Introduction
The two-spotted spider mite, Tetranychus urticae Koch, is a global polyphagous pest feeding on more than 1100 plant species, including many economically important ornamentals, fruits, vegetables, and agricultural crops, including hops (Humulus lupulus) (Grbic et al., Reference Grbic, Van Leeuwen, Clark, Rombauts, Rouze, Grbic, Osborne, Dermauw, Ngoc, Ortego, Hernandez-Crespo, Diaz, Martinez, Navajas, Sucena, Magalhaes, Nagy, Pace, Djuranovic, Smagghe, Iga, Christiaens, Veenstra, Ewer, Villalobos, Hutter, Hudson, Velez, Yi, Zeng, Pires-daSilva, Roch, Cazaux, Navarro, Zhurov, Acevedo, Bjelica, Fawcett, Bonnet, Martens, Baele, Wissler, Sanchez-Rodriguez, Tirry, Blais, Demeestere, Henz, Gregory, Mathieu, Verdon, Farinelli, Schmutz, Lindquist, Feyereisen and Van de Peer2011). Hops are a specialty crop cultivated mainly for their oils, which are used as a flavoring and stability ingredient in beer (Cranham, Reference Cranham, Helle and Sabelis1985). The US hop industry is estimated to be worth over US$375 million, with the key production area located in the Pacific Northwest (PNW), which comprises 99% of the USA's and 35% of the world's hop acreage (USDA-NASS, 2015). In PNW hopyards, T. urticae is the dominant pest species threatening hop quality and production (O'Neal et al., Reference O'Neal, Walsh, Gent, Barbour, Boydston, George, James and Sirrine2015; Piraneo et al., Reference Piraneo, Bull, Morales, Lavine, Walsh and Zhu2015). T. urticae management by hop growers in PNW and other production regions often includes the application of different classes of acaricides and insecticides. Based on records of acaricide applications in PNW hopyards, as many as ten acaricides with different modes of action are applied in rotation or combination over the course of the hop growing season (Piraneo et al., Reference Piraneo, Bull, Morales, Lavine, Walsh and Zhu2015). Among these acaricides, etoxazole (oxazoline compound), and hexythiazox (a thiazolidinone compound) represent a unique class of non-systemic acaricides that are commonly known as mite growth inhibitors (MGIs) (IRAC group 10) (Sparks & Nauen, Reference Sparks and Nauen2015). Clofentezine is a tetrazine compound currently being developed for registration on hops in the USA. Because MGIs show selective activities against spider mites vs. predatory mites (Hoy & Ouyang, Reference Hoy and Ouyang1986), and are also non-toxic to beneficial insects, mammals, and the environment (Van Leeuwen et al., Reference Van Leeuwen, Demaeght, Osborne, Dermauw, Gohlke, Nauen, Grbic, Tirry, Merzendorfer and Clark2012; Demaeght et al., Reference Demaeght, Osborne, Odman-Naresh, Grbic, Nauen, Merzendorfer, Clark and Van Leeuwen2014; Zhu et al., Reference Zhu, Merzendorfer, Zhang, Zhang and Muthukrishnan2016b ), they have proved to be valuable IPM tools for T. urticae management in both greenhouses (Abraham et al., Reference Abraham, Braman, Oetting and Hinkle2013) and field conditions for decades (Rathman et al., Reference Rathman, Beers, Flexner, Riedl, Hoyt, Westigard and Knight1990; Herron et al., Reference Herron, Edge and Rophail1993; Pree et al., Reference Pree, Bittner and Whitty2002; Bi et al., Reference Bi, Niu, Yu and Toscano2016). MGIs are commonly used in the middle of the hop season since they were first registered for use in hops (Piraneo et al., Reference Piraneo, Bull, Morales, Lavine, Walsh and Zhu2015). They are most often tank mixed by applicators with acaricides that target motile mites. However, the resistance status of T. urticae to MGIs in hop fields remains largely unknown. Consequently, when acaricide applications do not suppress T. urticae abundance, it is not clear why the applications failed.
T. urticae exhibits an extraordinary capability for developing resistance to the majority of acaricides used for its control due to its extremely short generation time, high fecundity, and arrhenotokous reproductive mode (Grbic et al., Reference Grbic, Van Leeuwen, Clark, Rombauts, Rouze, Grbic, Osborne, Dermauw, Ngoc, Ortego, Hernandez-Crespo, Diaz, Martinez, Navajas, Sucena, Magalhaes, Nagy, Pace, Djuranovic, Smagghe, Iga, Christiaens, Veenstra, Ewer, Villalobos, Hutter, Hudson, Velez, Yi, Zeng, Pires-daSilva, Roch, Cazaux, Navarro, Zhurov, Acevedo, Bjelica, Fawcett, Bonnet, Martens, Baele, Wissler, Sanchez-Rodriguez, Tirry, Blais, Demeestere, Henz, Gregory, Mathieu, Verdon, Farinelli, Schmutz, Lindquist, Feyereisen and Van de Peer2011). A crucial step in pesticide management programs is standardizing bioassay techniques to accurately characterize the pest's susceptibility to a pesticide. The bioassay method used to test the efficacy of most commonly used acaricides in hops has historically been topical exposure of adult gravid female T. urticae with a Potter precision spray tower or with a direct slide-dip method (Piraneo et al., Reference Piraneo, Bull, Morales, Lavine, Walsh and Zhu2015). However, these methods are not appropriate in the case of MGIs since MGIs are not toxic to deutonymph and adult stages of T. urticae (Dekeyser, Reference Dekeyser2005; Nauen & Smagghe, Reference Nauen and Smagghe2006). In the past, various types of bioassays have been employed to test the activities of MGIs (Aveyard et al., Reference Aveyard, Peregrine and Bryan1986; Knight et al., Reference Knight, Beers, Hoyt and Riedl1990; Rathman et al., Reference Rathman, Beers, Flexner, Riedl, Hoyt, Westigard and Knight1990; Marcic, Reference Marcic2003; Yorulmaz Salman et al., Reference Yorulmaz Salman, Aydinli and Ay2015). Most of these bioassay methods involve direct application of the MGI to eggs (Knight et al., Reference Knight, Beers, Hoyt and Riedl1990; Uesugi et al., Reference Uesugi, Goka and Osakabe2002; Demaeght et al., Reference Demaeght, Osborne, Odman-Naresh, Grbic, Nauen, Merzendorfer, Clark and Van Leeuwen2014). A few other studies were performed using immature or adult mites (Chapman & Marris, Reference Chapman and Marris1986; Suzuki et al., Reference Suzuki, Ishida, Kikuchi, Ito, Morikawa, Tsukidate, Tanji, Ota and Toda2002; Ay & Kara, Reference Ay and Kara2011). There is a need to develop and standardize bioassay methods to evaluate the efficacy of MGIs in hops and other agroecosystems. Furthermore, knowledge about cross-resistance among MGIs and between MGIs and other acaricides is crucial for developing acaricide resistance management programs (Asahara et al., Reference Asahara, Uesugi and Osakabe2008; Demaeght et al., Reference Demaeght, Osborne, Odman-Naresh, Grbic, Nauen, Merzendorfer, Clark and Van Leeuwen2014).
Although clofentezine, etoxazole, and hexythiazox have dissimilar chemical structures, they share the same mode of action: inhibition of the chitin synthase 1 (CHS1) enzyme during the final phases of chitin biogenesis (Nauen & Smagghe, Reference Nauen and Smagghe2006; Demaeght et al., Reference Demaeght, Osborne, Odman-Naresh, Grbic, Nauen, Merzendorfer, Clark and Van Leeuwen2014). The mechanisms of pesticide resistance are proposed to have evolved along several trajectories. Among these mechanisms, target site insensitivity and enhanced metabolic detoxification are two major known resistance mechanisms (Liu et al., Reference Liu, Zhu, Xu, Pridgeon and Gao2006; Zhu et al., Reference Zhu, Cui, Lavine, Walsh, Chandrasekar, Tyagi, Gui and Reeck2014; Van Leeuwen & Dermauw, Reference Van Leeuwen and Dermauw2016). Recent studies reported that a single non-synonymous mutation (I1017F) in the non-catalytic domain of CHS1 confers resistance to clofentezine, etoxazole, and hexythiazox in etoxazole and hexythiazox-resistant T. urticae populations (Van Leeuwen et al., Reference Van Leeuwen, Demaeght, Osborne, Dermauw, Gohlke, Nauen, Grbic, Tirry, Merzendorfer and Clark2012; Demaeght et al., Reference Demaeght, Osborne, Odman-Naresh, Grbic, Nauen, Merzendorfer, Clark and Van Leeuwen2014). Biochemical enzyme activity assays suggest that detoxification enzymes such as carboxylesterases (CoEs), glutathione S-transferases (GSTs), or cytochrome P450s may also contribute to resistance in a clofentezine resistant T. urticae strain and a etoxazole resistant Phytoseiulus persimilis (Acari: Phytoseiidae) strain (Ay & Kara Reference Ay and Kara2011; Yorulmaz Salman et al., Reference Yorulmaz Salman, Aydinli and Ay2015).
