Introduction
Body-size changes in insular species are a focus of enduring interest worldwide. This study evaluates large body size in Keen’s mouse, Peromyscus keeni, by comparing the sizes of Late Pleistocene, early Holocene, and modern specimens from Vancouver Island, Canada. It has long been suggested that large body size in Keen’s mouse is a relictual characteristic that developed in times of geographic separation from P. maniculatus. Based on this species’ limited coastal range, which extends along the Pacific Coast of northwestern North America from southeast Alaska to Oregon, McTaggart-Cowan (Reference McTaggart-Cowan1935) inferred that large body size in Keen’s mouse (then called P. sitkenensis) is a relictual characteristic. This model suggested that during Pleistocene glacials, Keen’s mouse evolved and persisted on unglaciated outer islands while ice covered inner islands and the adjoining mainland. Since this hypothesis was presented, a number of studies of body size in island faunas have been conducted, and additional models have been presented to explain variation in island species (Foster Reference Foster1964; MacArthur and Wilson Reference MacArthur and Wilson1967; Case Reference Case1978; Lomolino Reference Lomolino2005). However, the timing of body-size trends in Keen’s mouse have not been examined in detail.
This study describes the vertebrate faunal assemblage from Arch-2 Cave, a Late Pleistocene, early postglacial assemblage from north Vancouver Island, which includes abundant deer mice. Late Pleistocene specimens from Arch-2 Cave are supplemented with early Holocene deer mouse specimens from nearby Pellucidar Cave, and the sizes of these specimens are compared with those of modern P. keeni and P. maniculatus. If McTaggart-Cowan (Reference McTaggart-Cowan1935) was correct and large size is a relictual characteristic in Keen’s mouse, early postglacial deer mouse specimens are expected to be as large as modern Keen’s mice. Although early postglacial vertebrate faunas have been reported from several locations on Vancouver Island (Harington Reference Harington1996; Al-Suwaidi et al. Reference Al-Suwaidi, Ward, Wilson, Hebda, Nagorsen, Marshall, Ghaleb, Wigen and Enkin2006; Steffen et al. Reference Steffen, Hebda and McLaren2008), the Arch-2 Cave assemblage is significant because it expands the record of small vertebrates from this period, with implications for biogeography and body size. The first available radiocarbon age estimates from Arch-2 Cave are also provided.
Localities
Arch-2 Cave is hosted in Quatsino Limestone (Nixon et al. Reference Nixon, Kelman, Stevenson, Stokes and Johnston2006) and rests at an elevation of approximately 600 meters above modern sea level on the steep eastern slopes of Nimpkish Lake, northeast Vancouver Island (Fig. 1). A large west-facing walk-in entrance to the cave leads into an ~15 m long south-trending chamber to a branch passage on the east wall that connects through a narrow tube to a smaller second chamber. Evaluative excavations in these two chambers recovered the assemblage of 212 faunal specimens reported here. Seventeen deer mouse M1 molars from Pellucidar Cave, a locality within 2 km of Arch-2 Cave and at approximately the same elevation, are also included in this study. Pellucidar Cave consists of a number of connected passages, the first 60 m of which were surveyed and mapped in 2007. The Pellucidar Cave specimens included in this study date to the early Holocene and were recovered from a subsurface evaluative excavation unit, EU1 (Steffen et al. Reference Steffen, Hebda and McLaren2008). Nagorsen and Keddie (Reference Nagorsen and Keddie2000) conducted a previous survey of the locality and also provide a description.

Figure 1 A map of Vancouver Island, British Columbia, Canada, showing the sites discussed in the text.
Materials and Methods
Excavation and Sieving
Subsurface testing and sediment-processing methods were similar at both sites. At Arch-2 Cave, 12 subsurface evaluative units (EUs) that were 50 cm long by 50 cm wide to varying depths were configured into six excavation units. Sediment was excavated by hand with a trowel in 5 cm depth increments or natural layers, and was washed through a fine mesh screen with 0.25 mm openings for recovery of small faunal elements. Faunal specimens from Pellucidar Cave were excavated from a 50 cm by 50 cm evaluative unit, EU1, which was excavated to 80 cm depth below the unit datum. Sediment from EU1 at Pellucidar Cave was washed through fine mesh with <1 mm openings and, as with the material from Arch-2 Cave, bone specimens were picked from dried sediment.
