INTRODUCTION
The Franciscana dolphin, Pontoporia blainvillei (Gervais & d'Orbigny, Reference Gervais and d'Orbigny1844), is a small cetacean endemic to the South-western Atlantic Ocean, ranging in coastal waters from Itaúnas, Brazil (18°25′S 30°42′W) to Península Valdés, Argentina (42°35′S 64°48′W) (Crespo et al., Reference Crespo, Harris and González1998; Bastida et al., Reference Bastida, Rodríguez, Secchi and da Silva2007; Danilewicz et al., Reference Danilewicz, Secchi, Ott, Moreno, Bassoi and Borges-Martins2009). Due to the occurrence of incidental captures throughout much of its geographic distribution (Cappozzo et al., Reference Cappozzo, Negri, Perez, Albareda, Monzon and Corcuera2007; Negri et al., Reference Negri, Denuncio, Panebianco and Cappozzo2012), the Franciscana dolphin is considered the most threatened small cetacean in the western South Atlantic Ocean (Secchi et al., Reference Secchi, Zerbini, Bassoi, Dalla, Möller and Rocha-Campos1997; Bordino & Albareda, Reference Bordino and Albareda2004), and is classified as ‘Vulnerable A3d’ by the International Union for the Conservation of Nature (Reeves et al., Reference Reeves, Dalebout, Jefferson, Karczmarski, Laidre, O'Corry-Crowe, Rojas-Bracho, Secchi, Slooten, Smith, Wang, Zerbini and Zhou2008).
The occurrence of incidental captures is pervasive but not necessarily homogeneous along the species range, which may be due to local differences between coastal fisheries. In addition, the distribution of the species encompasses varied ecological habitats. Based on these considerations, Secchi et al. (Reference Secchi, Danilewicz and Ott2003) suggested the importance of defining stock boundaries in order to guide conservation and management procedures at a local level (Secchi et al., Reference Secchi, Danilewicz and Ott2003). Several authors proposed the existence of distinct management units or stocks within the Franciscana dolphins’ distribution (Pinedo, Reference Pinedo1991; Secchi et al., Reference Secchi, Wang, Murray, Rocha-Campos and White1998). Four distinct management areas (Franciscana Management Areas or FMAs) were established based on geographic distribution, contaminant and parasite loads, vital rates, genetics and information on phenotype. The FMA I ranges from Espírito Santo to Rio de Janeiro in Brazil, the FMA II from São Paulo to Santa Catarina in Brazil, and the FMA III from Rio Grande do Sul in Brazil to Uruguay. The FMA IV includes the coasts of Buenos Aires and Río Negro provinces in Argentina (Figure 1).