In this study, we developed three bioassay methods to establish baseline susceptibility levels of MGIs (clofentezine, etoxazole, and hexythiazox) in an acaricide susceptible T. urticae strain. Using the bioassay method that is most effective, we then tested populations of T. urticae collected from commercial hopyards for their resistance to these MGIs. Cross-resistance among MGIs, and between MGIs and other non-MGI acaricides used in hops, was also evaluated. In order to understand the mechanisms of MGI resistance in hopyards, we investigated the presence of the target site mutation (I1017F) in the CHS1 gene in four field-collected T. urticae populations. Lastly, we conducted synergist studies to examine the possible involvement of metabolic enzymes and transport proteins in the resistance to MGIs of T. urticae populations in hopyards.
Methods
Mite populations
The susceptible T. urticae strain was originally collected from weeds in Montana in 1995 and has since been maintained in the laboratory without exposure to any pesticides (Piraneo et al., Reference Piraneo, Bull, Morales, Lavine, Walsh and Zhu2015; Morales et al., Reference Morales, Mendoza, Lavine, Lavine, Walsh and Zhu2016). Four field T. urticae populations used in this study were collected from commercial hopyards located within the Yakima Valley of Washington State during the summer of 2015 from three locations. Two samples were collected in Prosser, WA (46°12′25″N, 119°45′56″W), one in Mabton, WA (46°12′42″N, 119°59′47″W), and one in Moxee, WA (46°33′23″N, 120°23′14″W). The pesticide application records for each of the sampled hop farms were obtained and recorded (table S1). Each sampled farm had sprayed either etoxazole or hexythiazox at least once in the 2015 growing season. Mite-infested hop leaves from yards were collected from upper, mid, and low hop branches to ensure that each sample was representative of the overall mite population. These leaves were stored in a plastic bag and transported back to the laboratory in a cooling box within a few hours of collection. Both the susceptible and field-collected T. urticae populations were reared on lima bean plants (Phaseolus lunatus L.) in a fine mesh BugDorm insect cage (MegaView Science Co., Ltd., Taiwan, China). Populations were kept at 25 ± 2°C, 70 ± 5% RH, and a photoperiod of 16:8 (Light: Dark) in an isolated walk-in growth chamber at Washington State University in Pullman, WA. Two new lima bean plants were provided for each spider mite population every 2–3 weeks.
Chemicals
Pesticides used in this study are summarized in table S2. To investigate a potential role for metabolic enzymes and transport proteins in the MGI resistance, four synergist compounds were used: piperonyl butoxide (PBO, purity 90%) (an inhibitor of cytochrome P450s) was purchased from MGK company (Minneapolis, MN); diethyl maleate (DEM, 95%) (an inhibitor of GSTs), triphenyl phosphate (TPP, 99%) (an inhibitor of CoEs), and verapamil (VER) (an inhibitor of ABC transporters) were all purchased from Sigma-Aldrich (St. Louis, MO) (Zhang et al., Reference Zhang, Liu, Yang, Dong and Han2012; Chouaibou et al., Reference Chouaibou, Zivanovic, Knox, Jamet and Bonfoh2014).
Bioassays
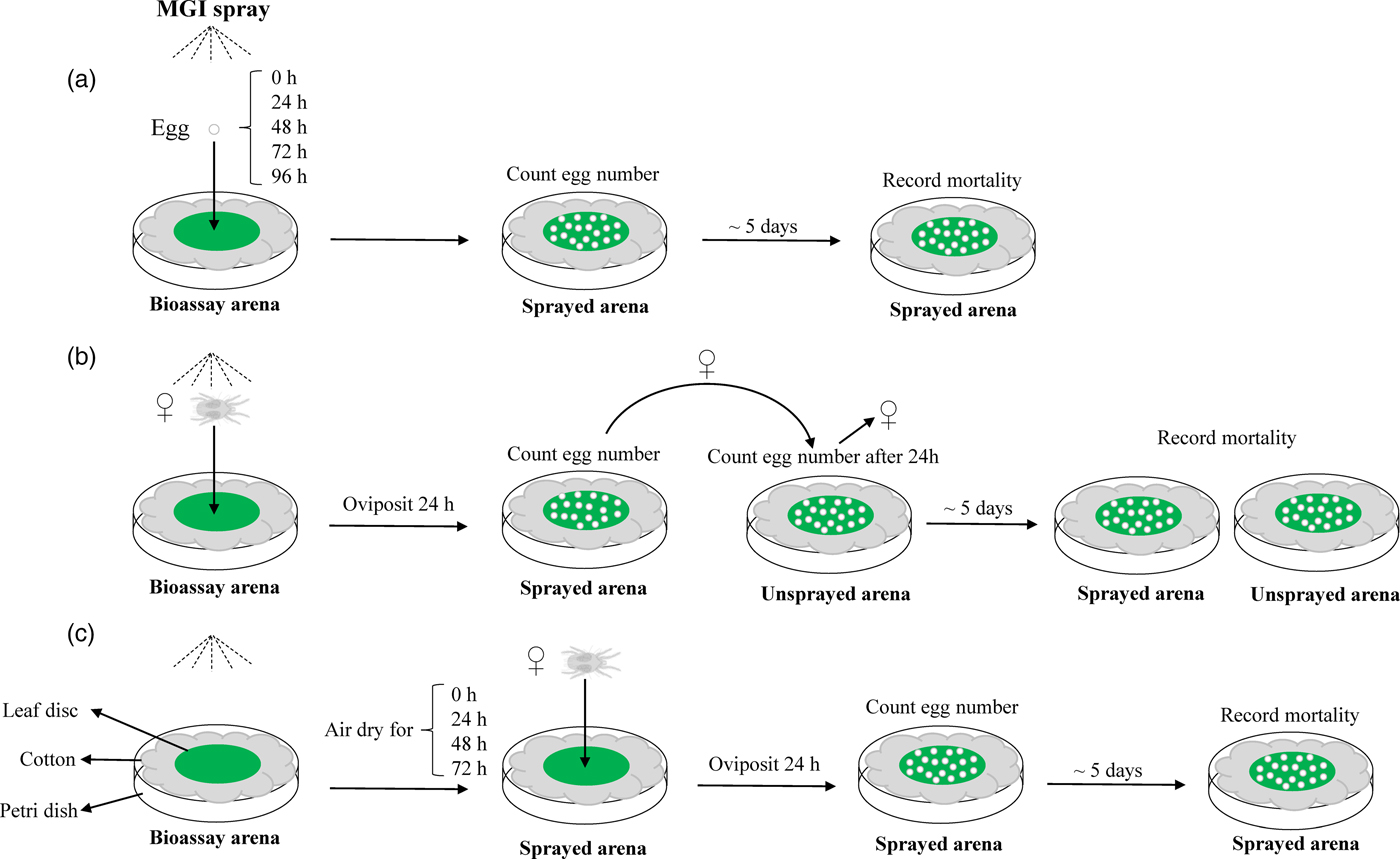
In order to understand the overall biological ovicidal activity of MGIs and identify the optimal bioassay method for determining ovicidal activity, a three-tiered approach was performed (fig. 1). The susceptible T. urticae strain was used for these bioassays.
