1. Introduction
Multicuspid teeth are known in various, distantly related groups of both extinct and living fishes and are generally indicative of algivorous/herbivorous diets (e.g. Gibson, Reference Gibson2015; Davis et al. Reference Davis, Unmack, Vari and Betancur2016). Despite a rich fossil record and a high ecomorphological diversity, no true multicuspid teeth have been clearly reported among pycnodont fishes, a widespread group known from the Upper Triassic to the Eocene. The anterior dentition of pycnodonts usually consists of monocuspid or slightly bifid prehensile teeth, very flattened and fully incisiform in derived taxa (Nursall, Reference Nursall, Arratia and Viohl1996; Poyato-Ariza & Wenz, Reference Poyato-Ariza and Wenz2002; Kriwet, Reference Kriwet2005; Poyato-Ariza, Reference Poyato-Ariza2005; Poyato-Ariza & Martín-Abad, Reference Poyato-Ariza and Martín-Abad2013). Nevertheless, Kriwet (Reference Kriwet2005: fig. 42e) figured dentary teeth of Nursallia with slightly incised crowns. In addition, the possible pycnodontiform genera Stephanodus and Hadrodus have large, broad incisiform teeth with, respectively, a denticulated occlusal edge (e.g. Zittel, Reference Zittel1888: fig. 310; Cappetta, Reference Cappetta1972: pl. 13, figs 1–3) and a bicuspid crown (e.g. Leidy, Reference Leidy1873: pl. 19, figs 17–20; Bell, Reference Bell1986: fig. 2.4–6).
The well-known Late Cretaceous (Maastrichtian) and Palaeogene (Danian–Ypresian) phosphate deposits of the Ouled Abdoun Basin (Morocco) have yielded abundant and diverse vertebrate remains, including many marine fish taxa (Arambourg, Reference Arambourg1952; Bardet et al. Reference Bardet, Gheerbrant, Noubhani, Cappetta, Jouve, Bourdon, Pereda Suberbiola, Jalil, Vincent, Houssaye, Solé, Elhoussaini Darif, Adnet, Rage, Lapparent de Broin, Sudre, Bouya, Amaghzaz and Meslouh2017). Among them, pycnodont fishes are well represented, with Phacodus and Eoserrasalmimus in the Maastrichtian, and Pycnodus and Serrasalmimus in the Palaeogene (Arambourg, Reference Arambourg1952; Kriwet, Reference Kriwet2005; Vullo et al. Reference Vullo, Cavin, Khalloufi, Amaghzaz, Bardet, Jalil, Jourani, Khaldoune and Gheerbrant2017). In addition, the enigmatic genera Stephanodus and Hadrodus are two other possible pycnodontiforms present in the Maastrichtian beds of the Ouled Abdoun Basin (Arambourg, Reference Arambourg1952, Reference Arambourg1964). We describe here two large pycnodontid premaxillae, each with one tooth preserved in situ, from the Thanetian of the Ouled Abdoun Basin. Both specimens show a similar incisor-like morphology; however, while the tooth crown of the first specimen (tentatively referred to Pycnodus cf. praecursor) shows a non-incised occlusal margin, the second specimen is characterized by a multicuspid crown and is assigned to a new species (Pycnodus multicuspidatus sp. nov.). These two pycnodont specimens illustrate the evolutionary transition between a typical generalist form and a new, previously undescribed adaptive form. This discovery suggests that the regulatory pathways that govern tooth shape formation and lead to multicuspid teeth in teleostean fishes may also have been present in non-teleost actinopterygians such as pycnodontiforms.
2. Systematic palaeontology
ACTINOPTERYGII Cope, Reference Cope1887
NEOPTERYGII Regan, Reference Regan1923
PYCNODONTIFORMES Berg, Reference Berg1937
PYCNODONTIDAE Agassiz, Reference Agassiz1833 (sensu Nursall, Reference Nursall, Arratia and Viohl1996)
PYCNODONTINAE Agassiz, Reference Agassiz1833 (sensu Poyato-Ariza & Wenz, Reference Poyato-Ariza and Wenz2002)
Pycnodus cf. praecursor Dartevelle & Casier, Reference Dartevelle and Casier1949
(Fig. 1a–d)

Fig. 1. Left premaxillae of Pycnodus cf. praecursor (MHNM.KHG229) (a–d) and P. multicuspidatus sp. nov. (holotype MHNM.KHG230) (e–h) from the Palaeocene of the Ouled Abdoun Basin, Morocco, in labial (a, e), lingual (b, f), mesial (c, g) and ventral (occlusal) (d, h) views. Abbreviations: ap – ascending process; blt – base of the (missing) lateral tooth; cs – cusps; of – olfactory fossa; tc – tooth crown; tn – tooth neck. Scale bar: 10 mm.
Material. MHNM.KHG229, a left premaxilla with one tooth preserved, housed at the Muséum d’Histoire Naturelle de Marrakech (MHNM).
Locality and horizon. Sidi Daoui area, Ouled Abdoun Basin, Province of Khouribga, Morocco. Upper Phosphorite Bed IIa – base of the Intercalary Bed II/I interval, Thanetian (Palaeocene) in age (Kocsis et al. Reference Kocsis, Gheerbrant, Mouflih, Cappetta, Yans and Amaghzaz2014).
Description. MHNM.KHG229 is a nearly complete left premaxilla with one tooth preserved (mesial tooth). A second, lateral tooth, broken at its base, was originally present. The preserved tooth crown is wider than high (see Table 1 for measurements). It is spatulate, typically incisiform in shape, with a markedly convex and asymmetrical occlusal margin in labial view. The occlusal margin is continuous, i.e. not incised. The labial face is convex and shows a dozen slight sub-vertical folds. The lingual face is concave and bears three wear facets. There is a high, well-developed tooth neck. The thick, sub-vertical ascending premaxillary process is broken apically (dorsally). There is no fenestra, but a large mesiolingual olfactory fossa is present; this depression was forming part of the nasal capsule, together with the olfactory fossa of the mesethmoid.
Table 1. Measurements of specimens MHNM.KHG229 and MHNM.KHG230

Remarks. Features of the premaxilla (i.e. two teeth originally present, elongated and almost vertical ascending process, large olfactory fossa for the nasal capsule) combined with the typical incisiform shape of the preserved tooth clearly indicate that MHNM.KHG229 belongs to Pycnodontidae (Poyato-Ariza & Wenz, Reference Poyato-Ariza and Wenz2002; Kriwet, Reference Kriwet2005). The well-developed olfactory fossa is similar in size, shape, and position to the pocket described in the premaxilla of Hadrodus marshi (Gregory, Reference Gregory1950: fig. 1a). By its size, MHNM.KHG229 might correspond to the premaxilla of Pycnodus praecursor, a large to gigantic nominal species from the Palaeocene of Africa (Angola, Niger) known only from isolated dentitions (Dartevelle & Casier, Reference Dartevelle and Casier1949; Cappetta, Reference Cappetta1972). MHNM.KHG229 is therefore tentatively referred to Pycnodus cf. praecursor. A large fragmentary prearticular dentition from Palaeocene of Togo, described as Pycnodus variabilis var. togoensis (Stromer, Reference Stromer1910: fig. 2), might correspond to the same taxon.
It is worth noting that the type species of Pycnodus (i.e. Pycnodus apodus from the early Eocene Bolca Lagerstätte, Italy), known from complete articulated specimens, is the only well-defined species of the genus (Poyato-Ariza & Wenz, Reference Poyato-Ariza and Wenz2002; Poyato-Ariza, Reference Poyato-Ariza2013; Cawley et al. Reference Cawley, Marramà, Carnevale and Kriwet2018). Most of the numerous Palaeogene nominal species based on isolated dentitions and traditionally referred to the genus Pycnodus (e.g. Longbottom, Reference Longbottom1984; for a short review, see Cawley et al. Reference Cawley, Marramà, Carnevale and Kriwet2018) need confirmation of their generic placement (Poyato-Ariza, Reference Poyato-Ariza2013); however, a systematic revision of these tooth-based species is beyond the scope of this paper. Therefore, the two specimens described here are provisionally assigned to Pycnodus.
Pycnodus multicuspidatus sp. nov.
(Fig. 1e–h)
Holotype and only known specimen. MHNM.KHG230, a fragmentary left premaxilla with one tooth preserved, housed at the MHNM.
Locality and horizon. Sidi Daoui area, Ouled Abdoun Basin, Province of Khouribga, Morocco. Upper Phosphorite Bed IIa – base of the Intercalary Bed II/I interval, Thanetian (Palaeocene) in age (Kocsis et al. Reference Kocsis, Gheerbrant, Mouflih, Cappetta, Yans and Amaghzaz2014).