Radiocarbon Dating
Eight bone samples, including six from Arch-2 Cave and two from Pellucidar Cave, were analyzed at the Keck Carbon Cycle Accelerator Mass Spectrometry (AMS) Laboratory, University of California, Irvine (Table 1). Results have been corrected for isotopic fractionation with δ13C values measured on prepared graphite using the AMS spectrometer. Radiocarbon was measured on ultrafiltered (>30 kD) collagen (Brown et al. Reference Brown, Nelson, Vogel and Southon1988; Beaumont et al. Reference Beaumont, Beverly, Southon and Taylor2010). δ13C and δ15N values were measured to a precision of <0.1‰ on aliquots of ultrafiltered collagen with a Fisons NA1500NC elemental analyzer/Finnigan Delta Plus isotope ratio mass spectrometer. Calendar age estimates from radiocarbon determinations were calculated with the IntCal3 calibration curve (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Grootes, Guilderson, Haflidason, Hajdas, HattŽ, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013) in OxCal, Version 4.2 (Bronk Ramsey Reference Bronk Ramsey2009).
Table 1 Radiocarbon age estimates from Pellucidar and Arch-2 caves on Vancouver Island, Canada. Calendar age ranges are calculated with the IntCal13 calibration curve (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Grootes, Guilderson, Haflidason, Hajdas, HattŽ, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013) in OxCal 4.2 (Bronk Ramsey et al. 2009). ~ indicates the bone specimen was too small to provide extra material for analyses of stable isotopes and the C:N ratio.

Identification and Taxonomic Assignment
Though closely related in genus Peromyscus, P. maniculatus and P. keeni have been separated by criteria of tail to head + body ratio and tail length (Sheppe Reference Sheppe1961; Allard et al. Reference Allard, Gunn and Greenbaum1987), karyotypes (Gunn and Greenbaum Reference Gunn and Greenbaum1986), and a mitochondrial cytochrome-b gene (Zheng et al. Reference Zheng, Abrogast and Kenagy2003; Lucid and Cook Reference Lucid and Cook2004). However, because no features of P. maniculatus and P. keeni molars consistently distinguish them, linear discriminant analysis was used on tooth measurements to assign the ancient specimens from Vancouver Island to one of the two species. For this, the Late Pleistocene and early Holocene deer mouse molars were identified by comparison with modern specimens of P. maniculatus and P. keeni, the two extant Peromyscus species on Vancouver Island (McTaggart-Cowan and Guiguet Reference McTaggart-Cowan and Guiguet1965; Wilson and Reeder Reference Wilson and Reeder2005). Then the size of ancient and modern specimens was compared statistically.
Modern specimens measured in this study are from two locations on north Vancouver Island: (1) Woss, located about 30 km SE of the fossil localities; (2) and Brooks Peninsula, located about 60 km to the SW. Specimens from Woss include 19 P. maniculatus and 7 P. keeni individuals. The Brooks Peninsula sample consists of 19 P. maniculatus and 15 P. keeni specimens. A commonly used criterion of tail length >96 mm in adult mice (Allard et al. Reference Allard, Gunn and Greenbaum1987; Zheng et al. Reference Zheng, Abrogast and Kenagy2003) differentiated P. maniculatus from P. keeni in the modern specimens. The specimens are housed in the permanent collections of the Royal BC Museum, Victoria, Canada, and are listed in the Appendix.
Ancient and modern specimens were compared based on two measurements of upper first molars (M1) in occlusal view: greatest anterioposterior diameter was measured from the anterior margin of the anterocone to the posterior margin of each tooth; and transverse diameter was measured across the hypocone and metacone. In most cases right-sided molars were measured in modern specimens; however, left sides were measured in a few specimens when right molars were damaged or not present. Sexes are pooled in the analysis because previous studies (Dice Reference Dice1949; Sheppe Reference Sheppe1961; Allard et al. Reference Allard, Gunn and Greenbaum1987) found insufficient morphological variation to justify separating deer mice into sex classes. The modern specimens are from adults; the ancient molars are from juveniles and adults. Each molar was viewed under magnification with a Leica MZ6 microscope and photographed with a Leica EC3 camera. Measurements were to the nearest 0.01 mm with LAS EZ software, Version 2.0. Statistical tests were conducted with the SPSS Statistics, Version 20 program.