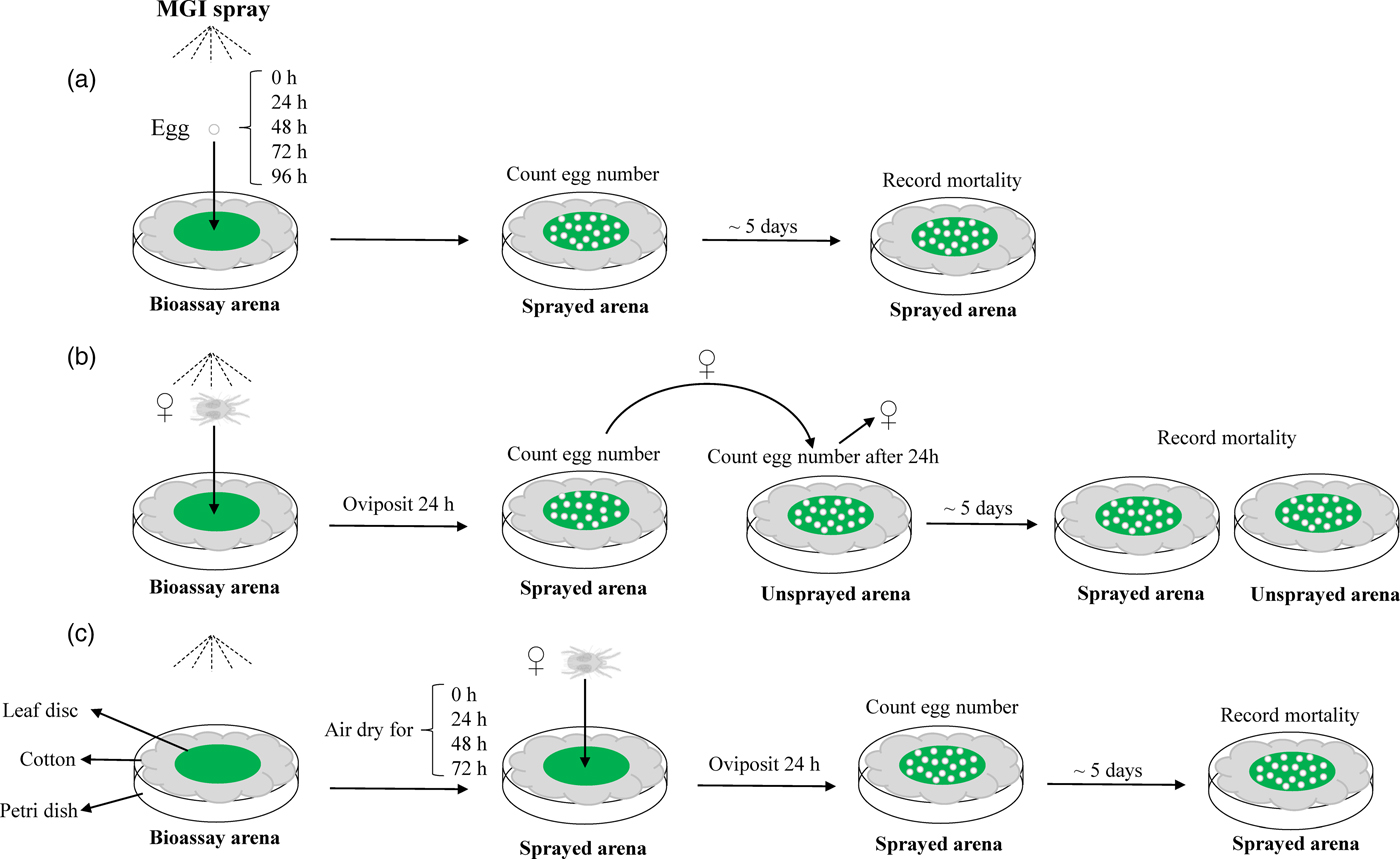
Fig. 1. Schematic diagram of a three-tiered bioassay approach for three MGIs. (a) Topical exposure of eggs; (b) topical exposure of gravid females; (c) residual exposure of gravid females.
The first bioassay method (fig. 1a) involved direct exposure of T. urticae eggs to serial dilutions of clofentezine, etoxazole, or hexythiazox. The concentrations ranged from 0 (control with Millipore-filtered water only) to the recommended field doses for hops with hexythiozox and etoxazole and the field dose under development for clofentezine (table S2). Leaf disc bioassay arenas were created by placing fresh lima bean leaf discs (22 mm in diameter) on wet cotton as previously described (Piraneo et al., Reference Piraneo, Bull, Morales, Lavine, Walsh and Zhu2015). Eight gravid adult female mites were placed on each leaf disc with a fine brush and allowed to lay eggs for 24 h. After 24 h, the female mites were removed. Eggs laid on bioassay arenas were used immediately (0 h) or permitted to mature for an additional 24, 48, 72, or 96 h to test if the age of the T. urticae egg (embryo development) affects MGI efficacy. After the indicated time, the eggs were subjected to direct topical exposure to MGIs using a Potter precision spray tower (Burkard Manufacturing, Richmansworth, Herts, UK). The Potter spray tower was calibrated to deposit 2 ± 0.1 mg cm−2 of liquid under 1.1 kg cm−2 of pressure, similar to the coverage of the recommend field dose. 2 ml of each concentration of the acaricides was used in the Potter spray tower. After spraying, the total number of eggs laid was counted under a light microscope. After 5 days, the number of eggs that successfully hatched into larvae were recorded. This experiment was replicated three to four times for each concentration of each MGI.
The second bioassay method was designed to check whether the exposure of gravid T. urticae females to MGIs has effects on the fecundity of females and the survival of their offspring (fig. 1b). Similar bioassay arenas were created as described in the first method. Six to eight gravid females were gently placed on a leaf disc with a fine brush and immediately sprayed with serial concentrations of MGIs ranging from 0 to field recommended doses. Mites were allowed to oviposit for 24 h on the sprayed arena. These mites were then transferred to a fresh unsprayed arena and allowed to oviposit for an additional 24 h, after which the adults were removed (fig. 1b). The total number of eggs laid on the sprayed and unsprayed arenas was counted. After 5 days, the number of eggs that successfully hatched into larvae was recorded for both sprayed and unsprayed arenas. The control was T. urticae females on leaf discs sprayed with distilled water alone. This experiment was repeated three to four times. Differences in the fecundity of T. urticae females and the survival of their offspring between the control and MGI treatments were analyzed using Student's t-test.
In the third method, we evaluated the ovicidal activity of MGI residue on eggs laid by gravid T. urticae females (fig. 1c). Leaf disc bioassay arenas were created as described above. The discs were pretreated with serial dilutions of each MGI and permitted to air dry in a fume hood for 0 (untreated control), 24, 48, or 72 h. Gravid female mites were allowed to oviposit on sprayed leaf discs for 24 h. This experiment was replicated three times for each MGI at each concentration. The dose–mortality response of eggs in these three bioassay methods were adjusted for mortality associated with the control treatment using Abbot's formula (Abbott, Reference Abbott1925) and then subjected to log-dose probit analysis (POLO Probit 2014) to estimate LC50. The statistical analysis of LC50 values was based on non-overlapping 95% CI (Liu & Yue, Reference Liu and Yue2000).
Lastly, we used the most effective bioassay method to evaluate the resistance ratio (RR) to MGIs of four field populations. These four T. urticae populations were collected from commercial hopyards during the summer of 2015 and kept in the lab on lima bean plants for 7–9 weeks before the initiation of bioassays. RRs for each T. urticae population to each MGI were calculated by dividing the LC50 value of the field population by the LC50 value of the susceptible population.
Selection for resistance
The Prosser 1 population was further selected with clofentezine, etoxazole, or hexythiazox to produce resistant strains named Prosser 1-CS, Prosser1-ES, and Prosser 1-HS strains, respectively. We chose the Prosser 1 population because it exhibited low-to-moderate resistance to MGIs and also has resistance associated I1017F mutation. Selection for resistance to MGIs was performed for 24 weeks (approximately 17 generations), using increasing concentrations of acaricide that each resulted in approximately 70–90% mite mortality (fig. S1). Initially, 40–60 lima bean leaves detached from plants with feeding mites of the Prosser 1 population were dipped into solutions of either clofentezine (Apollo®), etoxazole (Zeal®), or hexythiazox (Savey®) with concentrations listed in fig. S1 for 5 s. Leaves were allowed to air-dry in a fume hood and then placed in a clean plastic cup with lid for 24 h. The surviving mites were transferred to new plants in a clean BugDorm® insect cage to initiate a new population. This procedure was then repeated two weeks later. Starting at week 4, pesticide application was performed directly on whole plants using a hand sprayer until all the leaves on the plant were covered. Each population received MGI applications biweekly.
Evaluation of cross-resistance
Cross-resistance among the three candidate MGIs and also between MGIs and three non-MGI acaricides commonly used in hopyards was evaluated. The three non-MGI acaricides were abamectin (Epimek®), bifenazate (Acramite®), and bifenthrin (Bifenture®) (table S2). After 20 weeks’ selection (fig. S1), the populations Prosser 1-CS, Prosser 1-ES, and Prosser 1-HS along with the unselected Prosser 1 population were used for cross-resistance assays. The bioassay method used for evaluating cross-resistance among clofentezine, etoxazole, and hexythiazox was direct topical spray of the MGIs on eggs. Freshly laid eggs from Prosser 1, Prosser 1-CS, Prosser 1-ES, or Prosser 1-HS strains were sprayed with clofentezine, etoxazole, or hexythiazox by Potter spray tower. The leaf disc bioassay used for abamectin and bifenazate, and the sticky tape method used for bifenthrin, were described in our previous studies (Piraneo et al., Reference Piraneo, Bull, Morales, Lavine, Walsh and Zhu2015; Morales et al., Reference Morales, Mendoza, Lavine, Lavine, Walsh and Zhu2016). The only difference between this study and our previous study is that we recorded the mortality 48 h instead of 24 h after the spray in order to keep clear distinctions between mite survival and mortality. RRs for each T. urticae population to each acaricide were calculated by dividing the LC50 value of the selected field population by the LC50 value of the unselected population, Prosser 1.