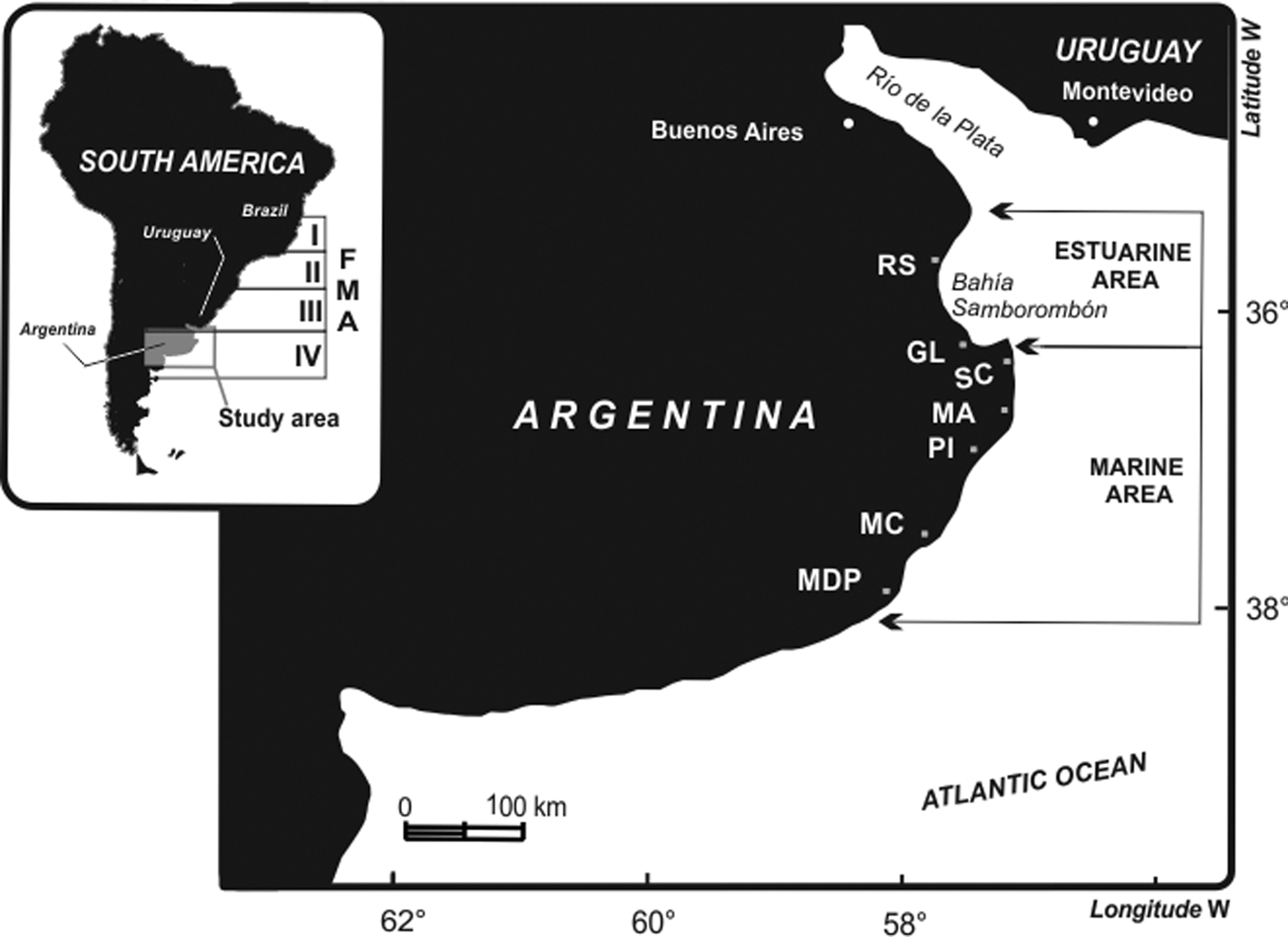
Fig. 1. Northern coast of Argentina showing estuarine and marine areas with artisanal fisheries communities where dolphins were incidentally captured. RS, Río Salado; GL, General Lavalle; SC, San Clemente del Tuyú; MA, Mar de Ajó; PI, Pinamar; MC, Mar Chiquita; MDP, Mar del Plata. In the left upper corner, FMA: Franciscana Management Areas (adapted from Secchi et al., Reference Secchi, Danilewicz and Ott2003).
Recently, several authors have proposed the existence of further population structure both in the species’ overall distribution and within the FMA IV (Cunha et al., Reference Cunha, Medeiros, Barbosa, Cremer and Marigo2014; Gariboldi et al., Reference Gariboldi, Túnez, Dejean, Failla, Vitullo, Negri and Cappozzo2015). Regarding the FMA IV, based on the analysis of microsatellite markers and mitochondrial DNA, Mendez et al. (Reference Mendez, Rossenbaum and Bordino2008, Reference Mendez, Rossenbaum, Subramaniam, Yackulic and Bordino2010) reported the existence of at least two (and probably three) populations in the coastal waters of Argentina, with a genetically isolated population in the estuarine area of Bahía Samborombón. Gariboldi et al. (Reference Gariboldi, Túnez, Dejean, Failla, Vitullo, Negri and Cappozzo2015), based on the analysis of the mitochondrial DNA control region, suggested that the FMA IV comprises four Franciscana dolphin populations: Bahia Samborombón, Cabo San Antonio and East Buenos Aires, South-west Buenos Aires and Rio Negro and Monte Hermoso.
Knowledge of growth patterns is also important for understanding the life history strategy of the species, which describes how individuals allocate resources to growth, reproduction and survival. Data on age-specific growth and reproductive rates, combined with survival rates, are essential for comparing life history strategies (Chivers et al., Reference Chivers, Dizon, Gearin and Robertson2002). Information on somatic growth is also very important as a tool to identify stocks. Morphological characteristics have commonly been used to define population boundaries in cetacean species with continuous geographic distributions (Messenger & McGuire, Reference Messenger and McGuire1998).