Results
Strata and Age
Arch-2 Cave
The sediment sequence varies in subsurface contexts but can be summarized as follows. In the east portion of the first cave chamber (EU5), rounded boulders, large cobbles, and gray silty-sand underlie diamicton of pea- to cobble-sized, subrounded to angular gravel with sand and silt. In the west and central areas of the first cave chamber (EU1 and EU6), bedrock and limestone cobbles underlie fluvial sand and gravel. A thin (<20 cm) stratum of largely disturbed dark-brown silt mixed with sand, gravel, and limestone clasts overlies deeper deposits. Near the back of the first chamber, EU2 was excavated into an existing pit that consists of disturbed sediments underlain at a depth of 1 m below the unit datum (DBD) by fluvial sand. In the second cave chamber, EU3 was a 100 cm long by 50 cm wide unit excavated to a maximum depth of 70 cm DBD (Fig. 2). Sediment in this unit consists of horizontally bedded tan-gray silt, clay, and sand lenses with few subrounded exotic rocks and angular limestone fragments that were more abundant in the west than in the east portion of the unit (stratum 1). Mottling indicated saturation in quiet water. Overlying deposits were an ~10 cm thick dark-brown silt and limestone fragments (stratum 2). Sediment in EU4 consisted of horizontally bedded tan-gray sandy-silt that coarsened upward, particularly from ~50 cm DBD, to diamicton consisting of subrounded to angular silty-sand and gravel with cobbles. These sediments were overlain, in turn, by a thin (<10 cm) layer of dark-brown silt with sand, gravel, and angular limestone fragments. Most of the small vertebrate specimens analyzed in this study were recovered from EU3 (Supplementary Table 1). These faunal specimens appear to have entered the cave chamber through an opening at the top of a sediment-choked upward-trending passage above EU3. Overlying dark-brown silt at EU3 and throughout the cave is windblown Holocene sediment.

Figure 2 A, Sediment profile of the of the east wall of evaluative unit 3 (EU-3) at Arch-2 Cave, Vancouver Island. B, Sediment profile of the south wall of evaluative unit 1 (EU-1) at Pellucidar Cave, Vancouver Island.
Six bone samples from subsurface deposits in EU3 and EU4 at Arch-2 Cave gave radiocarbon age estimates of 11,960±45 BP (14,004–13,637 cal BP) through 12,370±35 BP (14,695–14,148 cal BP) (Table 1). Because the mouse bone (dentaries without molars) samples UCIAMS 118259, UCIAMS 118260, and UCIAMS 118261 were small, there was insufficient >30 kDa collagen for additional isotopic analysis of δ13C and δ15N and the C:N atomic ratio of the purified collagen. The samples were small simply because the bone specimens were small, not because the yields were low. C:N atomic ratio can provide some information on the state of collagen preservation and the extent of contamination by exogenous carbon, where values of more than 5 indicate high levels diagenesis and or contamination (Tisnérat-Laborde et al. Reference Tisnérat-Laborde, Valladas, Kaltnecker and Arnold2003). Without C:N ratios the three small samples must be treated with caution. However, the collagen yields were relatively high (3–4%), and the product was white fluffy collagen, consistent with good bone preservation and an absence of any contamination by exogenous carbon.
The age of samples UCIAMS 118259, UCIAMS 118260, UCIAMS 118261, and UCIAMS 73321 (a long bone fragment of a mouse-sized mammal) that were recovered from silty sediment in stratum 2 at EU3 are statistically significantly younger at the 95% confidence level than the age of the U. arctos (m2) sample UCIAMS 118265 that was recovered from EU4 in overlying coarse diamicton, likely indicating that UCIAMS 118265 was old when it was deposited. Similarly, the large mammal (bear) rib sample UCIAMS 73320 that was recovered from EU3, 35–40 cm DBD, is statistically significantly older than the small mammal bone sample UCIAMS 118260 collected from the same depth and UCIAMS 73321 recovered below it at 60–65 cm DBD, suggesting that the bear rib UCIAMS 73320 was old when it was deposited. No pitting or disturbance of the sediments by rodents or other animals was observed during excavation.
Pellucidar Cave
Seventeen deer mouse M1s from Pellucidar Cave included in this study were recovered from EU1, a 50 cm square unit excavated into a sediment cone. The sediments from this unit were underlain by cave floor deposits of sand and gravel with cobbles and minor silt, which are a relict streambed (Fig. 2; stratum 1). A bone (small vertebrate postcranial) sample from this underling stratum gave a radiocarbon age estimate of 10,535±50 BP (12,658–12,250 cal BP) (Table 1). The cave floor deposit was overlain by layers of silt with minor sand and gravel containing calcite and charcoal, which were deposited gradually through a chimney opening in the ceiling of the cave (stratum 2). A thin (<1 cm) layer of moist, dark-brown eolian silt overlies sediment cone deposits. Radiocarbon analyses of a small mammal bone sample (a marmot-sized rib fragment) from the sediment cone deposit at 20–30 cm DBD, gave an age estimate of 8,660±30 BP (9,679–9,544 cal BP), confirming early Holocene age for the vertebrate specimens from the sediment cone (Table 1).