Detection of the I1017F substitution in T. urticae populations
Genomic DNA (gDNA) was extracted from pooled samples of ~50 adult mites for the susceptible, field-collected, and MGI-selected populations using DNeasy Blood & Tissue kit (QIAGEN®) and stored at −20°C until use. The gDNA was used as a template for polymerase chain reaction (PCR) performed in a ProFlex PCR system (Applied Biosystems, Life Technologies). The total reaction volume was 20 µl containing 4 µl 5X Phusion PCR buffer, 0.8 µl 10 mM dNTP mix, 0.8 µl Phusion High-Fidelity DNA Polymerase (2U µl−1) (Thermo Scientific, Pittsburgh, PA) and 0.6 µl of 10 mM forward primer (5′–TCCGCTTGTTATGCACTACTC–3′) and reverse primer (5′–ACCTGAACAAGTTTGCCAGAC–3′). PCR was performed under the following cycling parameters: an initial DNA template denaturation at 94°C for 3 min 50 s, 35 cycles of 94°C for 35 s, 58°C for 35 s, and 72°C for 1 min, with final extension for 10 min at 72°C. PCR products were purified using DNA Clean & Concentrator (Zymo Research, Irvine, CA) following the manufacturer's protocol. Each individual PCR product was sequenced using ABI Big Dye Terminator Version 3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA) on an ABI 3730 at the Center for Reproductive Biology Molecular Biology and Genomics Core facility at Washington State University. The sequence outputs were analyzed with BioEdit 7.0.1 software (Ibis Biosciences, Carlsbad, CA). The presence or absence of the I1017F mutation was determined by inspection of sequencing chromatographs. Each population or strain was sequenced three times with independently prepared gDNA pools.
Synergist assays
In order to investigate the potential involvement of cytochrome P450s, GSTs, CoEs, and ABC transporters in resistance to MGIs, freshly laid eggs by Prosser 1, Prosser 1-CS, Prosser 1-ES, or Prosser 1-HS were sprayed with 2 ml of PBO, DEM, TPP, or VER by Potter spray tower, 5–6 h prior to clofentezine, etoxazole, or hexythiazox application. Based on preliminary bioassays, the doses of the synergists were chosen as the highest doses which resulted in minimum mortality of T. urticae eggs of <10%. These doses were 0.2 g l−1 for PBO, 0.5 g l−1 for DEM, 1.0 g l−1 for TPP, and 0.5 g l−1 for VER. PBO (initially dissolved in dimethylformaldehyde) and DEM were dissolved in distilled water, TPP was initially dissolved in methanol. Mite eggs sprayed with MGIs only served as controls. The synergist ratios (SRs) and corresponding 95% confidence intervals were calculated for each synergist for each MGI (Van Pottelberge et al., Reference Van Pottelberge, Van Leeuwen, Khajehali and Tirry2009). SRs of PBO, DEM, TPP, and VER were determined through dividing the LC50 of the MGI alone by the LC50 of the synergist plus MGI. We considered there was no synergist effect if there was an overlap between LC50 confidence intervals of MGI alone and synergist plus MGI (Van Pottelberge et al., Reference Van Pottelberge, Van Leeuwen, Khajehali and Tirry2009). Synergist bioassays were done in March 2015 after 13 weeks of selection for resistance in the laboratory (fig. S1).
Results
Bioassay methods to evaluate ovicidal activity of MGIs
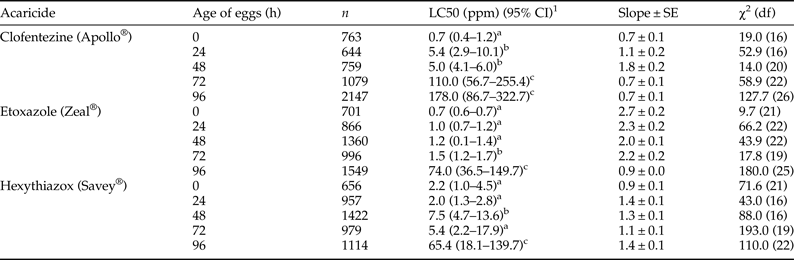