Information on growth curves for Franciscana dolphins from Argentina is scarce, consisting of mostly grey literature (i.e. conference papers, dissertations) (e.g. Negri, Reference Negri2010; Denuncio, Reference Denuncio2012) and one publication for the southernmost population (South FMA IV; Negri et al., Reference Negri, Denuncio and Cappozzoin press). This study presents new information on age composition and growth curves for Franciscana dolphins from the northern waters of Argentina (hereafter: North FMA IV), the area with the highest reported levels of incidental mortality of the species in Argentina (Bordino & Albareda, Reference Bordino and Albareda2004; Cappozzo et al., Reference Cappozzo, Negri, Perez, Albareda, Monzon and Corcuera2007).
MATERIALS AND METHODS
This study was conducted along the coastal waters of Northern Buenos Aires Province in Argentina (North FMA IV), from Bahía Samborombón (36°56′S 57°70′W) to Mar del Plata (38°00′S 57°33′W) (Figure 1). The study area includes two different habitats: an estuarine area and a marine coastal area. The estuarine area comprises the Bahía Samborombón, the southern margin of the La Plata River Estuary, characterized by shallow and brackish waters with a strong salinity gradient from 0–33 psu (Boschi, Reference Boschi1988, Guerrero et al., Reference Guerrero, Acha, Framiñan and Lasta1997). The marine area extends south of the Bahía Samborombón and is characterized by coastal marine waters with wide sandy beaches and a salinity range of 33.5–33.7 psu (Guerrero et al., Reference Guerrero, Acha, Framiñan and Lasta1997; Figure 1).
A total of 108 Franciscanas (64 males and 44 females; 42 from the estuarine area and 66 from the marine area) incidentally caught in artisanal fishing nets or stranded on the beach with clear external marks of fishing interaction between 2000 to 2011 were studied. Recorded information from each specimen included sex, weight and total length (TL). Sex was determined externally. TL was measured as a straight line from the tip of the rostrum to the fluke notch (Norris, Reference Norris1961).
Almost all specimens were incidentally captured animals collected in situ with the cooperation of fishermen from different localities (Figure 1). Fresh stranded animals on beaches of the marine coast with external evidence of interaction with fisheries were also included in this study. The information of stomach content of the specimens (Denuncio, Reference Denuncio2012) were used to corroborate the origin of the specimens, i.e. estuarine or marine. The presence of cephalopod beaks, which accumulate in the stomachs, was considered a strong evidence of Franciscanas from marine waters (Pineda et al., Reference Pineda, Aubone and Brunetti1996; Rodríguez et al., Reference Rodríguez, Rivero and Bastida2002).
Teeth were extracted from the centre of the lower left jaw for age estimation and processed following established procedures (Kasuya & Brownell, Reference Kasuya and Brownell1979; Pinedo & Hohn, Reference Pinedo and Hohn2000; Botta et al., Reference Botta, Müelbert, Secchi, Danilewicz, Negri, Cappozzo and Hohn2010). Teeth were decalcified in RDO® (a commercially available decalcifying agent) and sectioned longitudinally on a freezing microtome at a thickness of 25 μm. Sections were stained with Mayer's haematoxylin and mounted on microscope slides in 100% glycerin. Blind counts were made by two observers and the dataset was then compared between them. Age was estimated from the number of Growth Layer Groups (GLGs; Perrin & Myrick, Reference Perrin and Myrick1980) present in the dentine and cementum. There is evidence to support that each GLG represents 1 year of age in Franciscana dolphins (Kasuya & Brownell, Reference Kasuya and Brownell1979; Pinedo, Reference Pinedo1991; Pinedo & Hohn, Reference Pinedo and Hohn2000).
Growth models were fitted to length-at-age data, for the total dataset (i.e. North FMA IV), and for estuarine and marine areas separately: the von Bertalanffy (Reference von Bertalanffy1938; VBGM) and Gompertz (Ricker, Reference Ricker1975, Zullinger et al., Reference Zullinger, Ricklefs, Redford and Mace1984; GGM) growth models.
where:
• TL corresponds to the length-at-age t.