Taxonomy
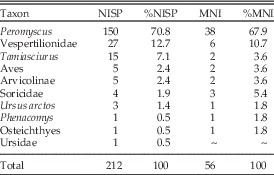
Subsurface evaluation at Arch-2 Cave recovered 212 bone specimens assignable to the taxonomic categories described here (Table 2).
Table 2 Number of identified specimens (NISP) and minimum number of individuals (MNI) per taxon for identified faunal specimens from Arch-2 Cave. MNI is calculated for the lowest possible taxonomic level.
Systematics
Phylum Chordata Haeckel, Reference Haeckel1874; Class Osteichthyes Huxley, Reference Huxley1880
Material
One fish vertebra (AC 1); number of identified specimens (NISP) 1, minimum number of individuals (MNI) 1.
Remarks
The fish vertebra is from a small individual, the maximum anterior diameter of the centrum is 1.94 mm. The bone is in poor condition. Deposition of the vertebra may have been as the scat or stomach contents of another animal. The nearest modern fish-bearing water source lies hundreds of meters below Arch-2 Cave and about 2 km W at Nimpkish Lake.
Systematics
Class Aves Linnaeus, Reference Linnaeus1758
Material
One partial beak (AC 11), four partial tarsometatarsals (AC 10, 12-14); NISP 5.
Remarks
The bird bones are from small-bodied individuals that are likely to be perching birds of Passeriformes (Campbell et al. Reference Campbell, Dawe, McTaggart-Cowan, Cooper, Kaiser, Stewart and McNall2001). The specimens are incomplete and display pitting suggestive of having been digested, which has limited identification beyond small Aves.
Systematics
Class Mammalia Linnaeus, Reference Linnaeus1758; Order Insectivora Linnaeus, Reference Linnaeus1758; Family Soricidae Fischer von Waldheim, Reference Fischer von Waldheim1814
Material
One partial left dentary with m1–m2 (AC 15), one partial left dentary with m1–m3 (AC 16), one partial left dentary with c1, p4–m3 (AC 17), one partial right dentary with m1–m2 (AC 18); NISP 4, MNI 3.
Remarks
Lower jaws and teeth do not distinguish the North American shrews Microsorex, Blarina, and Sorex in this family. Of these genera, Sorex and Microsorex currently live in British Columbia; the water shrew Sorex palustris brooksi (Anderson Reference Anderson1934), the vagrant shrew Sorex vagrans, and the dusky shrew Sorex monticolus are present on Vancouver Island (Wilson and Reeder Reference Wilson and Reeder2005). The Arch-2 Cave specimens compare favorably with Sorex and may be from this genus.
Systematics
Order Chiroptera Blumenbach, Reference Blumenbach1779; Family Vespertilionidae Gray, Reference Gray1821
Material
One partial rostrum that is broken anteriorly with right and left M1 (AC 25), one right partial dentary with i1, i2, p1–m3 (AC 22), one right partial dentary with m1–m3 (AC 19), one right dentary with i1–m3 (AC 30), one left dentary with i1–m3 (AC 35), one right partial dentary with p1–p3, m3 (AC 33), one right partial dentary with p1–m3 (AC 34), one left partial dentary with p1–p3, m2–m3 (AC 36), one right partial dentary with p3 and m2 (AC 37), one left partial dentary with m2–m3 (AC 44), seventeen individual teeth (AC 20, 21, 23, 24, 26-29, 31, 32, 38–43, 45); NISP 27, MNI 6.
Remarks
Of the North American genera of Vespertilionidae, Myotis and Corynorhinus have small body size and dentaries with a dental formula of I/3, C/1, P/3, P/3. Lasionycteris has this dental formula but larger (Hall Reference Hall1981). The Vespertilionidae specimens are small in size consistent with Myotis in this family. Vesper bats range onto Vancouver Island in modern times but do not currently inhabit the faunal locality.
Systematics
Order Rodentia Bowdich, Reference Bowdich1821; Family Sciuridae Fischer von Waldheim, Reference Fischer von Waldheim1817; Genus Tamiasciurus Trouessart, Reference Trouessart1880
Material
Two right P4 (AC 207, 209), four right M1 or M2 (AC 205, 206, 214, 215), two left M1 or M2 (AC 211, 212), one right p4 (AC 203), two left m1or m2 (AC 213, 216), one right m1 or m2 (AC 208), two left m3 (AC 210, 217), one right m3 (AC 204); NISP 15, MNI 2.
Remarks
Specimens are referred to Tamiasciurus following (Goodwin Reference Goodwin2004). Tamiasciurus currently include three species: (1) T. douglasii from western British Columbia, Washington, Oregon, and California; (2) T. hudsonicus that ranges from northern Alaska to Arizona and from the west coast of Canada to the eastern United States; and (3) T. mearnsi that live in northern Baja California (Abrogast et al. Reference Abrogast, Browne and Weigl2001). Tamiasciurus hudsonicus is endemic on Vancouver Island, and referral of the specimens to this species is likely on geographic grounds.
