = 523 eggs) and was unrelated to mother size (F 1,31 = 0.15, P = 0.70, n = 33 females). Eggs laid by a female within a single day did not hatch synchronously, variation of up to one week was observed. A two-way ANOVA showed that incubation time was significantly affected by individual female (F 32,457 = 5.01, P < 0.001), oviposition week (F 1,457 = 9.05, P < 0.01) and their interaction (F 32,457 = 3.27, P < 0.001). The interaction arose because differences among females in incubation time depended on the oviposition week (i.e. female age) which was evidenced by successive one-way ANOVAs for each female: in 24 females (73%) incubation time did not change with age, but in eight females (24%) incubation time decreased and even in one female (3%) increased. Neonate size (head width) was positively correlated with egg length (R 2 = 0.17, F 1,48 = 9.89, P < 0.01) but unexpectedly was unrelated to egg volume (R 2 = 0.01, F 1,48 = 0.41, P = 0.53). Lastly, the left mandible tended to be longer than the right one (F 1,49 = 5.26, P < 0.05, single larvae computed as blocks in the ANOVA) but mean mandible size was correlated with head width (R 2 = 0.17, F 1,48 = 9.99, P < 0.01).
C. welensii females were highly polyandrous and some of them mated more than 20 times during their lifetime. ANCOVA results showed that the number of matings did not significantly affect fecundity (F 6,37 = 1.35, P = 0.26) when controlling for female size (F 1,37 = 53.15, P < 0.001). ANCOVA assumptions were verified prior to analysis: there was linear independence between mating number and female length (F 6,38 = 0.82, P = 0.56), linear dependence between fecundity and female length (F 1,43 = 66.66, P < 0.001) and homogeneity of regression slopes (F 6,31 = 0.61, P = 0.72). Linear regression also showed that the number of matings did not affect fertility (R 2 = 0.06, F 1,43 = 2.71, P = 0.11) nor longevity (R 2 = 0.002, F 1,43 = 0.08, P = 0.78).