Diagnosis. Large to gigantic species of Pycnodus with incisiform premaxillary teeth characterized by a deeply incised, multicuspid crown (nine triangular cusps in the mesial tooth).
Description. MHNM.KHG230 is a fragmentary left premaxilla with one tooth preserved (mesial tooth). The lateral part of the bone (originally bearing a second, lateral tooth) is broken and worn. The labiolingually compressed, spatulate tooth crown is wider than high (see Table 1 for measurements). The crown is multicuspid, with seven well-defined cusps and a pair of poorly defined lateral cuspules. The cusps are triangular in shape and equally developed, albeit slightly decreasing in size laterally. The cuspidate occlusal margin is markedly convex in labial view. There is a well-developed, lingually inflated tooth neck. The mesiolingual olfactory fossa can be discerned along the thick, poorly preserved ascending premaxillary process. There is no fenestra.
Remarks. With the exception of the well-preserved tooth, MHNM.KHG230 is less complete and more abraded than MHNM.KHG229. Although MHNM.KHG230 clearly differs from MHNM.KHG229 by the multicuspidation of the preserved tooth crown, both specimens are similar in size and gross morphology (see Fig. 1; Table 1). Moreover, MHNM.KHG230 and MHNM.KHG229 share some characters (i.e. asymmetry and proportions of the crown, development of the tooth neck, thickness of the ascending premaxillary process, shape of the olfactory fossa; see Fig. 1), indicating that these two specimens belong to distinct but closely related pycnodontid species.
3. Discussion
3.a. Tooth shape development and evolution
Several studies have argued that only simple genetic changes were required for the rise of multicuspid teeth in the evolution of fishes (Streelman et al. Reference Streelman, Webb, Albertson and Kocher2003; Streelman & Albertson, Reference Streelman and Albertson2006; Jackman et al. Reference Jackman, Davies, Lyons, Stauder, Denton-Schneider, Jowdry, Aigler, Vogel and Stock2013). In their study on living African cichlids, Albertson et al. (Reference Albertson, Streelman and Kocher2003 a) found that interspecific differences in cusp number are determined by approximately one gene, suggesting that this character has the potential to respond to selection extremely quickly. A similar simple genetic basis of evolutionary novelty in the front dentition of pycnodontids can be assumed here, as suggested by the overall resemblance between specimens MHNM.KHG229 and MHNM.KHG230 as well as by the co-occurrence of the ancestral (MHNM.KHG229) and derived (MHNM.KHG230) morphotypes in the Thanetian strata of the Ouled Abdoun Basin. The peculiar tooth morphotype MHNM.KHG230 appears to be directly derived from the plesiomorphic monocuspid tooth morphotype MHNM.KHG229, and this may represent a case of punctuated equilibrium, with speciation between Pycnodus cf. praecursor and Pycnodus multicuspidatus sp. nov. by ‘budding cladogenesis’ (Wagner, Reference Wagner2000).
Whether multicuspid teeth have arisen during fish evolution either by concrescence, or by differentiation of tooth germs, is still debated (Trapani et al. Reference Trapani, Yamamoto and Stock2005; Jernvall & Thesleff, Reference Jernvall and Thesleff2012; Jackman et al. Reference Jackman, Davies, Lyons, Stauder, Denton-Schneider, Jowdry, Aigler, Vogel and Stock2013). However, this mainly concerns teleost taxa with numerous minute, closely spaced teeth (Trapani et al. Reference Trapani, Yamamoto and Stock2005; Jackman et al. Reference Jackman, Davies, Lyons, Stauder, Denton-Schneider, Jowdry, Aigler, Vogel and Stock2013). In the fossil record, the oldest evidence of similar increase in cusp number (from mono- or bicuspid to multicuspid small incisiform teeth) is known between two Late Triassic non-teleostean neopterygians, i.e. the dapediids Sargodon (Tintori, 1983, Reference Tintori1998) and Hemicalypterus (Gibson, Reference Gibson2015, Reference Gibson2016).