Systematics
Family Cricetidae Fischer von Waldheim, Reference Fischer von Waldheim1817; Genus Peromyscus Gloger, Reference Gloger1841
Material
Arch-2 Cave: two right partial maxillaries with M1–M2 (AC 57, 197), one partial right maxillary with M2–M3 (AC 58); one left partial maxillary with M2–M3 (AC 199); one left partial maxillary with M1–M3 (AC 154), four right M1 (AC 69, 79, 82, 186), two partial right M1 (AC 123, 124), nine left M1 (AC 59, 80, 107–109, 194, 196, 198, 200), eight right M2 (AC 62, 83, 127, 128, 155, 173, 187, 188), five left M2 (AC 71, 72, 84, 85, 159); two right M3 (AC 86, 87), one left M3 (AC 63), five right dentaries with incisor and m1 (AC 73, 88, 98, 110, 160), two right dentaries with incisor and m1–m3 (AC 168, 177), one right partial dentary with incisor and m1–m2 (AC 89), three left dentaries with m1 (AC 111, 129, 151), one right dentary with m2–m3 (AC 90), thirty right m1 (AC 53-55, 67, 75, 92, 99, 100, 112–115, 130–136, 146, 147, 161, 162, 169, 170, 178–180, 189, 201), twenty-three left m1 (AC 74, 91, 101, 102, 116, 117, 137–142, 148, 149, 152, 171, 172, 181–183, 191, 195, 202), twelve right m2 (AC 76, 103, 118, 119, 163–165, 174, 175, 184, 190, 192), six left m2 (AC 64, 77, 93, 104, 156, 193), seven right m3 (AC 68, 94, 95, 120, 157, 158, 185), three left m3 (AC 65, 143, 166), six right humeri (AC 56, 78, 97, 144, 145, 153), six left humeri (AC 96, 121, 122, 150, 167, 176); NISP 141, MNI 38. Pellucidar Cave: four right M1 (PC2 179, 181, 186, 212), four left M1 (PC2 177, 180, 221, 225); NISP 8.
Remarks
The specimens are assigned to genus Peromyscus because the molars are small with tuberculate cusps arranged in two alternating anterioposterior series, upper and lower third molars are reduced compared to first and second molars, upper incisors are not grooved on the labial face, and dentine spaces in molars are often confluent. Peromyscus is distinguished from its ancestor Copemys by the way in which the anterior arm of the hypocone in upper molars and the posterior arm of the protoconid in lower molars are aligned (Lindsay and Czaplewski Reference Lindsay and Czaplewski2011). Some sources recognize a subgenus Peromyscus based on the presence of one or more accessory cusps on the buccal side of M1 and M2, with a few exceptions in individuals that have undeveloped features or lack them (Osgood Reference Osgood1909; Hooper Reference Hooper1957; Hall Reference Hall1981). Others (Carleton Reference Carleton1989; Wilson and Reeder Reference Wilson and Reeder2005) emphasize genus-level assignment. Although accessory cusps are present on the buccal side of M1 and M2 specimens, the molars from Arch-2 and Pellucidar caves are assigned to genus Peromyscus.
Peromyscus molars can be described with respect to shape and the appearance of lophs and cusps. There are five main cusps on upper molars: the anterocone, protocone, paracone, hypocone, and metacone. The oval occlusal outlines of the M1 molars are slightly flattened buccally and posteriorly. The presence of an anteriomedian flexus results in a bilobed anterocone in many of the M1s from both localities. M1s have a thin and often short mesoloph, except AC 82, which lacks this feature, and AC 200, which is broken at the metacone. All of the M1 specimens analyzed in this study have accessory cusps on the buccal side that are visible in the lateral view. The M2s are oval to rectangular in occlusal outline. The cusps are similar to those of M1 except that instead of an anterocone, the molars have an anterior cingulum that extends buccally to join the paracone near its midline anteriobuccally. A mesoloph is often present in the M2s; a mesostyle is present in some specimens but is less common. As in the M1s, all of the M2s have accessory cusps on the buccal side. The M3 is a rounded triangle in occlusal outline and is reduced compared with M1 and M2. The M3 molars lack an anterocone and instead have a small anterior cingulum. Each M3 has a prominent paracone and protocone, whereas the hypocone and metacone are indistinct or absent. All upper molars have three roots in complete specimens.