• TL∞ is the asymptotic length.
• e is the base of the natural logarithm.
• t 0 is a parameter for a better fit of the curve and represents the theoretical age at which the animal's length equals zero.
• k is a parameter of the curve that determines the rate at which the animal reaches L∞.
Data for males and females were separated due to sexual dimorphism in total length (Kasuya & Brownell, Reference Kasuya and Brownell1979; Pinedo, Reference Pinedo1991) and data for specimens from estuarine and marine areas were separated to evaluate geographic differences within the study area (Figure 1). All growth model parameters were estimated using the non-linear iterative Quasi-Newton method. The goodness-of-fit of each model was assessed by examining residual sum of squares (SQ), the coefficient of determination (r 2), the level of significance (P < 0.05) and standard residual analysis. The maximum likelihood method was used to compare von Bertalanffy parameters among sexes and areas following Cerrato (Reference Cerrato1990).
In order to better adjust the growth curve, decimal ages were used for yearlings (<1 GLG). November was considered the birth month for all yearlings based on information regarding birthing dates of Franciscana dolphins in the coastal waters of Northern Argentina (Denuncio et al., Reference Denuncio, Bastida, Danilewicz, Morón, Rodríguez Heredia and Rodríguez2013).
RESULTS
Age and length data composition
Individuals up to 4 years old represented 92% of the specimens analysed. The modal age was less than 1 year (<1) in both sexes and areas (Table 1), and represented 60% of the total sample. Total length (TL) ranged from 62.3 to 144 cm for female and from 56.8 to 140 cm for male (Table 1).
Table 1. Age (GLG) and total length (TL in cm) ranges for Franciscana dolphins discriminated to estuarine and marine area.
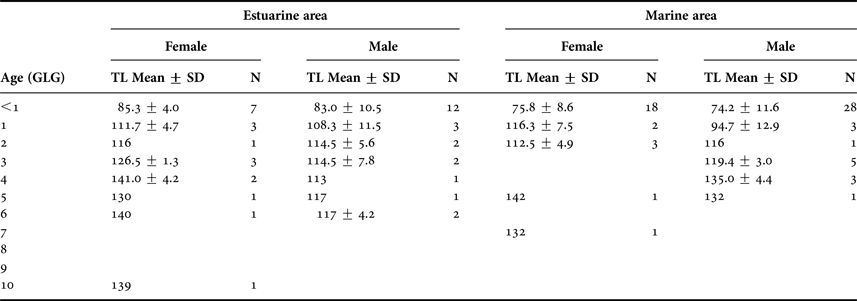
N, size sample; SD, standard deviance.
Franciscana dolphins from the estuarine area ranged from 0 to 10 years old, whereas animals from the marine area ranged from 0 to 7 years old. The oldest individual in the estuarine area was a 10-year-old female with a TL of 139 cm, while in the marine area, it was a 7-year-old female with a TL of 132 cm (Table 1). The age distribution of Franciscanas from the marine area was strongly biased toward younger specimens and included a large number of males <6 months old.
Growth models
The von Bertalanffy Growth Model (VBGM) and Gompertz Growth Model (GGM) provided growth curves with a good fit for both males and females of North FMA IV (all dataset) and for estuarine and marine ones (r 2 > 0.87, Table 2).
Table 2. Parameter values from the von Bertalanffy (VBGM) and Gompertz growth models (GMM) fit to age-at-length data of male and female Franciscanas from estuarine and marine areas in the northern waters of Argentina.
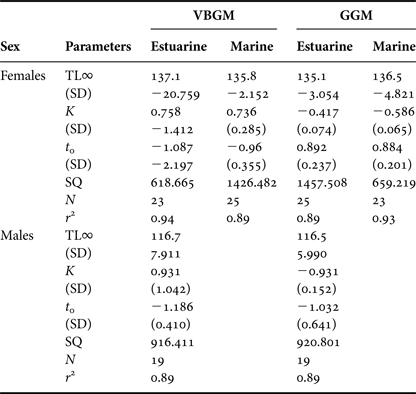
TL∞: asymptotic length (cm); K, growth constant; t 0, theoretical age at which the length of the animal is zero; SQ, residual sum of squares; r 2, the variance in the data explained by the model. Values in parentheses are standard errors of the estimates.