Males
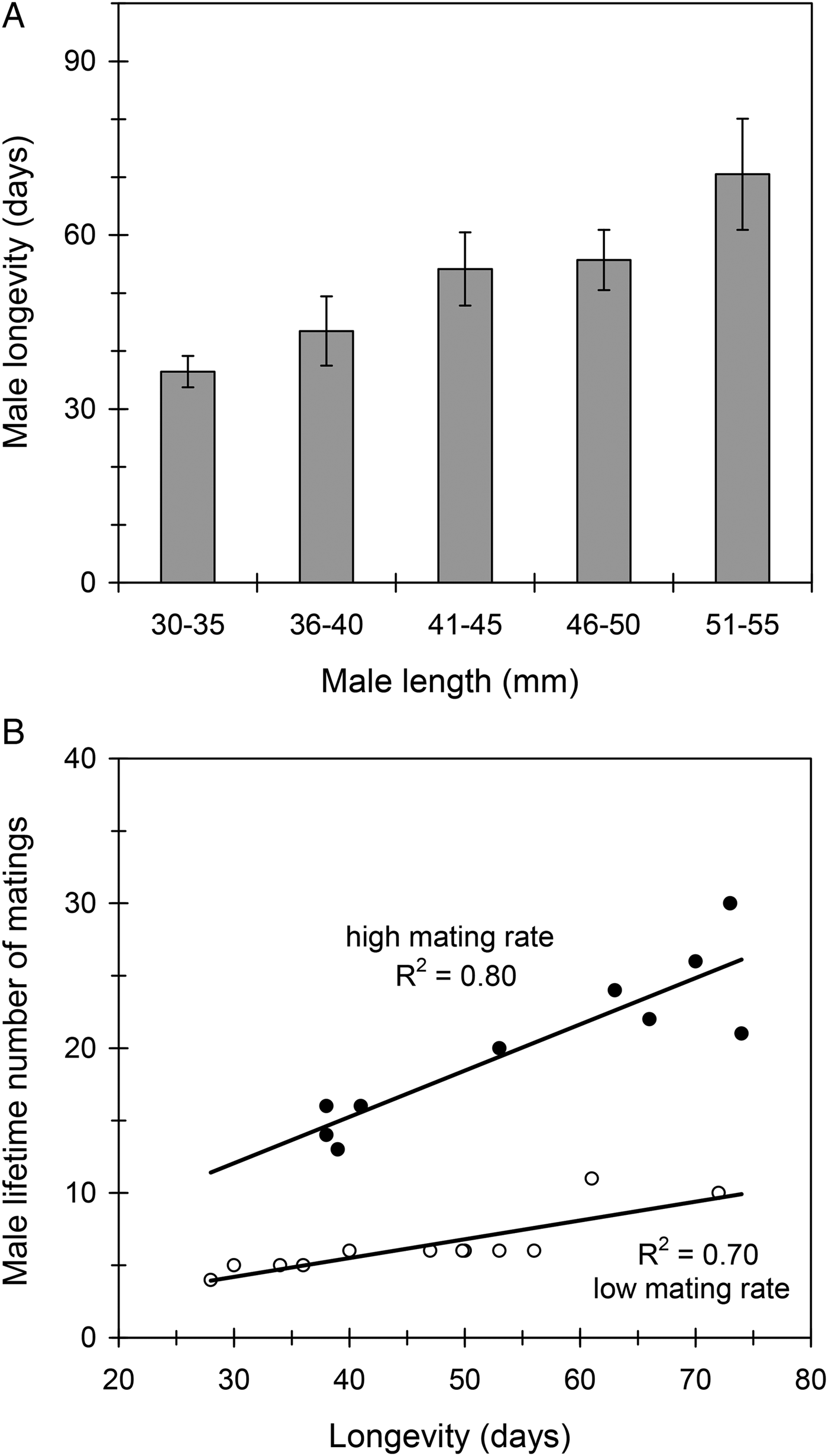
Male length (mean ± SE) was 43.7 ± 0.6 mm (range 31–54) in the studied sample (n = 78) and longevity was 52 ± 3 days (range 11–144). Thus, females were larger and lived longer than males (F 1,121 = 14.13, P < 0.001). Unlike what was observed with females, male longevity was positively correlated with body size, so that large males lived longer than small males (R 2 = 0.13, F 1,76 = 11.58, P < 0.01; fig. 5A). Results showed that C. welensii males were extremely polygynous, some of them being able to mate up to 30 times during their lifetime. Number of matings had no detrimental effect on male longevity (R 2 = 0.01, F 1,76 = 0.65, P = 0.42). Rather to the contrary, long-lived males mated more times throughout their lifetime because they had more mating opportunities (fig. 5B). Consistently, the longevity of males that mated once a week did not differ significantly from those that mated once every 2–3 days (F 1,20 = 2.21, P = 0.15). It follows that male mating history did not affect longevity, even if lifetime sexual activity was threefold higher in males that mated multiple times a week (20.2 ± 1.7 matings/male) than in those that just mated once a week (6.4 ± 0.6 matings/male) (fig. 5B).

Fig. 5. The effect of male size (male length) on longevity (A) and the effect of male longevity on lifetime number of matings (B) in C. welensii. Vertical lines represent the SE of the mean. The lower graph includes data from two experimental groups of males that were allowed to mate at either one mating every 2–3 days (full circles) or one mating per week (open circles). Regression equations and statistics were: (B) y = 0.32x + 2.45, F 1,8 = 30.98, P < 0.001 (high mating rate), and y = 0.13x + 0.32, F 1,10 = 23.05, P < 0.001 (low mating rate).