In lower molars there are two roots. The m1s are oval in shape with straightened buccal and posterior margins. The anteroconid is flexed slightly lingually and is anterior to the protoconid, metaconid, hypoconid, and entoconid. Many of the m1 specimens have a bilobed anteroconid. The teeth lack mesolophids and mesostylids, except specimens AC 116 and AC 191, which have rudimentary mesolophids. The m2s are oval to rectangular in occlusal outline and lack an anteroconid. Mesolophids and mesostylids appear absent in the m2s, with the exception of specimen AC 104, which has a small mesolophid. The m3s have a protoconid and metaconid as well as a reduced hypoconid, and the entoconid is indistinct or absent. A reduced anterior cingulum is present on the buccal side of these teeth, anterior to the protoconid.
In addition to teeth, 12 humeri are assigned to Peromyscus based on size and shape and the presence of an entepicondylar foramen that is absent in the humeri of similarly sized Arvicolinae (Lyman et al. Reference Lyman, Power and Lyman2001).
Systematics
Species Peromyscus cf. keeni Rhoads, Reference Rhoads1894
Material
Arch-2 Cave: six left M1 (AC 60, 61, 66, 70, 81, 126), three right M1 (AC 105, 106, 125); NISP 9. Pellucidar Cave: five right M1 (PC2 183, 189, 209, 213, 228), four left M1 (PC2 184, 196, 203, 226); NISP 9.
Remarks
Although species assignment cannot be fully resolved in this study because of the absence of species-diagnostic dental characters, statistical assignment to species was attempted based on measurements of the fossil M1s. To assess size differences between samples in ancient and modern data sets, the anterioposterior diameter and transverse diameter of Late Pleistocene M1s from Arch-2 Cave and early Holocene M1s from Pellucidar Cave were compared with those of 60 modern adult P. keeni and P. maniculatus. The application of t-tests to these measurements shows that Peromyscus vary significantly at the p<0.0001 level across modern and ancient samples in both measures (Table 3). The ancient specimens are large in comparison with the modern sample (Table 4, Fig. 3). There is a statistically significant positive relationship between body length and M1 anterioposterior diameter (p=0.005) in the modern Peromyscus specimens, indicating that, in general, as body size increases so does M1 length. Therefore, the ancient Peromyscus probably had larger bodies on average than those of the modern Peromyscus measured in this study.

Figure 3 A scatter plot of M1s anterioposterior diameter and transverse diameter of Late Pleistocene Peromyscus (black diamonds) and P. cf. keeni (white diamonds) from Arch-2 Cave; early Holocene Peromyscus (black circles) and P. cf. keeni (white circles) from Pellucidar Cave; modern P. maniculatus (star) and P. keeni (plus) from Woss and Brooks Peninsula on north Vancouver Island.
Table 3 Results of t-test comparisons between Late Pleistocene, early Holocene, and modern Peromyscus from Vancouver Island.
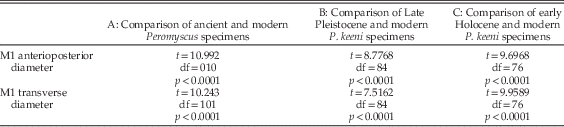
Table 4 M1 dimensions of ancient and modern samples of Peromyscus from Vancouver Island, Canada.
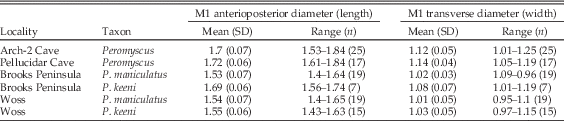
While it is possible that the large fossil M1 specimens include a distinct ice age species, it is more likely that the specimens are larger-bodied individuals of the Peromyscus species living on Vancouver Island today. A Shapiro-Wilk test did not reject the normality of M1 anterioposterior diameter and transverse diameter measurements on the modern comparative samples of P. maniculatus (p=0.54, 0.99) or P. keeni (p=0.76, 0.09). For statistical assignment of the fossil teeth to a species, the measurements were analyzed with linear discriminant analysis using the U-method, which correctly identified 66.7% of the modern P. maniculatus and P. keeni specimens. Then, classification assigned 9 of 25 M1 teeth from Arch-2 Cave (open circles plotted in Fig. 3) and 9 of 17 M1s from Pellucidar Cave (open diamonds plotted in Fig. 3) to P. keeni at the 95% confidence level or greater. These 18 M1s are reported as here P. cf. keeni. The remaining 24 M1s in the analysis could not be assigned to species with the same level of confidence and are therefore reported as genus Peromyscus.
Systematics
Subfamily Arvicolinae Gray, Reference Gray1821
Material
One right M1 (AC 51), two partial right m1 (AC 50, 52), two partial m3 (AC 48, 49); NISP 5, MNI 2.