For the North FMA IV specimens and according to the VBGM, female specimens reached the asymptotic length (TL∞) at 136.3 cm, whereas males specimens reached the TL∞ at 122.1 cm (Figure 2A; Table 2). The hypothetical size at birth (total length at age 0, t 0) was 70.3 and 68 cm for females and males respectively. The Maximum likelihood test results confirmed that differences in all growth parameters between sexes in this area were statistically significant (F 1.101 = 39.384; P < 0.001 for TL∞. F 1.101 = 5.848; P < 0.001 for k and F 1.101 = 2.464; P = 0.120 for t 0).
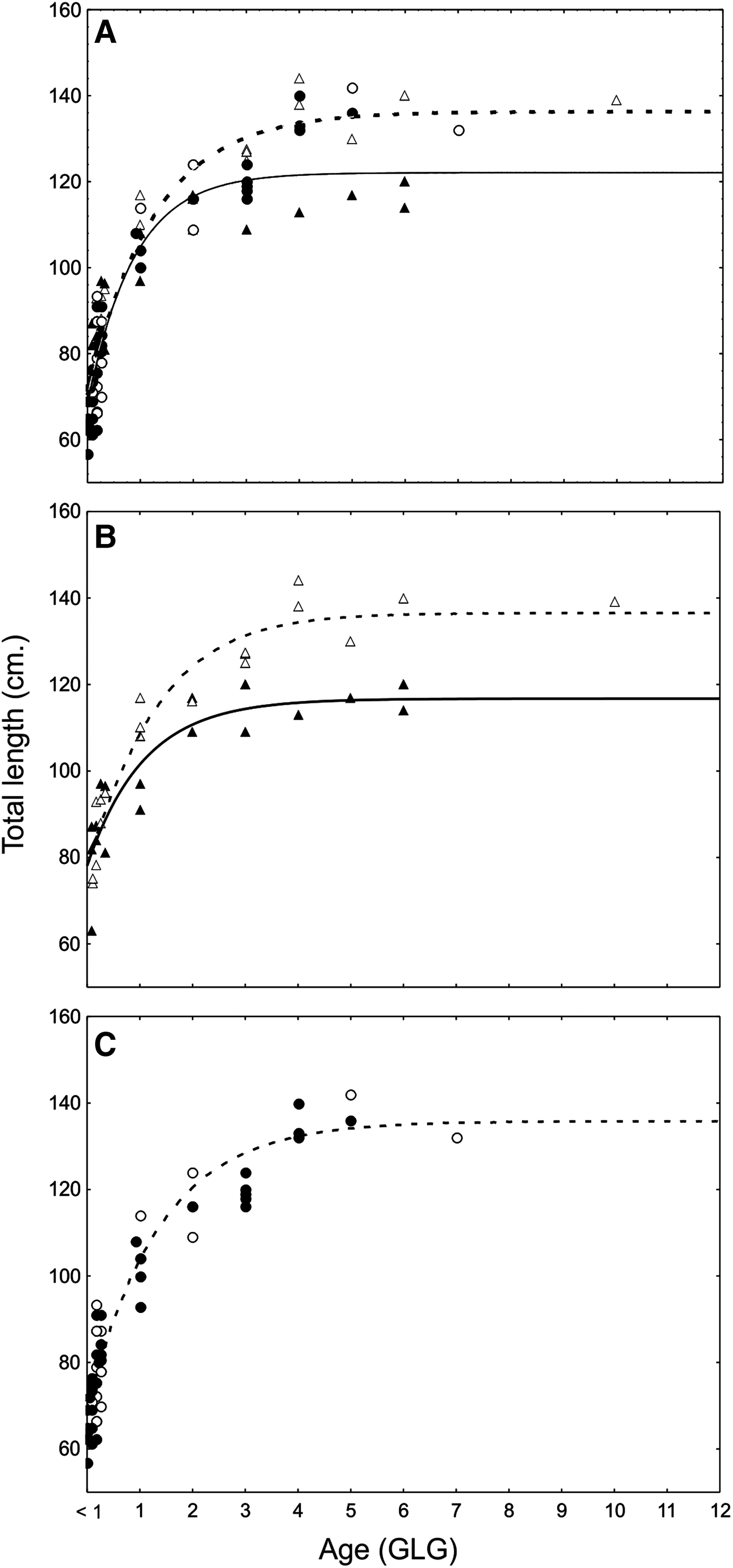
Fig. 2. von Bertalanffy growth curves of females (dashed lines and open symbols) and males (continuous line and full symbols) Franciscana dolphins from the northern coast of Argentina or North FMA IV (A), estuarine area (B and triangle symbols) and marine (C and circle symbols).
Estuarine female specimens reached the asymptotic length (TL∞) at 137.1 cm, whereas estuarine male specimens reached the TL∞ at 116.7 cm (Figure 2B; Table 2); and the hypothetical size at birth (total length at age 0, t 0) was 77.1 and 78 cm for females and males respectively. The Maximum likelihood test results confirmed that differences in all growth parameters between sexes in this area were statistically significant (F 1.36 = 71.976; P < 0.001 for TL∞; F 1.36 = 4.284; P = 0.003 for k; F 1.36 = 1.799; P = 0.041 for t 0).
Marine female specimens reached the asymptotic length (TL∞) at 135.8 cm (Figure 2C; Table 2) and the t 0 68.9 cm. The curve for marine males and its parameters are not presented because of the lack of adult specimens and the strong bias in sample composition toward yearling specimens.
Differences in the growth parameters between estuarine and marine females were not statistically significant (Maximum likelihood test results; F 1.38 = 0.147; P = 0.703 for TL∞; F 1.38 = 0.021; P = 0.884 for k; F 1.38 = 0.385; P = 0.539 for t 0).
DISCUSSION
The age composition of this study was strongly biased toward young specimens. More than 90% of the specimens were calves and juveniles <4 years old, and those specimens <1 year old account for over 50% of the total specimens. Based on the nature of our sample structure, we cannot rule out a possible effect on the growth models fitted. In this sense, Botta et al. (Reference Botta, Müelbert, Secchi, Danilewicz, Negri, Cappozzo and Hohn2010) studied the age and growth of Franciscanas from Rio Grande do Sul State in Brazil, finding similar growth deviation toward young specimens. In this study the authors fitted growth curves with and without yearling (<1 year old) specimens and no effect on growth trajectories was found.
This age composition may be related to the higher vulnerability of younger specimens to fishing nets (Secchi et al., Reference Secchi, Danilewicz and Ott2003; Negri et al., Reference Negri, Panebianco, Denuncio, Rodríguez and Cappozzo2014). The oldest male measured within the study area was 7 years old, and the oldest female was 10 years old. The maximum age in Argentinean waters was 13 years old in Southern Buenos Aires Province (South FMA IV, Negri et al., Reference Negri, Denuncio and Cappozzoin press). The maximum observed age for males was 16 years (Kasuya & Brownell, Reference Kasuya and Brownell1979) in Uruguayan waters and 17 years for a specimen from Rio Grande do Sul, Brazil (Botta et al., Reference Botta, Müelbert, Secchi, Danilewicz, Negri, Cappozzo and Hohn2010). The maximum observed age recorded for a female was 21 years for another specimen from Rio Grande do Sul, Brazil (Pinedo, Reference Pinedo1991). It should be noted that both males and females rarely exceeded 10 years old due to the high mortality rate (Secchi et al., Reference Secchi, Danilewicz and Ott2003).
The VBGM and GGM are the models most frequently used in growth studies of cetaceans (Zullinger et al., Reference Zullinger, Ricklefs, Redford and Mace1984; Hohn et al., Reference Hohn, Read, Fernandez, Vidal and Findley1996; Mattson et al., Reference Mattson, Mullin and Ingram2006) and are commonly used in growth studies of the Franciscana dolphin throughout its range (Di Beneditto & Ramos, Reference Di Beneditto and Ramos2001; Barreto & Rosas, Reference Barreto and Rosas2006; Botta et al., Reference Botta, Müelbert, Secchi, Danilewicz, Negri, Cappozzo and Hohn2010; Negri, Reference Negri2010) (Table 3).
Table 3. Comparative asymptotic total length (TL∞) for Franciscana dolphins from the FMAs proposed by Secchi et al. (Reference Secchi, Danilewicz and Ott2003) and reassessed by Cunha et al. (Reference Cunha, Medeiros, Barbosa, Cremer and Marigo2014).
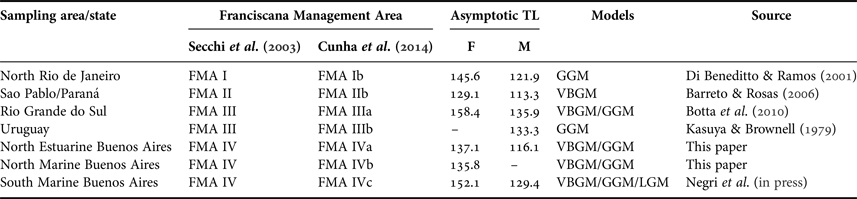
F, female; M, male; GGM, Gompertz Growth Model; VBGM, von Bertalanffy Growth Model; LGM, Logistic Growth Model.
The current study presents the first growth models published to date for Franciscana dolphins from the northern coast of Buenos Aires province in Argentina (North FMA IV in this paper). Based on information of the asymptotic length from these models, the data indicate that Franciscana dolphins from North FMA IV area were smaller than Franciscanas from South FMA IV Buenos Aires Province (Negri et al., Reference Negri, Denuncio and Cappozzoin press), Rio de Janeiro State in Brazil (Di Beneditto & Ramos, Reference Di Beneditto and Ramos2001), Uruguay (Kasuya & Brownell, Reference Kasuya and Brownell1979) and Rio Grande do Sul State in Brazil (Botta et al., Reference Botta, Müelbert, Secchi, Danilewicz, Negri, Cappozzo and Hohn2010). It should also be noted that the asymptotic values estimated for this study were similar to those estimated from Sao Paulo–Paraná State's Franciscana dolphins, from Brazil (Barreto & Rosas, Reference Barreto and Rosas2006) (Table 3). Similar differences were found in the external measures between adult specimens of the four FMAs (Barbato et al., Reference Barbato, Secchi, Di Beneditto, Ramos, Bertozzi, Marigo, Bordino and Kinas2012). However, the statistical confirmation of this could only be performed by comparing the set of rough data from all geographic areas.
Pinedo (Reference Pinedo1991) suggested the existence of two geographic forms of Franciscanas, with large individuals occurring in the south of the geographic distribution of the species and smaller ones in the north of the distribution. This is in accordance with the pattern predicted by Bergmann's Rule (Mayr, Reference Mayr1970), which states that animals of a species occupying colder regions are larger. However, Franciscana dolphins from the North FMA IV were smaller than nearby individuals from FMA III (Uruguay and Southern Brazil), suggesting that environmental factors were not solely responsible for the variation in size, as was previously suggested by Ramos et al. (Reference Ramos, Di Beneditto and Lima2000) and also discarding a mere clinal variation of the species.
Franciscana dolphins from the estuarine area exhibited reverse sexual size dimorphism, which has been reported throughout the species’ geographic distribution (see Table 3). Total length measurements of marine and estuarine Franciscana dolphins also demonstrated this dimorphism. The implications of this dimorphism for the reproductive behaviour of this species is unknown. Information regarding reproduction in Franciscana dolphins from Argentina is limited. Recent research suggested a monogamous mating system in marine Franciscanas (Danilewicz et al., Reference Danilewicz, Claver, Carrera, Secchi and Fontoura2004; Panebianco et al., Reference Panebianco, Negri and Cappozzo2012). This is supported by the absence of male secondary sexual characteristics and the supposed absence of male fighting. Behavioural studies reported pairings of unrelated female and male adult Franciscanas, which further supports the hypothesis of a monogamous mating system (Wells et al., Reference Wells, Bordino and Douglas2013).
Not only did the asymptotic length demonstrate that Franciscana dolphins from North FMA IV are smaller than in nearby areas (FMA III and South FMA IV): the hypothetical size at birth in this study (67–78 cm total length at age 0) was also lower than those estimated for individuals from the south of Buenos Aires Province (79.6–81.6 cm) (Negri, Reference Negri2010), and from Uruguay and Brazil (70 to 85 cm) (Kasuya & Brownell, Reference Kasuya and Brownell1979; Harrison et al., Reference Harrison, Bryden, McBrearty and Brownell1981; Danilewicz, Reference Danilewicz2003; Ramos et al., Reference Ramos, Di Beneditto and Lima2000; Botta et al., Reference Botta, Müelbert, Secchi, Danilewicz, Negri, Cappozzo and Hohn2010). Moreover, the estimated size-at-birth through this method were higher than direct measurements of neonates (64 cm) from the same study area and also exceeded values estimated with the overlap method of Borjesson & Read (Reference Borjesson and Read2003) (60.7 cm) (Denuncio et al., Reference Denuncio, Bastida, Danilewicz, Morón, Rodríguez Heredia and Rodríguez2013).