In the first bioassay method, we examined toxicity of MGIs by topical application to T. urticae eggs (fig. 1a). All MGIs exhibited high ovicidal activity to eggs laid by the susceptible T. urticae (table 1). Toxicity of all the MGIs varied greatly with the age of the eggs. Freshly oviposited eggs (0 h) exhibited the greatest susceptibility to all three MGIs. However, the ovicidal efficacy decreased dramatically with egg maturity. 96 h post-oviposition eggs required 254, 106, and 30 times the concentration of clofentezine, etoxazole, and hexythiazox, respectively, than the 0 h eggs to cause 50% mortality (table 1).
Table 1. Response of susceptible T. urticae eggs to three MGIs at different stages of development.
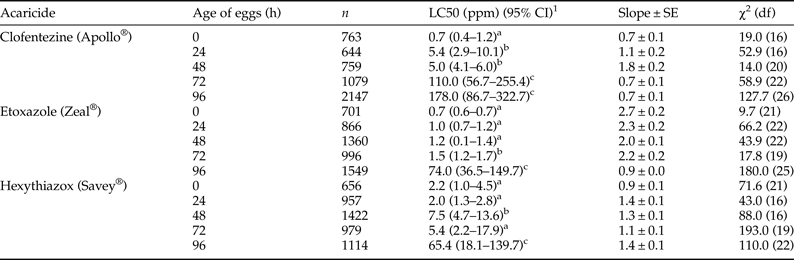
1 95% confidence interval, toxicity of acaricide is considered significant difference when the 95% CI fail to overlap.
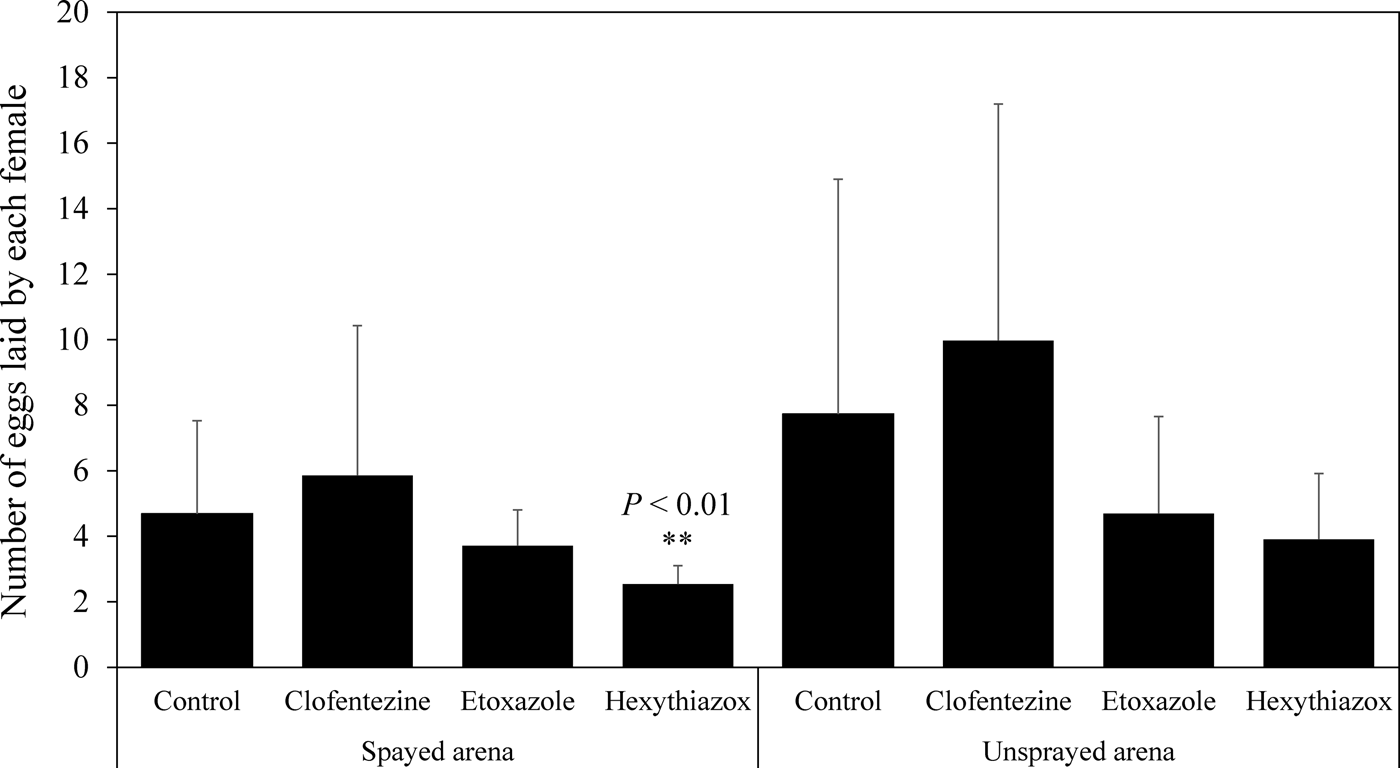
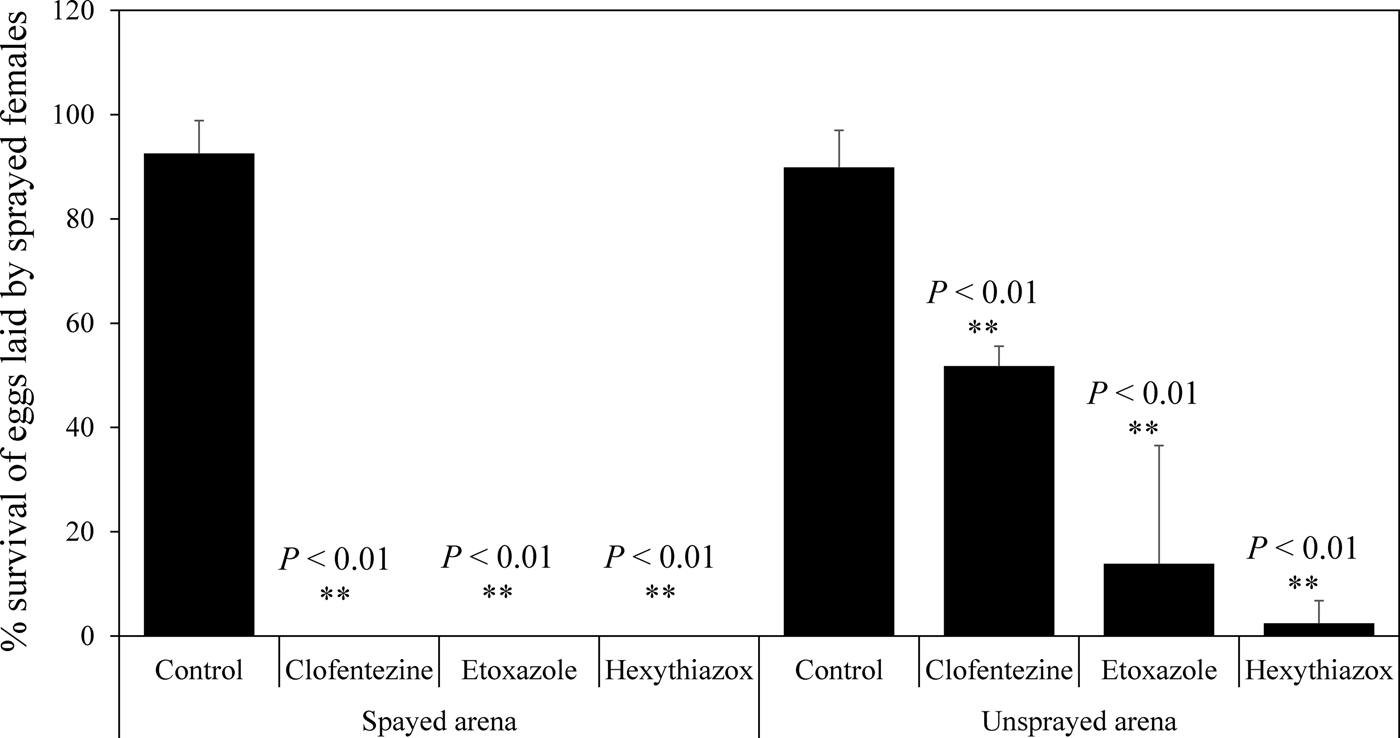
The second bioassay method was designed to investigate the effects of MGIs on the fecundity of T. urticae gravid females and the survival of their eggs (fig. 1b). Our data showed that clofentezine and etoxazole had no significant effect on the fecundity in sprayed bioassay arenas within the 24 h time frame (P > 0.05) (fig. 2). In contrast, hexythiazox significantly decreased the fecundity on sprayed bioassay arenas compared with the control (P < 0.01) (fig. 2). None of the MGIs significantly affected the fecundity in unsprayed bioassay arenas (P > 0.05) (fig. 2). All three MGIs at the field recommended dose for hops caused 100% mortality of eggs on the sprayed bioassay arenas (the mortality in the controls was 7.5%) (fig. 3). When the sprayed gravid female mites were transferred to unsprayed bioassay arenas, clofentezine, etoxazole, and hexythiazox caused 48.2, 86.2, and 97.6% mortality, respectively, in the newly oviposited eggs (the average mortality in the non-treated controls was 10.1%) (fig. 3).
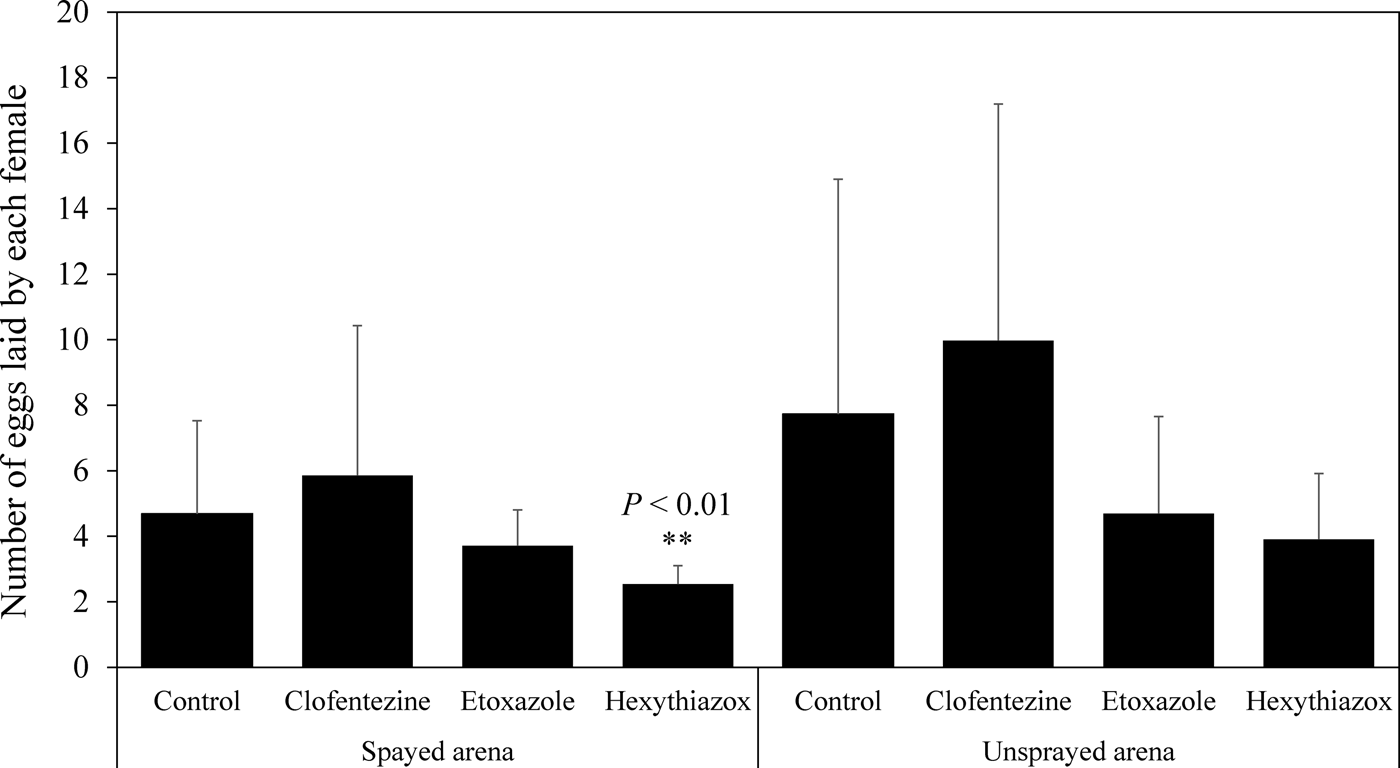
Fig. 2. Effect of topical exposure of MGIs on the fecundity of T. urticae gravid females on sprayed and unsprayed bioassay arenas. The concentrations of MGIs here were field doses used in hop fields. Clofentezine was used in 626 ppm a.i.; etoxazole was used in 300 ppm a.i.; hexythiazox was used in 460 ppm a.i. (table S2). Statistical significance of the egg numbers between the control and each MGI treatment was calculated using Student's t test. ** P < 0.01.

Fig. 3. Effect of topical exposure of MGIs on the survival of eggs laid by sprayed T. urticae gravid females on sprayed and unsprayed bioassay arenas. The concentrations of MGIs here were field doses used in hop fields (table S2). Statistical significance of survival of eggs between the control and each MGI treatment was calculated using Student's t test. ** P < 0.01.
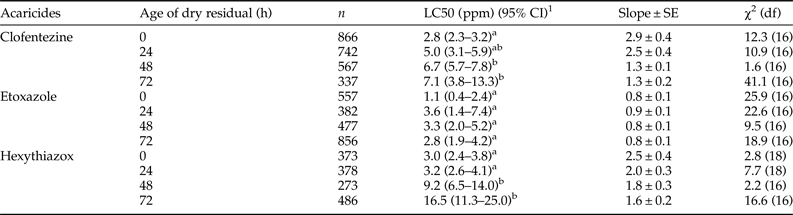
The third bioassay method was designed to examine ovicidal effects on the susceptible T. urticae eggs exposed to residual MGIs (fig. 1c). There was no significant difference in the toxicity of etoxazole to eggs laid by gravid females exposed to 0 to 72 h aged residuals (table 2). However, the toxicities of clofentezine and hexythiazox to eggs laid by gravid females exposed to 48 and 72 h residuals decreased significantly compared to the toxicities on 0 and 24 h residuals (table 2). A significantly greater amount of hexythiazox (~3-fold) was required to cause 50% mortality of eggs on 48 and 72 h aged residuals compared with 0 and 24 h aged residuals (table 2).