Remarks
The Arvicolinae, including voles, lemmings, muskrats, and their extinct relatives, have molars with prismatic cusps that are arranged in a series of alternating triangles. The number of triangles on lower first molars characterizes some members of this subfamily. In the Arch-2 Cave teeth, the presence of ever-growing molars, the size of the specimens, and the presence of cementum in the re-entrant angles are consistent with genus Microtus; however, the m1s are incomplete, so the total number of triangles on these molars is unknown and assignment to this genus would be tentative. The Arch-2 Cave specimens are consistent with Microtus townsendii, the only arvicoline rodent with ever-growing molars that now lives on Vancouver Island (McTaggart-Cowan and Guiguet Reference McTaggart-Cowan and Guiguet1965; Wilson and Reeder Reference Wilson and Reeder2005). Early Holocene specimens consistent with M. townsendii have been reported from Whidbey Island in Puget Sound (Mustoe et al. Reference Mustoe, Harington and Morlan2005), and late Holocene specimens have been reported in archaeological assemblages on south Vancouver Island (Blacklaws Reference Blacklaws1979; Hanson Reference Hanson1991).
Systematics
Genus Phenacomys Merriam, Reference Merriam1889
Material
One partial left M1 (AC 46); NISP 1, MNI 1.
Remarks
The partial left M1 (AC 46) is referred to Phenacomys because the molar is rooted, re-entrant angles lack cement, and inner angles are asymmetrically elongated (Hall Reference Hall1981). Although characters of the molars do not distinguish the species in this genus, this specimen may belong to Phenacomys intermedius, the heather vole, which is geographically closer to Vancouver Island than any other species from this genus. A Phenacomys specimen was reported from ~16,000 BP deposits at Port Eliza Cave on northwest Vancouver Island (Al-Suwaidi et al. Reference Al-Suwaidi, Ward, Wilson, Hebda, Nagorsen, Marshall, Ghaleb, Wigen and Enkin2006). The genus is now extirpated from Vancouver Island.
Systematics
Order Carnivora Bowdich, Reference Bowdich1821; Family Ursidae Linnaeus, Reference Linnaeus1758
Material
One partial rib (AC 222); NISP 1.
Remarks
The specimen AC 222 is a fourth or fifth left rib that is fragmented at both ends. The size and shape this specimen is consistent with Ursidae. Specimen AC has a direct radiocarbon age estimate of 12,110±45 BP (Table 1).
Systematics
Species Ursus arctos Linnaeus, Reference Linnaeus1758
Material
One right I2 (AC 218), one right P4 (AC 219), one right m2 (AC 220); NISP 3, MNI 1.
Remarks
The P4 AC 219 is assigned to Ursus because the metacone is conical and lacks a shearing blade enamel ridge along the apices of the paracone and metacone as in tremarctine bears (Kurtén Reference Kurtén1967). Though the specimen is larger than modern brown bear P4s measured in this study, size does distinguish specimen AC 219 from Ursus americanus (Table 5). An I2 and m2 are referred to U. arctos based on shape and size (Table 5). The m2 AC 220 has a direct radiocarbon date of 12,370±35 BP (Table 1).
Table 5 Comparison of ursid specimens from Arch-2 Cave, Vancouver Island, with Arctodus simus, Ursus arctos, and U. americanus. *Measurements from Richards et al. (Reference Richards, Churcher and Turnbull1996).

Discussion
Faunas
The Arch-2 Cave faunal assemblage has low overall taxonomic diversity. Small mammals are the most numerous and diverse group, with Peromyscus comprising 71% of the total assemblage as the most abundant genus (Table 2). Of the 150 Peromyscus specimens recovered from subsurface deposits, 140 were excavated from EU3. Specimens in EU3 were deposited into the cave from above through a sediment-choked opening. High numbers of Peromyscus specimens in EU3 suggest that Peromyscus were abundant relative to other small mammals in the Late Pleistocene environment around the site. An age estimate of 11,960±45 BP (14,004–13,637 cal BP) on Cricetidae bone from EU3, 60–65 cm DBD, indicates that these small mammals were present at the site when glaciers were retreating from this region near the end of the Pleistocene (Clague and James Reference Clague and James2002). Deer mice are pioneer species that tolerate a wide range of climates (Zwolak Reference Zwolak2009; Blois et al. Reference Blois, McGuire and Hadly2010). During Late Pleistocene climate changes, these mice may have had an advantage over other small mammals with less environmental flexibility. By the early Holocene on north Vancouver Island, relative abundance was more equitable across small mammals. The Arch-2 Cave faunal assemblage and the early Holocene assemblage from Pellucidar Cave (Steffen et al. Reference Steffen, Hebda and McLaren2008) include many of the same small vertebrate taxa, such as deer mouse, vesper bat, and arvicoline vole; however, the early Holocene assemblage from Pellucidar Cave shows a more balanced relative abundance.