Several authors highlighted the need to re-evaluate the current Franciscana Management Areas. Cunha et al. (Reference Cunha, Medeiros, Barbosa, Cremer and Marigo2014) proposed that the FMAs should be subdivided into a larger number of units than previously established by Secchi et al. (Reference Secchi, Danilewicz and Ott2003) and urgently recommended re-assessment of the current FMA boundaries.
The population structure of Franciscanas within FMA IV is thought to be complex. Mendez et al. (Reference Mendez, Rossenbaum and Bordino2008) suggested the existence of at least two distinct populations along the coast of Argentina, and suggested that Bahía Samborombón (the estuarine area in this study) contained an isolated population. More recently, based on the analysis of the mitochondrial control region, Gariboldi et al., Reference Gariboldi, Túnez, Dejean, Failla, Vitullo, Negri and Cappozzo2015, suggested that four different populations are found in FMA IV: Bahia Samborombón (FMA IV-1) (estuarine area in this study), Cabo San Antonio (FMA IV-2) (marine areas in this study), South-west Buenos Aires (FMA IV-3) and Rio Negro and Monte Hermoso (FMA IV-4). Biological and ecological factors may contribute to the differences between estuarine and marine specimens within the north part of FMA IV (diet: Rodríguez et al., Reference Rodríguez, Rivero and Bastida2002; Denuncio et al., Reference Denuncio, Bastida, Dassis, Giardino, Gerpe and Rodríguez2011; Denuncio, Reference Denuncio2012; body condition: Denuncio, Reference Denuncio2012), however, no observable differences in asymptotic growth values or the size of newborn animals were found in our study.
We suspect that the absence of differences is related to the age distribution of the samples previously mentioned. Further analyses including a higher number of adult animals from both areas should be run once more adults are available.
The North FMA IV has the highest reported mortality levels of Franciscana dolphins within the FMA IV (Bordino & Albareda, Reference Bordino and Albareda2004; Cappozzo et al., Reference Cappozzo, Negri, Perez, Albareda, Monzon and Corcuera2007; Negri et al., Reference Negri, Denuncio, Panebianco and Cappozzo2012). Caswell et al. (Reference Caswell, Brault, Read and Smith1998) speculated that the growth rate of species under high levels of bycatch might increase. While our results demonstrated that Franciscana dolphins from this area were smaller than the counterpart of the south, the information is insufficient to make a conclusive statement on the effect of incidental capture on the growth of this species. Further investigation into this topic is warranted.
To conclude, this paper contributes to the existing pool of information on the growth parameters from North FMA IV Franciscana dolphins. In addition, this work may contribute to differentiate between distinct populations of Franciscana dolphins and to the re-assessment of the species’ management unit boundaries in Argentina.
ACKNOWLEDGEMENTS
We gratefully acknowledge Fundación Mundo Marino and our local fishermen. We especially thank M. Marchi, L. Mulder, L. Ramos, J. Arce and F. Spina for their valuable cooperation during our studies. We also thank our colleagues M. Dassis, G. Giardino, A. Mandiola and J. Bastida from the Marine Mammal Research Group of UNMDP for their assistance with the necropsies. A very special thanks to Marité from the Coastal Marine Station and ‘J.J. Nagera’ from UNMDP who helped organize logistics for the necropsies. Also many thanks to William Rossiter from Cetacean Society International. Finally, we thank Peter Miles for the thorough review of the manuscript and the English. This study was conducted as part of the Post-PhD thesis research of PD at UNMDP and CONICET.
FINANCIAL SUPPORT
Financial support for this work was provided by Universidad Nacional de Mar del Plata (UNMDP15/E335), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET PIP 0348/10), Agencia Nacional de Promoción Científica y Tecnológica de Argentina (PICT 1834/11) and Cetacean Society International (CSI).