Table 2. Residual toxicity of MGIs to freshly laid eggs of susceptible T. urticae.
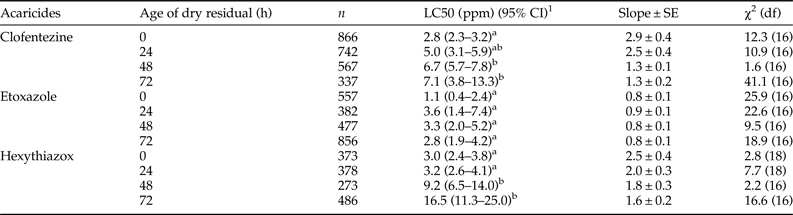
1 95% confidence interval, toxicity of acaricide is considered significant difference when the 95% CI fail to overlap.
Comparison of the three bioassay methods revealed that topical exposure of freshly laid eggs is the most efficient method, as it requires the lowest concentration of active ingredient of clofentezine (0.7 ppm) and etoxazole (0.7 ppm) to kill 50% of the eggs (tables 1 and 2; table S3). Hexythiazox had a slightly lower LC50 (2.0 ppm) in the second bioassay method than the first bioassay method (2.2 ppm) (table 1, table S3). Given that the high mortality it generates and the ease of the first bioassay method, it was used for the subsequent bioassays in this study. The second most efficient method is the third bioassay method with LC50 values of 2.8 and 1.1 ppm for clofentezine and etoxazole, respectively (tables 1 and 2, table S3). However, the efficiency of hexythiazox in the third method is the lowest one (LC50 = 3.0 ppm). Among these three MGIs, etoxazole required the least amount of active ingredient to kill freshly laid spider mite eggs in all three bioassay methods.
Response of field-collected T. urticae populations to three MGIs
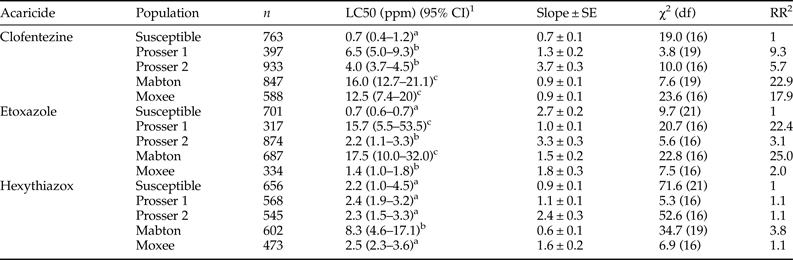
The dose–mortality response of four T. urticae populations collected from hopyards in central Washington was determined using a range of concentrations from 0 to the recommended field dose of each MGI (table S2). Based on spray records, each of these locations received at least one application of either etoxazole or hexythiazox during the 2015 hop growing season (table S1). All field populations exhibited low (RRs between 2 and 10) to moderate (RRs between 10 and 100) levels of resistance to clofentezine and etoxazole when compared with the susceptible strain (table 3). Among all four field-collected populations, the Mabton population exhibited the greatest level of resistance to clofentezine (RR = 22.9), etoxazole (RR = 25.0), and hexythiazox (RR = 3.8). The Prosser 1 population showed low level resistance to clofentezine (RR = 9.3) and moderate level resistance to etoxazole (RR = 22.4) (table 3). The Moxee population also exhibited moderate level resistance to clofentezine (RR = 17.9) and low level resistance to etoxazole (RR = 2.0) (table 3). The Prosser 2 population showed low level resistance to clofentezine (RR = 5.7) and etoxazole (RR = 3.1) (table 3). Interestingly, the Mabton population is the only population tested that exhibited any resistance to hexythiazox (RR = 3.8) (table 3). The other three populations were as susceptible to hexythiazox as the acaricide susceptible laboratory population. It should be noted that the maximum LC50 values for all three ovicides across the tested populations, i.e. 16.0 ppm (clofentezine), 17.5 ppm (etoxazole), and 8.3 ppm (hexythiazox), are still far less than the respective recommended field application doses for hops (table 3 and S2).
Table 3. Response of eggs (0 h old) laid by field-collected T. urticae gravid females to three MGIs.
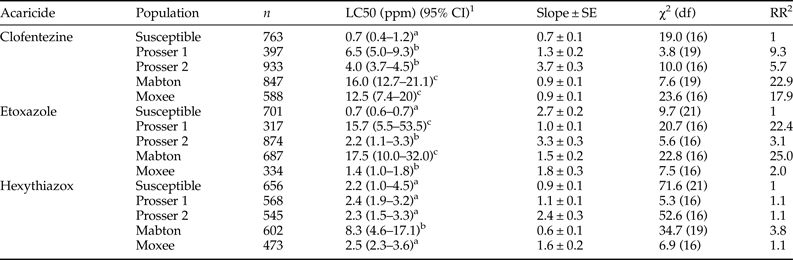
1 95% confidence interval, toxicity of acaricide is considered significant difference when the 95% CI fail to overlap.
2 RR, resistance ratio = LC50 of the field population/LC50 of the susceptible population.
Cross-resistance studies
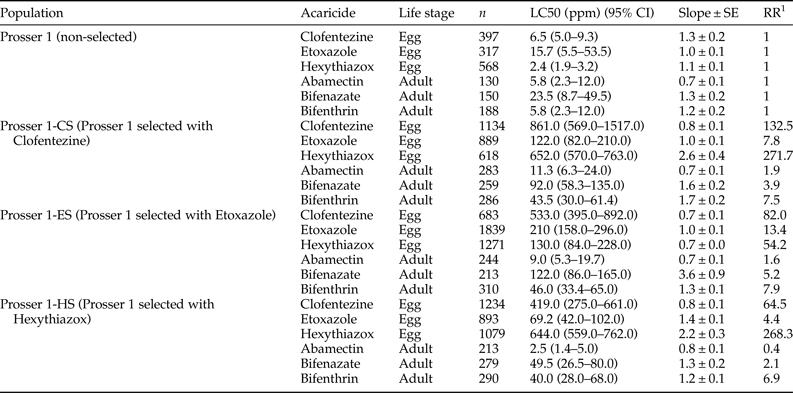
We examined the toxicity of MGIs to T. urticae eggs laid by gravid females of the three MGI-selected populations (Prosser 1-CS, Prosser 1-ES, and Prosser 1-HS), and compared them with the unselected Prosser 1 population (table 4). We also compared the toxicity of three non-MGI acaricides (abamectin, bifenazate, and bifenthrin) that are commonly used in hopyards between MGI-selected and unselected populations (table 4). After 20 weeks of selection, the three MGI-selected populations Prosser 1-CS, Prosser 1-ES, and Prosser 1-HS exhibited significantly higher resistance to clofentezine (RR = 132.5), etoxazole (RR = 13.4), or hexythiazox (RR = 268.3), respectively, than the control population Prosser 1 (table 4). The clofentezine-selected population (Prosser 1-CS) showed high resistance to hexythiazox (RR = 271.7), but only low resistance to etoxazole (RR = 7.8), bifenazate (RR = 3.9), and bifenthrin (RR = 7.5) (table 4). The etoxazole-selected population (Prosser 1-ES) showed moderate levels of resistance to clofentezine (RR = 82.0) and hexythiazox (RR = 54.2) but low levels of resistance to bifenazate (RR = 5.2) and bifenthrin (RR = 7.9) (table 4). The hexythiazox-selected population (Prosser 1-HS) showed moderate resistance to clofentezine (RR = 64.5), but only low levels of resistance to bifenthrin (RR = 6.9), and no significant resistance to any of the other compounds. The abamectin RRs of Prosser 1-CS, Prosser 1-ES, and Prosser 1-HS populations were 1.9, 1.6, and 0.4, respectively (table 4), suggesting there was no cross-resistance detected between MGIs and abamectin.
Table 4. Toxicity of MGIs, abamectin, bifenazate and bifenthrin to eggs (0 h old) laid by T. urticae population (Prosser 1) and three ovicidal acaricide-selected populations.
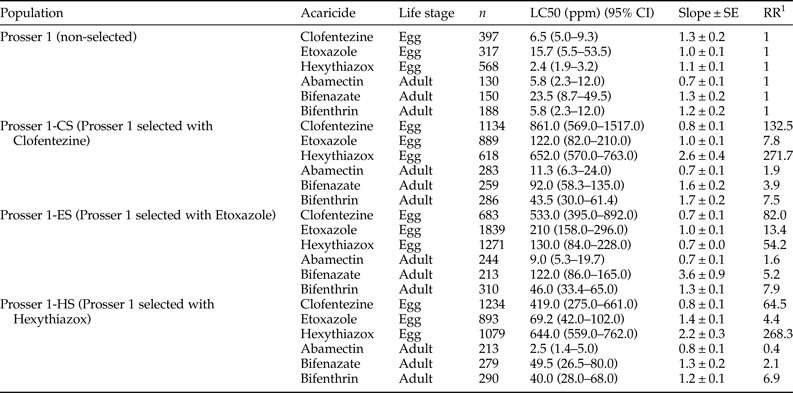
1 RR, resistance ratio = LC50 of the selected field population/LC50 of the non-selected field population, Prosser 1.
Screening for resistance-associated mutation CHS 1 I1017F
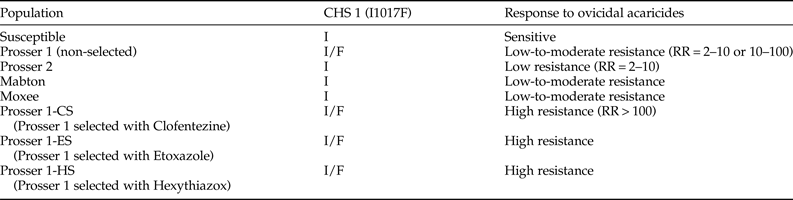
The presence or absence of the nonsynonymous mutation I1017F in the CHS 1 gene, which has been shown to confer resistance to ovicidal MGIs, was examined in different field populations of T. urticae. All of our field-collected T. urticae populations harbored the wild-type allele only, with the exception of the Prosser 1 population, which contained both the wild-type and resistant alleles (table 5). The Prosser 1 population exhibited low-to-moderate resistance to MGIs and was used in our laboratory for resistance selection. After 20 weeks under selection with clofentezine, etoxazole, or hexythiazox, each of our laboratory-selected strains exhibited high resistance to the corresponding acaricides (RR > 100 fold) (fig. S1), we still detected both alleles in all strains (table 5).
Table 5. The amino acid substitution in CHS 1 from the susceptible and field-collected (non-selected and ovicidal acaricide-selected) T. urticae populations.
Synergist studies
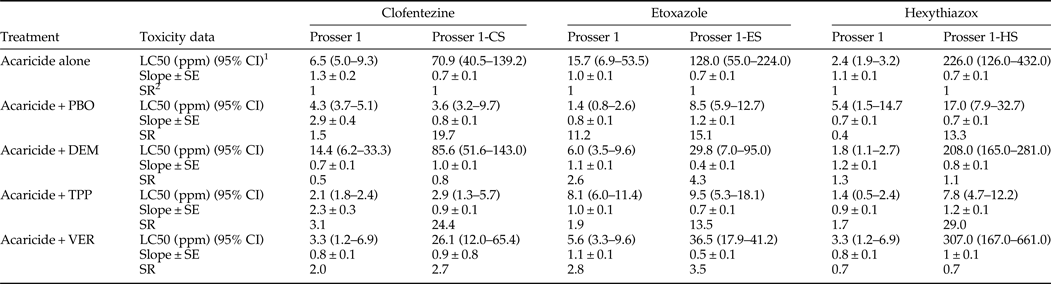
Pre-treatment of the three ovicidal acaricide-selected populations (after 13 weeks of selection) and the control – Prosser 1 population with inhibitors of metabolic enzymes and transport proteins (PBO, DEM, TPP, and VER) was performed prior to acaricide bioassays to investigate the possibility of involvement of metabolic enzymes and transport proteins in the resistance to MGIs. VER had no significant synergistic effect on the toxicity of clofentezine, etoxazole, and hexythiazox in the three ovicidal acaricide-selected populations and the control Prosser 1 population (table 6). Similarly, DEM did not show synergistic effect on the toxicity of most of the populations tested except the Prosser 1-ES (table 6). However, PBO significantly increased the toxicity of clofentezine to Prosser 1-CS by 19.7-fold (table 6), and reduced the RR from 101.3 to 5.1 (table S4). PBO caused 11.2- and 15.1-fold increases in toxicity of etoxazole to Prosser 1 and Prosser 1-ES, respectively (table 6 and S4). PBO also showed a significant synergistic effect on hexythiazox to Prosser 1-HS by increasing the toxicity 13.3-fold (table 6), and reduced the RR from 102.7 to 7.7 (table S4). This suggests that cytochrome P450-mediated detoxification plays an important role in resistance to MGIs by these populations. TPP significantly increased the toxicity of clofentezine to Prosser 1 and Prosser 1-CS by 3.1-fold and 24.4-fold (table 6), and reduced the RR from 9.3 to 3.0 and from 101.3 to 4.1, respectively (table S4). TPP caused a 13.5-fold increase in toxicity of etoxazole to Prosser 1-ES (table 6) and reduced the RR from 182.9 to 13.6 (table S4). Similarly, TPP significantly increased the toxicity of hexythiazox to Prosser 1-HS by 29.0-fold (table 6), and reduced the RR from 102.7 to 3.5 (table S4). Thus, CoE-mediated detoxification appears to also be important in the resistance of these populations to MGIs.
Table 6. Synergism of PBO, DEM, TPP, and VER on MGIs’ toxicity to eggs laid by T. urticae population (Prosser 1) and three ovicidal acaricide-selected populations.
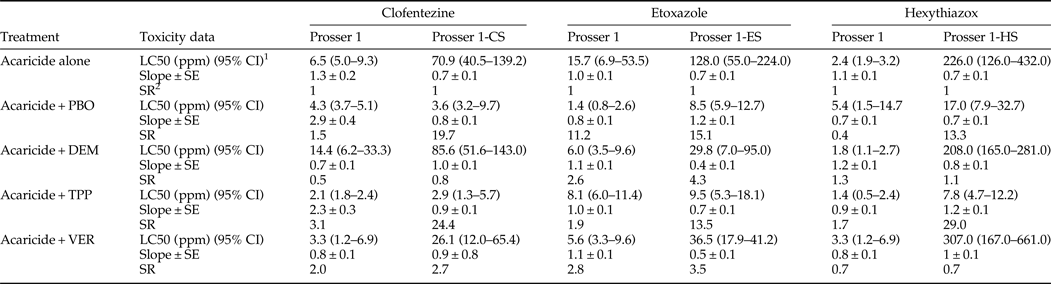
1 95% confidence interval, toxicity of acaricide is considered significant difference when the 95% CI fail to overlap.
2 SR, synergist ratio = LC50 of acaricide/LC50 of synergist + acaricide.
Discussion
The chitin biosynthesis pathway has become a desirable target for selective control of arthropods in agricultural and urban environments because of its uniqueness and importance in forming the cuticle and midgut peritrophic matrix (Merzendorfer, Reference Merzendorfer2006; Van Leeuwen et al., Reference Van Leeuwen, Demaeght, Osborne, Dermauw, Gohlke, Nauen, Grbic, Tirry, Merzendorfer and Clark2012). The three MGIs used in this study, clofentezine, etoxazole, and hexythiazox, share the same mode of action by inhibiting chitin deposition in mite embryos and immature stages (Van Leeuwen et al., Reference Van Leeuwen, Demaeght, Osborne, Dermauw, Gohlke, Nauen, Grbic, Tirry, Merzendorfer and Clark2012; Demaeght et al., Reference Demaeght, Osborne, Odman-Naresh, Grbic, Nauen, Merzendorfer, Clark and Van Leeuwen2014). Due to the broad application of MGIs for T. urticae control in hops and other economic crops, it is critical to develop and standardize bioassay methods to evaluate the efficacy of these acaricides, monitor resistance to MGIs, and reveal the mechanisms underlying MGI resistance in field-collected populations.
In our study, we tested three bioassay methods to evaluate ovicidal activity of MGIs on T. urticae (fig. 1). These three methods simulate different MGI exposure scenarios in the field: through direct exposure to eggs, direct exposure to gravid females, or exposure of females and their eggs to MGI residues on leaves. Based on the LC50 values, the first bioassay method, topical exposure of MGIs to freshly laid eggs (0 h), exhibited the most efficiency except that hexythiazox had a slightly lower LC50 in the second method than the first one (tables 1 and 2 and S3), as well as being the quickest and least labor intensive method. The age of the eggs (embryo development) had a significant effect on the efficacy of MGIs. It required at least 30 times the amount of MGIs to kill 50% of fully matured eggs (96 h old) compared to freshly laid eggs (0 h old) (table 1). This result is consistent with a previous study that the toxicity of hexythiazox decreased with egg aging (Marris, Reference Marris1988). We hypothesize that as the eggs age, the increase in eggshell thickness reduces the penetration of MGIs and provides additional protection to the developing embryo. Work is in progress to investigate the developmental expression of the CHS 1 gene in T. urticae. Another study reported a reduction in efficacy of clofentezine toward eggs of laboratory-reared T. cinnabarinus and overwintering Panonychu ulmi as they matured (Aveyard et al., Reference Aveyard, Peregrine and Bryan1986). Furthermore, the duration of exposure also played a crucial role because freshly laid eggs were exposed to MGIs longer than mature eggs.