In pycnodontiform fishes, the number of premaxillary and dentary teeth never exceeds three and five, respectively (Poyato-Ariza & Wenz, Reference Poyato-Ariza and Wenz2002). In Pycnodus and other derived pycnodontids, these bones bear two monocuspid incisiform teeth (Nursall, Reference Nursall, Arratia and Viohl1996; Poyato-Ariza & Wenz, Reference Poyato-Ariza and Wenz2002), except Polazzodus, Sylvienodus and Tergestinia, which have a premaxilla bearing a single tooth (Capasso, Reference Capasso2000; Poyato-Ariza, Reference Poyato-Ariza2010, Reference Poyato-Ariza2013). Therefore, it is obvious that the nine-cusped tooth of Pycnodus multicuspidatus sp. nov. described here arose from complex folding of a single tooth germ (Differentiation Theory) rather than from early fusion of several tooth germs (Concrescence Theory). This interpretation is clearly supported by the equally sized premaxillary tooth MHNM.KHG229, which shows a similar overall morphology and differs only by its simple, non-incised crown contour (ancestral condition).
During the development of mammalian teeth, the folding of the enamel epithelium leading to the formation of multicuspid crowns is regulated by signalling centres called secondary enamel knots (Jernvall et al. Reference Jernvall, Kettunen, Karavanova, Martin and Thesleff1994; Vaatokari et al. Reference Vaatokari, Åberg, Jernvall, Keränen and Thesleff1996). The presence of enamel knot-like signalling centres controlling the cusp number in teleostean fishes has been suggested by several authors (Streelman et al. Reference Streelman, Webb, Albertson and Kocher2003; Fraser et al. Reference Fraser, Bloomquist and Streelman2008, Reference Fraser, Bloomquist and Streelman2013; Jernvall & Thesleff, Reference Jernvall and Thesleff2012; Atukorala & Franz-Odendaal, Reference Atukorala and Franz-Odendaal2014; Debiais-Thibaud et al. Reference Debiais-Thibaud, Chiori, Enault, Oulion, Germon, Martinand-Mari, Casane and Borday-Birraux2015). Recently, Smith et al. (Reference Smith, Johanson, Butts, Ericsson, Modrell, Tulenko, Davis and Fraser2015) showed that the development genes shh and bmp4 operating in the dentition of teleosts are similarly expressed in the dentition of the basal actinopterygian Polyodon, thus extending this conserved developmental pattern within the Actinopterygii. Therefore, the evolutionary transition observed between the monocuspid pycnodont tooth MHNM.KHG229 and the multicuspid pycnodont tooth MHNM.KHG230 may be explained by minor genetic changes leading to the formation of secondary enamel knot-like structures in the derived form, as for the difference in cusp number observed today between the adult teeth of two closely related species of cichlid fishes, i.e. Metriaclima zebra and Labeotropheus fuelleborni (Albertson et al. Reference Albertson, Streelman and Kocher2003 a,b; Streelman et al. Reference Streelman, Webb, Albertson and Kocher2003). The symmetrical formation of four lateral cusps on each side of the central cusp of MHNM.KHG230 could be compared with that of the tricuspid teeth of Labeotropheus fuelleborni, which is due to the uniform development of secondary enamel knots (and thus lateral cusps) on both the mesial and distal sides of the central cusp (Streelman et al. Reference Streelman, Webb, Albertson and Kocher2003).
3.b. Feeding habits and palaeoecological implications
The large size of MHNM.KHG230 contrasts with the diminutive size characterizing the multicuspid teeth of many modern fish taxa (Jernvall & Thesleff, Reference Jernvall and Thesleff2012). However, the dental morphology observed in MHNM.KHG230 is strikingly similar to that observed in modern algivorous/herbivorous forms, such the characids Hemigrammus, Hyphessobrycon and Phycocharax (Lima et al. Reference Lima, Wosiacki and Ramos2009; Ohara et al. Reference Ohara, Abrahão and Espíndola2017 a, b), the acanthurid Acanthurus nigrofuscus (Fishelson & Delarea, Reference Fishelson and Delarea2013), the sparid Crenidens (Fishelson et al. Reference Fishelson, Golani and Diamant2014), the terapontid Helotes (Davis et al. Reference Davis, Unmack, Vari and Betancur2016) and the cichlid Labeotropheus (Fraser et al. Reference Fraser, Bloomquist and Streelman2008). Interestingly, a similar tooth morphology is also found in various groups of herbivorous reptiles, such as pareiasaurs (e.g. Jalil & Janvier, Reference Jalil and Janvier2005; Tsuji, Reference Tsuji2013), ankylosaurian dinosaurs (Ősi et al. Reference Ősi, Prondvai, Mallon and Bodor2017), the extinct crocodyliform Simosuchus (Kley et al. Reference Kley, Sertich, Turner, Krause, O’Connor and Georgi2010) and the algae-eating Galápagos marine iguana Amblyrhynchus (Melstrom, Reference Melstrom2017). Among mammals, similar fan-shaped multicuspid incisors are present in an extinct macroscelidid tentatively referred to Miorhynchocyon gariepensis (Senut, Reference Senut2003) and in some hyracoids (De Blieux & Simons, Reference De Blieux and Simons2002).
Although the vomerine and prearticular dentitions of Pycnodus multicuspidatus sp. nov. still remain unknown and may have had a crushing function, all the aforementioned morphological similarities clearly indicate that at least one pycnodontine species has evolved an anterior dentition well suited for benthic macroalgal scraping. Pycnodus multicuspidatus sp. nov. was likely able to feed on the heavily calcified macroalga Halimeda, a chlorophyte abounding in the shallow marine ramp facies of the Palaeogene formations of the central High Atlas (Dragastan & Herbig, Reference Dragastan and Herbig2007).
The multicuspid incisiform teeth of Pycnodus multicuspidatus sp. nov. are rather similar to those of the front dentition of Stephanodus splendens and Hadrodus belinkoi, two putative pycnodontiform taxa occurring in the underlying Maastrichtian strata of the Ouled Abdoun Basin (Arambourg, Reference Arambourg1952, Reference Arambourg1964). However, the incisiform teeth of Stephanodus and Hadrodus can be easily distinguished from those of Pycnodus multicuspidatus sp. nov. The incisiform teeth of Stephanodus have a lower crown with a rectilinear or slightly concave occlusal margin showing numerous (up to 14), smaller cuspules (Zittel, Reference Zittel1888: fig. 310; White, Reference White1934: pl. 10, fig. 11; Tabaste, Reference Tabaste1963: pl. 13, fig. 3; Cappetta, Reference Cappetta1972: pl. 13, figs 1–3). The incisiform teeth of Hadrodus are usually bifid, with a crown showing two well-separated and well-developed cusps sometimes flanked by one or two additional cuspules in dentary teeth (Arambourg, Reference Arambourg1964; Bell, Reference Bell1986). In pycnodont fishes, premaxillary and dentary teeth may have distinct crown morphologies (e.g. Szabó et al. Reference Szabó, Gulyás and Ősi2016). As suggested by the dignathic heterodonty observed between premaxillary and dentary teeth of Hadrodus hewletti (Bell, Reference Bell1986: fig. 2.4–6), teeth of Hadrodus belinkoi and Stephanodus splendens may correspond, respectively, to premaxillary and dentary teeth of the same species (with large hook-shaped branchial teeth originally described under the name Ancistrodon libycus; Dames, Reference Dames1883; Arambourg, Reference Arambourg1952). A complete systematic revision of this group of putative pycnodonts is clearly needed but is beyond the scope of this paper; therefore, pending further investigation, Hadrodus belinkoi and Stephanodus splendens are considered here as two distinct taxa.
In the Palaeocene epicontinental seas of Morocco, Pycnodus multicuspidatus sp. nov. may have occupied, by opportunistic replacement, the trophic niche filled by Stephanodus and Hadrodus before the K/Pg boundary and vacated by the end-Cretaceous extinction of these two taxa (Fig. 2). This case of parallelism emphasizes the high morphological plasticity of pycnodont fishes and their ability to adapt to sudden palaeoenvironmental and palaeoecological changes (Poyato-Ariza, Reference Poyato-Ariza2005). Pycnodont fishes appear to have been trophically diverse in the Ouled Abdoun Basin ecosystem during the Palaeogene, with generalist, shell-crushing forms (Pycnodus spp., including P. cf. praecursor), a macroalgal scraper (Pycnodus multicuspidatus sp. nov.) and a predatory, flesh-eating form (Serrasalmimus secans).
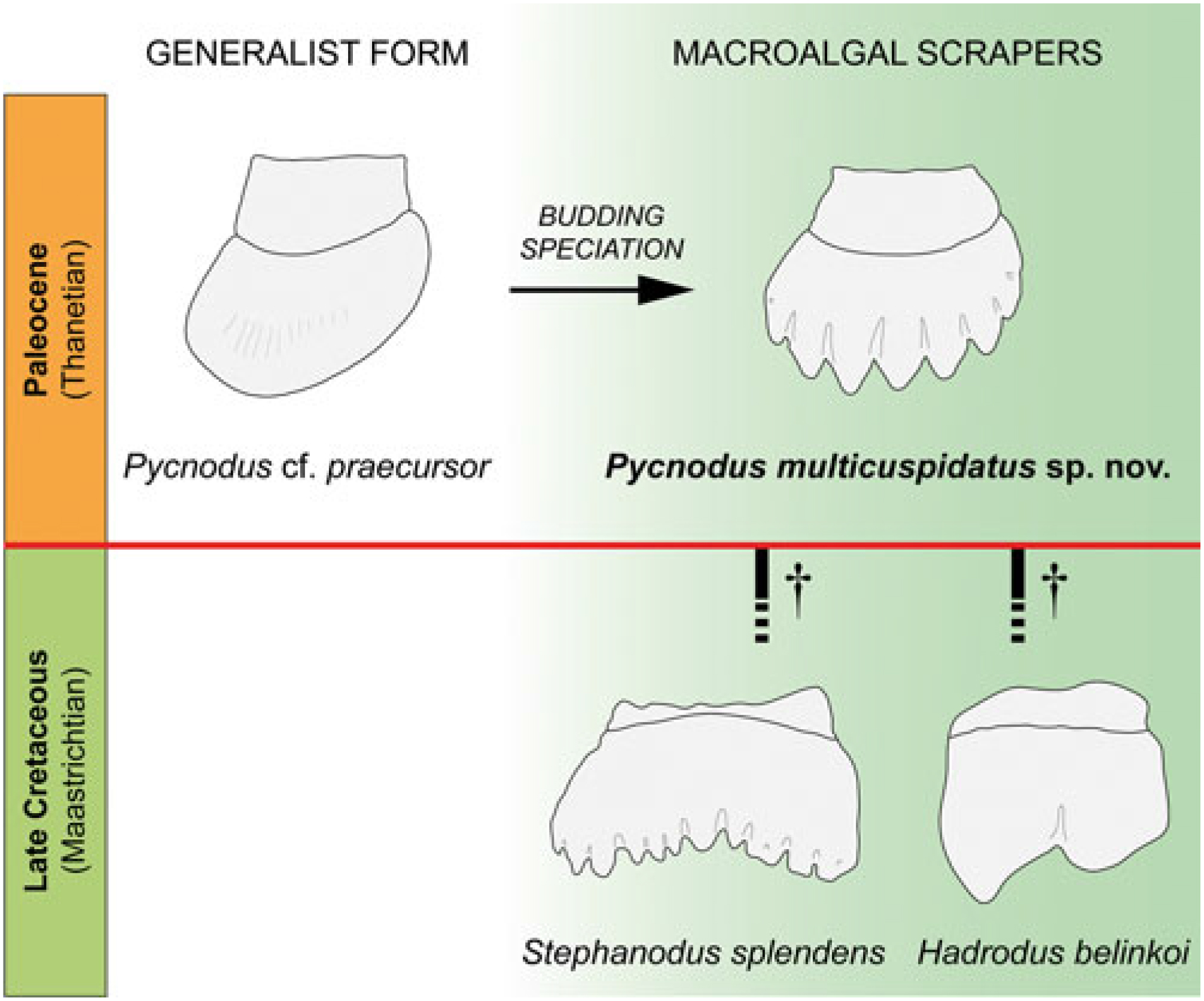
Fig. 2. End-Cretaceous extinction of the macroalgal scrapers Stephanodus and Hadrodus (?Pycnodontiformes) followed by opportunistic refilling of vacated ecospace by the Palaeocene pycnodontid Pycnodus multicuspidatus sp. nov. Line drawings of Stephanodus and Hadrodus incisiform teeth after Tabaste (Reference Tabaste1963: pl. 13, fig. 3) and Arambourg (Reference Arambourg1964: fig. 1a), respectively. Not to scale.
Author ORCIDs
Romain Vullo, 0000-0002-1900-9991
Acknowledgements
We would like to thank Patrick Catto for kindly donating the holotype of the new species described in this paper, and Damien Gendry for his help with the photographs of the specimens. Márton Szabó and an anonymous reviewer provided constructive comments that improved the manuscript. There are no sources of financial support.