Discussion
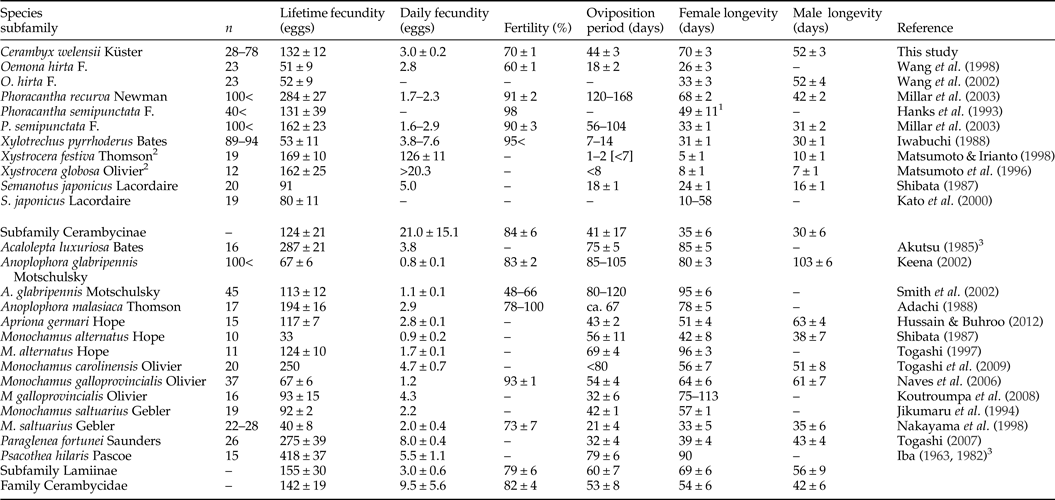
Mean fecundity of C. welensii was quite close to the average of the subfamily Cerambycinae (table 1), but individual values covered a quite broad range between 20 and 286 eggs/female, depending largely on female size. A positive correlation between fecundity and female size is widespread in insects (Honěk, Reference Honěk1993) irrespective of nutritional background (Torres-Vila et al., Reference Torres-Vila, Rodríguez-Molina, Roehrich and Stockel1999) and specifically in longhorns beetles, both Cerambycinae (Iwabuchi, Reference Iwabuchi1988; Matsumoto & Irianto, Reference Matsumoto and Irianto1998; Wang et al., Reference Wang, Shi and Davis1998, Reference Wang, Shi, Song, Rogers, Davis and Chen2002; Kato et al., Reference Kato, Yamada and Shibata2000) and Lamiinae (Lawrence, Reference Lawrence1990; Keena, Reference Keena2002; Togashi, Reference Togashi1997, Reference Togashi2007; Togashi et al., Reference Togashi, Appleby, Oloumi-Sadeghi and Malek2009). Fitness benefits for females of being large are likely to be especially important in these and other wood-boring species. A wide variation in adult size frequently occurs within these species because larvae (usually legless) cannot move between hosts and have restricted mobility within host, so that they are incapable of improving their nutritional status if host quality is poor. When adverse conditions arise, the larvae of these species produce smaller adults rather than fail to complete their development (Andersen & Nilssen, Reference Andersen and Nilssen1983). In this scenario, females able to choose the best egg-laying sites would have a significant fitness advantage.
Table 1. Reproductive and biological traits of Cerambycidae species belonging to the Cerambycinae and Lamiinae subfamilies.

Values are the mean, mean ± SE or variation range. Averaged values are given in those studies in which several experimental treatments were tested (e.g. larval feeding, adult feeding, female mating number and temperature). In those studies in which the numerical data were not provided, relevant values were recovered by measuring the figures. Note that the reproductive and biological traits compiled in this table are merely illustrative, as experimental conditions were variable among species/studies.
1 Sexes pooled.
2 X. festiva and X. globosa have very short-lived adults and females often laid eggs in a single cluster.
3 In Togashi (Reference Togashi2007).
Adults of C. welensii were long-lived (around 2 months), longevity records being in most cases higher than in other Cerambycinae and more similar to Lamiinae species (table 1). However, C. welensii longevity showed to be extremely variable in both sexes, from 2 to 3 weeks to almost 5 months. Mean longevity recorded in this study was much higher than the 2 weeks reported in a previous work conducted in the same experimental conditions, but in which adults remained grouped throughout lifetime (Torres-Vila et al., Reference Torres-Vila, Sánchez-González, Ponce-Escudero, Martín-Vertedor and Ferrero-García2012a). This suggests that C. welensii longevity was reduced by the stress derived from the continuous interaction between adults, competition between females for egg-laying sites, male fights to gain mates and intense male harassment of females. Longevity records in the present study were also much higher than the 2–3 weeks of lifespan estimated under field conditions with mark–recapture methods (Torres-Vila et al., Reference Torres-Vila, Sánchez-González, Merino-Martínez, Ponce-Escudero, Conejo-Rodríguez, Martín-Vertedor and Ferrero-García2013), which was attributed to a better adult feeding in the laboratory and the predation risk in the field. Female longevity was positively correlated with fecundity as in other longhorn species (Iwabuchi, Reference Iwabuchi1988; Wang et al., Reference Wang, Shi and Davis1998; Togashi et al., Reference Togashi, Appleby, Oloumi-Sadeghi and Malek2009) although there are also exceptions (Jikumaru et al., Reference Jikumaru, Togashi, Taketsune and Takahashi1994; Keena, Reference Keena2002).
The oviposition period was highly variable among females but the mean value (one month and a half) was similar to the average of the subfamily Cerambycinae (table 1). The postoviposition period was very irregular and unexpectedly long in some individuals, suggesting a non-adaptive situation derived from an imbalance between the reproductive and somatic reserves. Many females did not oviposit for several weeks at the end of their life (up to 10 weeks) but their fecundity was not significantly reduced. Superior adult nutrition in our experimental conditions could partly explain these unexpected results. The irregularity among females in the postoviposition period determined both (1) the lack of correlation between longevity and female size, and (2) the better correlation of fecundity with oviposition period than with longevity, as also reported in Monochamus galloprovincialis Olivier (Koutroumpa et al., Reference Koutroumpa, Vincent, Roux-Morabito, Martín and Lieutier2008).
Daily fecundity in C. welensii showed a typical synovigenic pattern, characterized by females that produce, mature and lay eggs throughout an extended oviposition period. Synovigeny is characteristic of species that rely on feeding in adult stage (the so-called income breeders) to gather vital energetic resources for both reproduction and somatic maintenance (Stearns, Reference Stearns1977). There was a slight decreasing trend over time in daily fecundity (both mean and maximum values) with oviposition rate being always higher in large than small females. Thus, daily fecundity was positively correlated with female size, but negatively correlated with female age, as observed in other cerambycids (Lawrence, Reference Lawrence1990; Keena, Reference Keena2002; Smith et al., Reference Smith, Bancroft and Tropp2002). Mean daily fecundity (3 eggs/day) was similar to the values reported in most longhorn beetles when Xystrocera species were not taken into account given their quite dissimilar reproductive biology (table 1). Large fluctuations in oviposition rate occurred because many females did not laid eggs every day, and moreover, after a day without oviposit, egg-laying was often accentuated the next day. A high fluctuation in oviposition rate is often reported in cerambycids (Shibata, Reference Shibata1987; Jikumaru et al., Reference Jikumaru, Togashi, Taketsune and Takahashi1994; Keena, Reference Keena2002; Koutroumpa et al., Reference Koutroumpa, Vincent, Roux-Morabito, Martín and Lieutier2008) and attributed to intraspecific differences in female size, oogenesis rate and even ovariole number (Togashi et al., Reference Togashi, Appleby, Oloumi-Sadeghi and Malek2009). In the case of C. welensii, also keep in mind that mating duration is extremely long (8 h in average), so that females are constrained by males on mating days, even if pair-bonded females are able to feed and oviposit between intromissions (Luis M. Torres-Vila, personal observation).
Eggs of C. welensii were large (length × width: 4.2 × 2.3 mm2), values being similar to those reported in previous studies: 4.5 × 2.3 mm2 (Hernández, Reference Hernández1991) and 4.7 × 2.3 mm2 (Vitali, Reference Vitali2001). A large size of C. welensii eggs was explained by the allometric scaling between female size and egg size, the so-called ‘Bauplan’ relationship (Wiklund & Karlsson, Reference Wiklund and Karlsson1984). However, in relative terms (in relation to female size) the eggs of C. welensii – as those other Cerambycinae – were proportionally smaller than in Lepturinae and Lamiinae species (Hernández, Reference Hernández1991), which evidences a phylogenetic background regulating the trait. In any case, larger C. welensii females produced larger eggs as in most insects (Fox & Czesak, Reference Fox and Czesak2000) and particularly in cerambycids (Togashi et al., Reference Togashi, Akita, Nakane, Shibata and Nakai1997; Kato et al., Reference Kato, Yamada and Shibata2000). Egg size remained large and relatively constant throughout most of the oviposition period and just decreased on the fourth week, probably by a depletion of energetic resources not compensated for by adult feeding (Torres-Vila & Rodríguez-Molina, Reference Torres-Vila and Rodríguez-Molina2002).
There was a positive correlation between neonate size (head width) and egg size (length) as reported in others cerambycids (Kato et al., Reference Kato, Yamada and Shibata2000; Walczyńska, Reference Walczyńska2008). However, head width was unrelated to egg volume, signifying that yolk surplus in large eggs was incorporated to larval body rather than to increase head size. The adaptive significance of egg size in insects is controversial as there is not a generalizable relationship between neonate size and larval performance. In some species, however, large neonates have an adaptive advantage over small ones especially in adverse environments (Torres-Vila & Rodríguez-Molina, Reference Torres-Vila and Rodríguez-Molina2002; Torres-Vila et al., Reference Torres-Vila, Cruces Caldera, Rodríguez-Molina and Cauterruccio2012b). The adaptive advantage of larger progeny size could be especially important in C. welensii and other xylophagous cerambycids that develop on hardwood trees. A better performance of large neonates could result from the larger head/mandible size (Murphy et al., Reference Murphy, Launer and Ehrlich1983; Nakasuji, Reference Nakasuji1987) conferring advantage in the host perforation during the first tunnelling stages. Larger neonates could also be more resistant when facing the numerous physical and biochemical countermeasures that a host tree displays in response to borer injury, either to directly kill or compartmentalise the intruder. Tree resistance mechanisms include lignification of cell walls, formation of impervious tissue and necrophylactic periderm, callus formation in the cambial zone, cytosolic changes with accumulation of secondary chemicals (phenolic and isoprenoid compounds), high tissue moisture content, bark turgor pressure and increased tree sap flow (Hanks, Reference Hanks1999; Kato et al., Reference Kato, Yamada and Shibata2000; see Sallé et al., Reference Sallé, Nageleisen and Lieutier2014 for a review). There is little evidence available in cerambycids to support this idea. In Monochamus alternatus Hope the larvae kill each other under the bark at high densities and the winners are larger than the losers, suggesting that the initial size of progeny affects early survival (Togashi et al., Reference Togashi, Akita, Nakane, Shibata and Nakai1997). In Semanotus japonicus Lacordaire larval survivorship to adulthood is greater in large than small progeny (Kato et al., Reference Kato, Yamada and Shibata2000). The adaptive advantage of larger neonates could also explain why small females laid larger eggs relative to their body size compared with larger females in M. alternatus (Togashi et al., Reference Togashi, Akita, Nakane, Shibata and Nakai1997) and C. welensii (this study). The effect of neonate size on larval performance and early survival merits further research in C. welensii given its potential adaptive significance.
There were huge differences in incubation time (from 7 to 22 days) among females under a constant temperature (25°C) suggesting heritable variation in the trait. Variation in incubation time was unrelated to female size but could be linked to the geographical origin of individuals tested. We observed that some C. welensii larvae, perfectly formed inside the chorion, delayed some days their emergence contributing to lengthen incubation time, but this variable was not measured. Similar variation in incubation time has been reported in the longhorn Psacothea hilaris Pascoe, in which there is also a negative trade-off between incubation time and postembryonic development time (Yumino & Togashi, Reference Yumino and Togashi2015). What is more, in cerambycids there is evidence that incubation time is heritable, as this trait depends on phylogeny and varies among subfamilies (Lamiinae < Cerambycinae < Prioninae) (Hanks, Reference Hanks1999). Interestingly, in C. welensii there were also large differences in the incubation time within the egg compliment of a single female. This could derive from a risk-spreading (bet-hedging) strategy, because a female may increase her fitness if a part of her progeny manages to avoid unfavourable and unpredictable environmental conditions (Hopper, Reference Hopper1999; Yumino & Togashi, Reference Yumino and Togashi2015).
Both females and males of C. welensii were extremely promiscuous, accomplishing up to 20 and 30 lifetime matings, respectively. Note that our experiments were not designed to define the maximum mating potential, so they could underestimate what occurs in the wild. Male fights for mates were a commonly observed behaviour. Male coercion was not a rare event including male harassment of females and punishing resisting females, especially when males were allowed to mate with previously multiple mated females in which sexual receptivity was lower. Pair-bonded males forcefully guarded their mates, while unreceptive females walked quickly and climbed the box walls, trying to get rid of the males, hiding their genitalia under the elytra or even extruding the ovipositor and laying eggs, trying in every way possible to prevent male intromission attempts. Male coercion was early described (e.g. Rothschild, Reference Rothschild1978) and male cerambycids often harass and mount females to induce mating, although it has been questioned whether such behaviour implies forced copulation (Eberhard, Reference Eberhard2002).
Male mating history did not affect longevity. Rather to the contrary, long-lived males mated more times throughout their lifetime because they had more mating chances. In females in turn, multiple mating did not affect longevity, fecundity or fertility. Our results suggest that the adaptive significance of extreme polygyny and polyandry in C. welensii would be to promote sperm competition and cryptic female choice rather than the acquisition of energy as nuptial gifts to increase fecundity (Arnqvist & Nilsson, Reference Arnqvist and Nilsson2000; Torres-Vila et al., Reference Torres-Vila, Rodríguez-Molina and Jennions2004; Torres-Vila, Reference Torres-Vila2013). However, this notion requires further experimental support since the good adult nutrition in our tests could have biased the effects. The energetic resources gathered via nuptial gifts could become significant when adult feeding is limited or non-existent, a common situation in summer in Mediterranean dehesa woodlands. The effect of multiple mating on fecundity in cerambycids is unclear because few and contradictory studies are available. For instance, in Tetraopes tetraophthalmus Forster (Lawrence, Reference Lawrence1990) and Oemona hirta F. (Wang et al., Reference Wang, Shi and Davis1998) there is a positive relationship between polyandry and fecundity, but it is lacking in Xylotrechus pyrrhoderus Bates (Iwabuchi, Reference Iwabuchi1988), Phoracantha semipunctata F. (Bybee et al., Reference Bybee, Millar, Paine and Hanlon2005) and C. welensii (this study).
Species-specific values of fecundity, daily fecundity, oviposition period and longevity in cerambycids are quite variable both between and within subfamilies (table 1). Data showed the effect of the phylogenetic background, supporting that Lamiinae tend to live longer than Cerambycinae (Hanks, Reference Hanks1999). Cerambycids are a very diverse group, so that an array of biological, behavioural and environmental variables may account for differences in reproductive traits. These include the main energetic source – income or capital breeders –, mate location pattern, adult dispersal/sedentary behaviour, female gnawing or not into bark for oviposition, single (iteroparity) versus clustered (semelparity) egg laying, feeding versus non-feeding adults or the occurrence/absence of adult maturation feeding (Linsley, Reference Linsley1959; Hanks, Reference Hanks1999; Allison et al., Reference Allison, Borden and Seybold2004). Environmental conditions are also extremely important, including weather conditions: temperature, drought (Bybee et al., Reference Bybee, Millar, Paine, Campbell and Hanlon2004; Keena, Reference Keena2006), host-plant quality: tree species, age, phenology, physiological and health status (Hanks, Reference Hanks1999; Keena, Reference Keena2002; Smith et al., Reference Smith, Bancroft and Tropp2002; Wang et al., Reference Wang, Shi, Song, Rogers, Davis and Chen2002), feeding resources for adults (Hanks et al., Reference Hanks, McElfresh, Millar and Paine1993; Millar et al., Reference Millar, Paine, Joyce and Hanks2003) and even sublethal entomophatogenic infections (Hajek et al., Reference Hajek, Lund and Smith2008). Differences between studies on the same target species (seven cases in table 1) support both the effects of genetic background and environmental conditions. It follows that detailed comparisons of reproductive variables between species (or populations) should be made with caution.
Conclusions
Our study provides insight on the reproductive output of C. welensii. Larger males lived longer and mated more times than small males while larger females had greater overall reproductive fitness than did smaller females in terms of lifetime fecundity, daily fecundity, oviposition period and offspring size. These biological data will be useful to improve pest management methods and to establish new action guidelines to prevent or mitigate the increase of C. welensii populations in dehesa open woodlands and other oak forests.
Acknowledgements
We are grateful to F. Ponce Escudero, F. Fernández Moreno, E. Cruces Caldera, D. Martín-Vertedor, J. Merino-Martínez, F. Barrena Galán, J.J. Ferrero-García, J.A. Rebollo Nieto and L. López Pajares for their technical assistance in both laboratory and field, to L. Jiménez for improving the English, to Dr K. Togashi for his kindness in sending bibliography, and to two anonymous reviewers for their valuable comments. This research was supported by the Servicio de Sanidad Vegetal (Gobierno de Extremadura).