Background
Aortic coarctation occurs in 6–8% of patients with CHD. Several surgical techniques have been used to repair aortic coarctation in the neonatal period. The most frequently used technique for isolated aortic coarctation is an extended end-to-end anastomosis, with the incision extended under the distal part of the arch. When there is severe aortic arch hypoplasia associated, a radical extended anastomosis is usually performed, with the incision reaching the middle portion of the arch, including the origins of both left carotid and subclavian arteries.Reference Jonas1
Aneurysms or pseudoaneurysms are a possible complication following repair.Reference Jonas1 While a true aneurysm is bound by all three layers of the vessel wall – intima, media, and adventitia, a pseudoaneurysm occurs when there is a breach in the vessel wall and blood leaks through the wall but is contained by the adventitia or surrounding perivascular soft tissue. Following coarctation repair, these typically occur at the ascending aorta or at the site of repair. In neonates, this is a rare complication, especially in the early period following repair.Reference Fiore, Fischer and Schwartz2 Aneurysms/pseudoaneurysms may develop due to wall abnormalities, increased wall stress at the site of repair, aortic dissection, or the development of a mycotic aneurysm.Reference Serfontein and Kron3, Reference Bogaert, Gewillig and Rademakers4 This is a particular concern since when left untreated the risk of rupture is high.Reference Kenny and Hijazi5
Clinical case
A male newborn was admitted to hospital at 10 days of life with prostration and poor feeding over the previous 24 hours. The neonate was pale, poorly perfused, tachypnoeic, and with absent femoral pulses. He weighed 3330 g, heart rate was 156 bpm, and blood pressure was 98/66 mmHg on the right arm. It was not possible to measure blood pressure in the inferior limbs. A holosystolic heart murmur grade III/VI was present as well as bilateral basal pulmonary crackles and mild hepatomegaly. Transthoracic echocardiogram documented a hypoplastic aortic arch with isthmic aortic coarctation and a large apical muscular ventricular septal defect. Two-dimensional echocardiogram measurements were aortic valve 5.7 mm (z-score −1.68), sinotubular junction 6.98 mm (z-score −0.49), transverse aortic arch 2.89 mm (z-score −6.15), and isthmus 1.96 mm (z-score −6.03). Chest X-ray showed bilateral reinforced vascular markings, and blood tests were unremarkable.
The newborn was started on prostaglandin infusion and stabilised. Surgery was performed at 13 days of life with coarctation resection and radically extended end-to-end anastomosis by left thoracotomy. Pulmonary artery banding was also performed. Immediate post-operative course was unremarkable. Transthoracic echocardiogram showed a mildly narrow aortic arch – transverse aortic arch 6 mm (z-score −1.6) with laminar flow and no residual coarctation (peak gradient 18 mmHg and no diastolic extension of flow). Pulmonary artery banding was well positioned with a gradient of 50 mmHg.
At day 5 after surgery, the infant developed sepsis. Enterobacter cloacae was isolated on blood cultures, and this was treated according to antimicrobial susceptibility testing with meropenem for 14 days.
On day 18 after surgery, the infant had failure to thrive and had developed tachypnoea over the previous week. A transthoracic echocardiogram documented a vascular structure in continuity with the transverse aortic arch, with a diameter superior to that of the aortic arch and with a low velocity of flow within Figure 1. A CT scan showed a large transverse aortic arch pseudoaneurysm with 28 mm × 24 mm of largest diameters that wrapped around the transverse aortic arch (Figs 2, 3 and 4). The pseudoaneurysm originated from the inferior aspect of the aorta distal to the origin of the brachiocephalic artery. There is a clearly defined site of arterial wall breach, the structure is large and polycyclic, and has a thin outline – characteristic of a pseudoaneurysm. The left common carotid artery and the left subclavian artery originated at the aneurysmatic area with no obstruction. There were no signs of compression of adjacent structures.
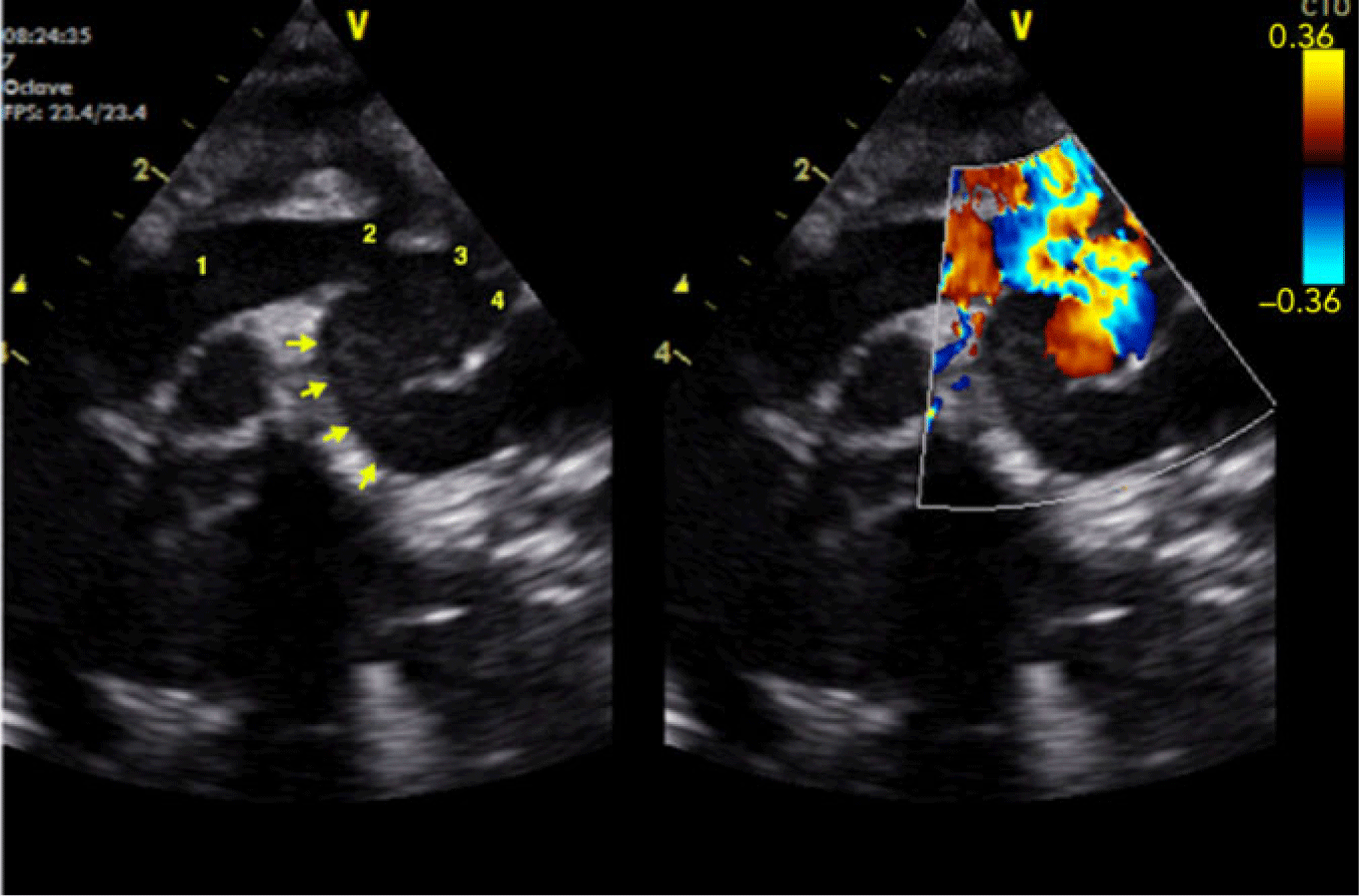
Figure 1. Transthoracic echocardiogram depicting the pseudoaneurysm of the transverse aortic arch. 1: ascending aorta, 2: brachiocephalic artery, 3: left common carotid artery, 4: left subclavian artery. Arrows point at the pseudoaneurysm.

Figure 2. Posterior projection of a 3D rendered image from a CT scan of the thoracic aorta and pseudoaneurysm. 1: ascending aorta, 2: brachiocephalic artery, 3: left common carotid artery, 4: left subclavian artery, 5: pseudoaneurysm, 6: descending aorta.

Figure 3. Left anterior multi-planar reformatted image from a CT scan of the thoracic aorta depicting the site of communication of the aortic arch with the pseudoaneurysm. 1: ascending aorta, 2: brachiocephalic artery, 3: left common carotid artery, 4: pseudoaneurysm, 5: descending aorta, *: pseudoaneurysm neck - site of communication with the aortic arch.

Figure 4. Left anterior multi-planar reformatted image from a CT scan of the thoracic aorta depicting the aortic arch and extent of the pseudoaneurysm. 1: ascending aorta, 2: brachiocephalic artery, 3: left common carotid artery, 4: pseudoaneurysm, 5: descending aorta.
Surgical correction was performed on day 43 of life with cardiopulmonary bypass, deep hypothermia, and a short period of circulatory arrest. The aortic arch was repaired with an autologous pericardial patch excluding the false aneurysm from the circulation and filling of the lumen of the pseudoaneurysm with fibrin glue. Surgery confirmed the origin of the aneurysm at the proximal site of the previous repair. The infant recovered slowly from surgery – he was extubated on day 6 and remained on inotropes for 10 days.
After surgery, there was dilatation of the aorta at the site of patch implantation, with no signs of recoarctation. Transverse arch: 6.7 mm (z-score −0.84), largest diameter at the site of repair: 12 mm (z-score + 4.3). This dilatation remained stable on sequential echocardiographic assessments.
A CT scan 1 year after correction showed no evidence of new aneurysm/pseudoaneurysm formation or other complications. The infant has remained asymptomatic, with normal development and no signs of re-coarctation or other complications at 24-month follow-up.
Discussion
Early correction of a large muscular apical ventricular septal defect is sometimes impossible, and in this case, a two-staged correction was preferred, with an initial correction of aortic coarctation with arch hypoplasia and pulmonary artery banding and second-stage ventricular septal defect closure.
This infant developed a rare complication in the early period following repair. Pseudoaneurysms at the site of anastomosis are usually a late complication, related to the type of surgical repair.Reference Von Kodolitsch, Aydin and Koschyk6, Reference Bromberg, Beekman and Rocchini7 In a study comparing surgery to angioplasty, none of the 34 neonates surgically treated developed a pseudoaneurysm during the 38 months of follow-up.Reference Fiore, Fischer and Schwartz2 As a late complication, the incidence of aneurysms in children following coarctation repair is 3–9%.Reference Von Kodolitsch, Aydin and Koschyk6, Reference Parikh, Hurwitz, Hubbard, Brown, King and Girod8
In this case, the pseudoaneurysm seems to be more likely related to haemodynamic effects with a residual mildly narrow aortic arch favouring wall tension at the site of repair and turbulent flow. A hypoplastic aortic arch increases the technical challenge of the repair and is a known risk factor for the development of aneurysms/pseudoaneurysms.Reference Bogaert, Gewillig and Rademakers4, Reference Quaegebeur, Jonas, Weinberg, Blackstone and Kirklin9 This increases wall tension at the site of repair and creates blood flow acceleration and post-stenotic turbulence that may favour pseudoaneurysm development. The infant developed sepsis in the early period following surgery, so this could possibly be a mycotic aneurysm. However, at the time of repair, the surgeon reported no signs of infection. No tissue was sent for microbial analysis. Although a pseudoaneurym with this size and location could possibly cause compression or deviation of adjacent structures, this was not the case.
Although the surgery was successful and the infant is doing well, this was an unusual bailout procedure and so long-term follow-up of the aortic arch is needed.
We present the case of a rare complication following coarctation repair in the neonatal period: an aortic pseudoaneurysm at the site of repair, with echocardiogram and CT scan documentation. Although this is a serious complication, surgical correction was possible and the infant has remained clinically well.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.