Our bioassays assessing ovicidal activity of MGIs via different exposure methods provide insights on the spatial and temporal dynamics of application of MGIs for mite control in the field. Our study showed that clofentezine, etoxazole, and hexythiazox applied at the recommended field doses for hops provided effective control of mite eggs on both sprayed and unsprayed leaves (figs 2 and 3). Among these three MGIs, hexythiazox was the most effective acaricide in reducing egg survival when sprayed on gravid females (fig. 3). Hexythiazox was also the only acaricide of the three MGIs tested that caused a significant decrease in the number of eggs laid by gravid females on the sprayed arena within 24 h exposure (fig. 2). In a previous study, etoxazole strongly affected the fecundity of T. urticae gravid females after 72 h exposure (Van Leeuwen et al., Reference Van Leeuwen, Demaeght, Osborne, Dermauw, Gohlke, Nauen, Grbic, Tirry, Merzendorfer and Clark2012). Saenz-de-Cabezon Irigaray et al. reported that etoxazole caused a significant increase in the mortality of eggs laid by sprayed T. urticae females, and etoxazole also affected the fecundity of predatory mites that consumed these eggs (Saenz-de-Cabezon Irigaray & Zalom, Reference Saenz-de-Cabezon Irigaray and Zalom2012). Since the fecundity of T. urticae gravid females and the survival of their eggs can be suppressed by exposure of gravid females with MGIs, it may be feasible to reduce the amount of acaricides used for crop protection and the number of sprays, which would help in maintaining the efficacy of MGIs and delaying the development of MGI resistance in the field.
T. urticae management is largely based on the use of various acaricides with different modes of action. However, the development of cross-resistance to acaricides with the same mode of action and multiple resistance to acaricides with different modes of action is a threat to successful T. urticae management (Asahara et al., Reference Asahara, Uesugi and Osakabe2008). Cross-resistance between clofentezine and hexythiazox was reported in T. urticae (Herron et al., Reference Herron, Edge and Rophail1993) as well as other mite species, such as Panonychus ulmi and P. citri (Thwaite, Reference Thwaite1991; Yamamoto et al., Reference Yamamoto, Yoneda, Hatano and Asada1995). Cross-resistance between etoxazole and hexythiazox has also been reported (Asahara et al., Reference Asahara, Uesugi and Osakabe2008). Our study, for the first time, fully demonstrated that strains selected for resistance to clofentezine, etoxazole, or hexythiazox can develop cross-resistance to the other two MGIs that were not directly used in the selection (table 4). This is not surprising given that clofentezine, etoxazole, and hexythiazox share a common mode of action and bind to the same target site (Van Leeuwen et al., Reference Van Leeuwen, Demaeght, Osborne, Dermauw, Gohlke, Nauen, Grbic, Tirry, Merzendorfer and Clark2012; Demaeght et al., Reference Demaeght, Osborne, Odman-Naresh, Grbic, Nauen, Merzendorfer, Clark and Van Leeuwen2014). Our study showed that T. urticae strains resistant to clofentezine, etoxazole, and hexythiazox also possess resistance to bifenazate and bifenthrin, which are commonly used for mite control on hops (table 4). However, abamectin did not show cross-resistance (table 4), suggesting that abamectin can be used as a rotation partner to cope with resistance to MGIs.
Our bioassay data showed all field-collected T. urticae populations exhibited low-to-moderate level of resistance to clofentezine, etoxazole, and hexythiazox (table 3). However, the target site mutation screening revealed that the I1017F mutation in CHS 1 was found only in one (Prosser 1) of the four field-collected T. urticae populations examined (table 5). Similarly, in previous studies, the I1017F mutation was identified in about 30% T. urticae populations that were associated with etoxazole resistance originating from a wide geographic range, including Europe, Asia, and Africa (Ilias et al., Reference Ilias, Vontas and Tsagkarakou2014). In addition, even after applying strong MGI selection pressure on a subset of the Prosser 1 population for 20 weeks, we still detected both the wild-type and resistant alleles; i.e. despite strong and constant selection pressure the mutant allele was not fixed in the population (table 5). Thus it seems very likely that there are other mechanisms that contribute to MGI resistance in these populations, besides I1017F-mediated target site insensitivity.
Beyond target site insensitivity, elevated expression of detoxification enzymes (P450, GSTs, and CoEs) and transport proteins (ABC transporters) are known to confer pesticide resistance in arthropods (Zhu et al., Reference Zhu, Gujar, Gordon, Haynes, Potter and Palli2013b ). Previous studies have been suggested that detoxification enzymes play roles in clofentezine resistance in T. urticae (Ay & Kara, Reference Ay and Kara2011) and etoxazole resistance in Ph. Persimilis (Yorulmaz Salman et al., Reference Yorulmaz Salman, Aydinli and Ay2015). Cytochrome P450s constitute one of the largest and oldest gene superfamilies in all living organisms (Liu & Zhu, Reference Liu, Zhu, Liu and Kang2011; Feyereisen, Reference Feyereisen and Gilbert2012). Cytochrome P450-mediated detoxification is a very important mechanism in pesticide resistance of arthropods (Zhu et al., Reference Zhu, Parthasarathy, Bai, Woithe, Kaussmann, Nauen, Harrison and Palli2010; Feyereisen, Reference Feyereisen and Gilbert2012; Zhu et al., Reference Zhu, Moural, Shah and Palli2013a ; Riga et al., Reference Riga, Tsakireli, Ilias, Morou, Myridakis, Stephanou, Nauen, Dermauw, Van Leeuwen, Paine and Vontas2014; Van Leeuwen & Dermauw, Reference Van Leeuwen and Dermauw2016; Zhu et al., Reference Zhu, Moural, Nelson and Palli2016a ). The T. urticae genome revealed 86 cytochrome P450 genes, and many of them have been shown to be associated with acaricide resistance (Grbic et al., Reference Grbic, Van Leeuwen, Clark, Rombauts, Rouze, Grbic, Osborne, Dermauw, Ngoc, Ortego, Hernandez-Crespo, Diaz, Martinez, Navajas, Sucena, Magalhaes, Nagy, Pace, Djuranovic, Smagghe, Iga, Christiaens, Veenstra, Ewer, Villalobos, Hutter, Hudson, Velez, Yi, Zeng, Pires-daSilva, Roch, Cazaux, Navarro, Zhurov, Acevedo, Bjelica, Fawcett, Bonnet, Martens, Baele, Wissler, Sanchez-Rodriguez, Tirry, Blais, Demeestere, Henz, Gregory, Mathieu, Verdon, Farinelli, Schmutz, Lindquist, Feyereisen and Van de Peer2011; Dermauw et al., Reference Dermauw, Wybouw, Rombauts, Menten, Vontas, Grbic, Clark, Feyereisen and Van Leeuwen2013). For example, functional characterization of CYP392E10 demonstrated that it can metabolize the acaricides spirodoclofen and spiromesifen (Demaeght et al., Reference Demaeght, Dermauw, Tsakireli, Khajehali, Nauen, Tirry, Vontas, Lummen and Van Leeuwen2013). CYP392A16 and CYP392A11 proved to be able to metabolize abamectin (Riga et al., Reference Riga, Tsakireli, Ilias, Morou, Myridakis, Stephanou, Nauen, Dermauw, Van Leeuwen, Paine and Vontas2014) and METIs (Riga et al., Reference Riga, Myridakis, Tsakireli, Morou, Stephanou, Nauen, Van Leeuwen, Douris and Vontas2015), respectively. Another class of metabolic enzymes, CoEs, belongs to the carboxyl/cholinesterase family, a branch of the α/β-hydrolase fold superfamily. CoE-mediated detoxification has been extensively studied as one of the major mechanisms of pesticide resistance in many arthropod species (Nauen, Reference Nauen2007; Van Leeuwen & Tirry, Reference Van Leeuwen and Tirry2007). In a field-collected multiresistant strain of T. urticae, CoE-mediated metabolic resistance was shown to be the most likely mechanism involved in bifenthrin resistance (Van Leeuwen & Tirry, Reference Van Leeuwen and Tirry2007). In another study, increased synthesis of CoEs was hypothesized to play important roles in clofentezine resistance in T. urticae (Ay & Kara, Reference Ay and Kara2011). Our synergist experiments indicated that cytochrome P450s and CoEs play roles in MGI resistance to clofentezine, etoxazole, and hexythiazox in laboratory-selected and field-collected T. urticae populations (tables 6 and S4). However, identifying the individual P450 and CoE genes that contribute to MGI resistance will require further investigation through transcriptome analysis and functional studies.
In conclusion, our study clearly demonstrated that clofentezine, etoxazole, and hexythiazox are valuable management tools for T. urticae management on hops. Low-to-moderate MGI resistance in T. urticae populations in hop fields appears to be mediated by multiple genes and mechanisms. Positive selection pressure on the I1017F chitin synthase mutation does not appear to be strong in T. urticae populations in hopyards. Instead, metabolic detoxification genes may play important roles in conferring resistance to MGIs both in field-collected populations and in populations that experienced strong selection pressure in the laboratory.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485317000414
Acknowledgements
We thank Tora Brooks, Dan Groenendale, Bianca Mendoza, and Christina Nguyen (Washington State University) for their help on field mite collection and rearing mite populations in the lab. This project was funded by United States Department of Agriculture National Institute of Food and Agriculture Specialty Crop Research Initiative (SCRI) award number 2014-51181-22381, the Hop Research Council, the Washington Hop Commission, and the Washington State Commission on Pesticide Registration. We also appreciate BARTH HAAS for their support in this study.