The stratigraphic context of the faunas may provide some insight into the relative age of the specimens. Abundant deer mice were found with bones of vesper bat, arvicoline vole, and brown bear at Arch-2 Cave EU3 and EU4 in the second cave chamber (Supplementary Table 1). Radiocarbon age determinations on bones from these EUs range from 11,460±60 BP (13,406–13,281 cal BP) to 12,370±35 BP (14,510–14,205 cal BP). Tamiasciurus and Aves bones were not found in EU3 or EU4 and were present only in the first chamber closest to the cave entrance. Soricidae were only recovered from the dark-brown silt stratum at the top of the sediment sequence and in uppermost underlying sediments. That squirrel and shrew specimens were not found in deeper sediments farther into the cave may indicate these faunas were deposited later in time than deer mice, vesper bats, arvicoline voles, and brown bear from EU3 and EU4. These differences may reflect the postglacial arrival of these faunas on Vancouver Island at the Arch-2 site, although additional faunal specimens with age estimates are needed for more information about the relative age of these faunas in the postglacial environment.
Paleoenvironment
The present fauna indicate a cool and moist arboreal setting. Soricidae live in forested habitats and tolerate cold winters and cool to moderately warm summer temperatures with sufficient moisture to sustain ground cover vegetation (Harris Reference Harris1985). Vesper bats live in a range of habitats that include moist forests and dry grasslands (Nagorsen and Brigham Reference Nagorsen and Brigham1993). Tree squirrels indicate wooded habitat as these rodents often occur in mountainous areas with spruce–fir or other mixed forests. Small perching birds in the Arch-2 Cave assemblage also indicate an arboreal setting. Arvicolinae inhabit subalpine, montane, and moist coniferous forests as well as dry grasslands in the Pacific Northwest (Maser and Storm Reference Maser and Storm1970). Within this family, Phenacomys are typically a boreal forest species that currently lives on the British Columbia mainland, though not on Vancouver Island or in other coastal areas (Hall Reference Hall1981). Brown bears live in diverse environments where there are sufficient resources (Churcher Reference Churcher1999). These bears were present at the end of the Pleistocene on north Vancouver Island, where they no longer maintain a regular range. Pollen spectra for north Vancouver Island are consistent with the Arch-2 Cave fauna and indicate an open woodland in a cool, moist environment from ~12,000–11,700 BP (~13,800–13,500 cal BP) with increasingly closed coniferous forests into the early Holocene (Lacourse Reference Lacourse2005).
Biogeography, Glaciation, and Relative Sea-Level Change
The Late Pleistocene glaciation and relative sea-level history of Vancouver Island provide significant constraints on the arrival of terrestrial faunas with limited over-water dispersal capabilities. During the Vashon Stade of the late Wisconsinan Fraser glaciation, ice forming along the Coast Mountains and Vancouver Island coalesced and moved west toward the continental shelf as well as south toward the Puget Lowland (Porter and Swanson Reference Porter and Swanson1998; Clague and James Reference Clague and James2002). Glaciation reached a maximum extent in this region by ~14,500 BP (~17,000 cal BP). Glaciers began to retreat rapidly from west Vancouver Island by ~14,000–13,500 BP (~17,000–16,200 cal BP) and from the Puget Lowland by 14,400 BP (Cosma et al. Reference Cosma, Hendy and Chang2008; Clague and James Reference Clague and James2002). Deglaciation began before 12,930±160 BP (WSU-1710; ~15,500 cal BP) on northeast Vancouver Island (Howes Reference Howes1983). South Vancouver Island was ice free by ~13,000 BP, and the Strait of Georgia was free of ice soon thereafter (Clague and Ward Reference Clague and Ward2011). Ice cover was at modern levels throughout this region by between 9500 BP (10,800 cal BP) and 8800 BP (~10,200 cal BP) (Clague and James Reference Clague and James2002; Cosma et al. Reference Cosma, Hendy and Chang2008).
Early postglacial relative sea-level histories vary somewhat around Vancouver Island due to differences in local ice loading (Clague Reference Clague1981; Howes Reference Howes1983; Clague and James Reference Clague and James2002; Hutchinson et al. Reference Hutchinson, James, Clague, Barrie and Conway2004; James et al. Reference James, Hutchinson, Barrie, Conway and Mathews2005, Reference James, Gowan, