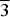I. INTRODUCTION
Recently, we reported a crystal structure study of a new silver palladium selenide with metallic behaviour, AgPd3Se (Laufek et al., Reference Laufek, Vymazalová, Chareev, Kristavchuk, Lin and Drahokoupil2011). The crystal structure of this compound is formed by two kinds of interpenetrating networks of Ag, Pd, and Se atoms. Another known silver palladium selenide is the Ag2Pd3Se4 phase, which is known from natural occurrence as a mineral chrisstanleyite (Paar et al., Reference Paar, Roberts, Criddle and Topa1998). Its crystal structure is composed of two distinct frameworks of polyhedra (elongated AgSe4 tetrahedra and PdSe4 squares) that interpenetrate and support each other (Topa et al., Reference Topa, Makovicky and Balič-Žunič2006). During the further research of phase relations in the Ag–Pd–Se system, another ternary metal-rich selenide–the (Ag,Pd)22Se6 phase–was described. According to Vymazalová et al. (Reference Vymazalová, Chareev, Kristavchuk, Laufek and Drábek2012), this phase crystallizes in the Fm ![]() $\overline3$m space group and forms a solid solution showing an extensive substitution of silver for palladium, whereas the selenium content is almost constant.
$\overline3$m space group and forms a solid solution showing an extensive substitution of silver for palladium, whereas the selenium content is almost constant.
Since no crystal structure data of the (Ag,Pd)22Se6 phase are hitherto known, we carried out a crystal structure study of this compound. Powder diffraction data up to 140°2θ (CoKα) are also reported. Part of this work was presented in a conference abstract (Laufek et al., Reference Laufek, Vymazalová, Drábek and Drahokoupil2012).
II. EXPERIMENTAL
The ternary (Ag,Pd)22Se6 phase was prepared, using solid-state techniques, in silica glass tubes. Pure elements (silver 99.999%, selenium 99.999%, and palladium 99.95%) were used as starting materials. The evacuated tube with carefully weighted charge was at first annealed at 650°C for 2 days. The charge was grinded in acetone and again heated at 500°C for 35 days, after which the sample was quenched in a cold-water bath.
Chemical analyses were carried out on a Cameca SX-100 microprobe, in the wave-length-dispersion mode using a focused beam (size 1–2 μm). Accelerating voltage was set to 15 keV and the beam current to 10 nA. The sample was analysed using Pd L α, Ag L β, and Se L α lines; pure metals were used as standards. The data were collected on several different crystals and then averaged. The chemical analysis yielded an overall composition of (Ag10.61(7)Pd11.72(4))Σ22.33Se6.00 (based on 6 Se per formula unit).
III. STRUCTURE REFINEMENT
The powder X-ray diffraction pattern used for the structure determination was collected in Bragg-Brentano geometry on an X'Pert Pro PANalytical diffractometer, equipped with an X'Celerator detector using CoKα radiation. To minimize background, the specimen was placed on a flat low-background silicon wafer. The data were collected in the range from 10 to 140°2θ. A full-width at half maximum of 0.074°2θ was obtained at 44.339°2θ showing good crystallinity of the studied sample.
The crystal structure of (Ag,Pd)22Se6 was refined by the Rietveld method for powder X-ray diffraction data using the FullProf program (Rodriguez-Carvajal, Reference Rodriguez-Carvajal1990). A pseudo-Voigt function was used to generate the line shape of the diffraction peaks; the background was determined by linear interpolation between consecutive breakpoints in the powder pattern. A preliminary Rietveld refinement using the crystal structure of Pd (Fm ![]() $\overline3$m, a = 4.1056 Å, Pd atom at 4a position) (Maeland and Flanagan, Reference Maeland and Flanagan1964) fitted the most intensive reflections (R p = 7.86%, R wp = 14.3%) in the powder diffraction pattern; however, many other relative weak reflections were not fitted by this simple cubic model. Indexing of the powder pattern by means of the program DICVOL06 (Louër and Boultif, Reference Louër and Boultif2007) revealed that all reflections can be fitted by a cubic supercell with a′ = 3a (a = 12.31 Å). Therefore, the 3a.3a.3a superstructure of Pd (fcc structure) was created using the Wycksplit program from Bilbao Crystallographic Server (Kroumova et al., Reference Kroumova, Perey-Mato and Aroyo1998). This superstructure with Fm
$\overline3$m, a = 4.1056 Å, Pd atom at 4a position) (Maeland and Flanagan, Reference Maeland and Flanagan1964) fitted the most intensive reflections (R p = 7.86%, R wp = 14.3%) in the powder diffraction pattern; however, many other relative weak reflections were not fitted by this simple cubic model. Indexing of the powder pattern by means of the program DICVOL06 (Louër and Boultif, Reference Louër and Boultif2007) revealed that all reflections can be fitted by a cubic supercell with a′ = 3a (a = 12.31 Å). Therefore, the 3a.3a.3a superstructure of Pd (fcc structure) was created using the Wycksplit program from Bilbao Crystallographic Server (Kroumova et al., Reference Kroumova, Perey-Mato and Aroyo1998). This superstructure with Fm ![]() $\overline3$m symmetry is a k-subgroup at index 27 of the Fm
$\overline3$m symmetry is a k-subgroup at index 27 of the Fm ![]() $\overline3$m space group; the 4a crystallographic position in the Pd structure is split into the 4a, 24e, 32f, and 48h positions. Since it is nearly impossible to distinguish between Ag and Pd atoms in the conventional powder diffraction experiments, all metallic positions in the superstructure were treated as Ag/Pd mix positions with 0.5/0.5 occupancy. In order to comply with the measured stoichiometry of (Ag10.61(7)Pd11.72(4))Σ22.33Se6.00, the 24e position was assigned as Se; the 4a, 32f, and 48h positions as Ag/Pd mix positions. The stoichiometry of this structure model is (Ag,Pd)21Se6. The subsequent Rietveld refinement converged to R p = 6.12%, R wp = 10.8%. Nevertheless the difference Fourier map revealed one large positive peak (32.2 e A−3) at coordinates ½, 0, 0 (i.e. at the 4b position) suggesting that some atoms are missing in the current structure model. From the comparison of stoichiometry of the current structure model (Ag,Pd)21Se6 and the measured chemical composition of (Ag10.61(7)Pd11.72(4))Σ22.33Se6.00, it was evident that this 4b position corresponds to the next Ag/Pd mix site. Thus, this position was assigned as next Ag/Pd mixed position in subsequent refinements, during which the values of R p and R wp significantly decreased to 3.85 and 5.06%, respectively.
$\overline3$m space group; the 4a crystallographic position in the Pd structure is split into the 4a, 24e, 32f, and 48h positions. Since it is nearly impossible to distinguish between Ag and Pd atoms in the conventional powder diffraction experiments, all metallic positions in the superstructure were treated as Ag/Pd mix positions with 0.5/0.5 occupancy. In order to comply with the measured stoichiometry of (Ag10.61(7)Pd11.72(4))Σ22.33Se6.00, the 24e position was assigned as Se; the 4a, 32f, and 48h positions as Ag/Pd mix positions. The stoichiometry of this structure model is (Ag,Pd)21Se6. The subsequent Rietveld refinement converged to R p = 6.12%, R wp = 10.8%. Nevertheless the difference Fourier map revealed one large positive peak (32.2 e A−3) at coordinates ½, 0, 0 (i.e. at the 4b position) suggesting that some atoms are missing in the current structure model. From the comparison of stoichiometry of the current structure model (Ag,Pd)21Se6 and the measured chemical composition of (Ag10.61(7)Pd11.72(4))Σ22.33Se6.00, it was evident that this 4b position corresponds to the next Ag/Pd mix site. Thus, this position was assigned as next Ag/Pd mixed position in subsequent refinements, during which the values of R p and R wp significantly decreased to 3.85 and 5.06%, respectively.
Finally, 17 parameters including six profile and eight structural variables were refined. The maximum and minimum peaks in the final difference Fourier map were 1.21 and −1.85 e A−3. The structure derived chemical composition (Ag,Pd)22Se6 is in a good agreement with the chemical composition of (Ag10.61(7)Pd11.72(4))Σ22.33Se6.00 measured by electron microprobe. The details of the final refinement are summarized in Table I, the refined atomic coordinates and isotropic displacement parameters are given in Table II. Figure 1 shows the final Rietveld plot.

Figure 1. Observed (circles), calculate (solid line) and difference Rietveld profiles for (Ag,Pd)22Se6. The upper reflection bars correspond to (Ag,Pd)22Se6 and the lower bars to 3 mass percent (Ag,Pd) alloy impurity.
Table I. Experimental conditions.

Table II. Refined parameters for (Ag,Pd)22Se6 [room temperature, space group Fm ![]() $\overline3$m, a = 12.3169(2) Å, V = 1862.55(5) Å3, Z = 4, D c = 10.01 g/cm3, R p = 3.85%, R wp = 5.06%, and R B = 3.51%].
$\overline3$m, a = 12.3169(2) Å, V = 1862.55(5) Å3, Z = 4, D c = 10.01 g/cm3, R p = 3.85%, R wp = 5.06%, and R B = 3.51%].

The M1–M4 sites represent the Ag/Pd mixed positions with 0.5/0.5 occupancy.
IV. RESULTS AND DISCUSSION
The powder diffraction data are listed in Table III. The observed values of diffraction positions, d-spacing, and intensities were extracted by the XFIT program (Coelho and Cheary, Reference Coelho and Cheary1997) employing the split Pearson VII profile function. 2θ obs and d obs were corrected for the refined zero-point shift of 0.027°2θ.
Table III. Powder diffraction data for (Ag,Pd)22Se6 (CoK α radiation). Reflection with I obs and I calc < 0.5% are not shown in the table.

The refined structure has four cation sites, all of which were refined as mixed Ag/Pd sites, and one Se position. All atomic sites are in different types of special positions, with 0 and 1 degree of freedom for positional parameters. The cation sites were labelled from M1 to M4 because of their Ag/Pd mixed character; all occupancy parameters for Ag and Pd were set to 0.5/0.5.
The crystal structure of (Ag,Pd)22Se6 can be described as a stuffed 3a.3a.3a superstructure of cubic close packing of atoms, where only four from 108 octahedral holes are occupied. The corresponding group–subgroup relation is ![]() $Fm \overline{3} m \displaystyle{k27 \over 3a} Fm \overline{3} m$ and is shown in Figure 2.
$Fm \overline{3} m \displaystyle{k27 \over 3a} Fm \overline{3} m$ and is shown in Figure 2.

Figure 2. Group–subgroup relation between (Ag,Pd)22Se6 and cubic close packing (ccp).
The unit cell of (Ag,Pd)22Se6 as well as the selected coordination polyhedra of all atoms are presented in Figure 3. The M1 site is coordinated by 12 M4 atoms in a cuboctahedral arrangement. These cuboctahedra are regular with the M(4)–M(4) edge distance of 2.922(2) Å. The M2 atoms are in the centre of regular octahedra with M2–Se distances of 2.664(1) Å. These [M(2)Se6] octahedra are isolated from each other and alternate with [M(1)M(4)12] cuboctahedra in the NaCl-type fashion. The M3 and M4 atoms also show cuboctahedral coordination, however, considerably distorted. The Se atoms are inside monocapped tetragonal antiprism built by the M3, M4 and M4 positions. As is also shown in Figure 4(a), the M3 atoms also form regular cubes, which are centred by the M2 atoms. However, there are no short M4–M2 interactions (3.587 Å). The Se atoms are placed slightly above the centres of the faces of these [M(3)8] cubes (Figure. 4a). It is interesting to note that the edge-length of the [M(3)8] cubes (4.142 Å) is very close to the thickness of the [M(1)M(4)12] cuboctahedra (4.134 Å). The selected bond lengths are summarized in Table IV.

Figure 3. Polyhedral representation of (Ag,Pd)22Se6 crystal structure emphasizing the [M(1)M(4)12] cuboctahedra and [M(2)Se6] octahedra (M = Ag/Pd mix sites). Unit-cell edges are highlighted.

Figure 4. Comparison of (Ag,Pd)22Se6 and Cr23C6 (Bowman et al., Reference Bowman, Arnold, Storms and Nereson1972) crystal structures. The [M(3)8] cubes (orange) and [M(1)M(4)12] cuboctahedra (green) are emphasized (M = Ag/Pd or Cr). Note the difference (4a vs. 8c) in the occupied Wyckoff positions in both structures. Unit-cell edges are highlighted.
Table IV. Selected interatomic distances (Å) for (Ag,Pd)22Se6. The M1–M4 sites represent the Ag/Pd mixed positions with 0.5/0.5 occupancy.

The crystal structure of the (Ag,Pd)22Se6 represents a unique structure; no exact structural analogues were found in the ICSD database (Fachinformationszentrum Karlsruhe, 2012). However, it shows close structural relation to the Cr23C6 type structure (Bowman et al., Reference Bowman, Arnold, Storms and Nereson1972). Both phases exhibit the same symmetry (Fm ![]() $\overline3$m) and very similar stoichiometry (Pearson symbols are cF112 and cF116, respectively). In both compounds, the electronegative atoms (C or Se) occupy the position 24e, while the 4a, 32f, and 48h positions are occupied by electropositive atoms (Cr or Ag and Pd). The characteristic distinction between these two structures is different occupancy of the 4b and 8c positions. As is evident from Figure 4, the 4b position is occupied in the (Ag,Pd)22Se6 structure (M2 atoms in centres of the [M(3)8] cubes), whereas the 8c position is vacant. Contrary to that, the Cr23C6 structure has exactly opposite occupation scheme of these two positions; the vacant 4b position (note the empty cubes in Figure 4b) and occupied the 8c position. Remarkable is also the difference in the ratio between the edge-length of the cubes and thickness of the cuboctahedra (see Figure 4). This ratio is 0.70 for the empty cube in Cr23C6, while the ratio for the [M(3)8] cube filled by the M2 atoms is 1.00. As discussed in detail by Adelsberger and Jansen (Reference Adelsberger and Jansen1999), the Cr23C6 structure type also shows the group–subgroup relation to the cubic close packing of atoms.
$\overline3$m) and very similar stoichiometry (Pearson symbols are cF112 and cF116, respectively). In both compounds, the electronegative atoms (C or Se) occupy the position 24e, while the 4a, 32f, and 48h positions are occupied by electropositive atoms (Cr or Ag and Pd). The characteristic distinction between these two structures is different occupancy of the 4b and 8c positions. As is evident from Figure 4, the 4b position is occupied in the (Ag,Pd)22Se6 structure (M2 atoms in centres of the [M(3)8] cubes), whereas the 8c position is vacant. Contrary to that, the Cr23C6 structure has exactly opposite occupation scheme of these two positions; the vacant 4b position (note the empty cubes in Figure 4b) and occupied the 8c position. Remarkable is also the difference in the ratio between the edge-length of the cubes and thickness of the cuboctahedra (see Figure 4). This ratio is 0.70 for the empty cube in Cr23C6, while the ratio for the [M(3)8] cube filled by the M2 atoms is 1.00. As discussed in detail by Adelsberger and Jansen (Reference Adelsberger and Jansen1999), the Cr23C6 structure type also shows the group–subgroup relation to the cubic close packing of atoms.
It is also worth mentioning that the Se atoms in the (Ag,Pd)22Se6 structure form an octahedral network (Figure 5a). This network consists of large and small corner-sharing octahedra, which alternate in the NaCl-type fashion. The M1 (4a) and M2 (4b) atoms are located at the centres of large and small octahedra, respectively. As shown in Figure 5b, analogical arrangement of C atoms was described for Cr23C6-type structures by Ohodnicky et al. (Reference Ohodnicky, Cates, Laughlin, McHenry and Widom2008). However, while the large octahedra have the Cr atoms in their centres, the small octahedra are vacant.

Figure 5. Polyhedral representation of the (a) (Ag,Pd)22Se6 and (b) Cr23C6 crystal structures (Bowman et al., Reference Bowman, Arnold, Storms and Nereson1972). The corner-sharing octahedral networks of Se and C atoms are shown. (M = Ag/Pd).
ACKNOWLEDGEMENTS
This work was funded through the project LA 11125/KONTAKT II from the Ministry of Education, Youth and Sports of the Czech Republic and the grant from the President of the Russian Federation for State Support of Young Russian Scientists (MK-1557.2011.5).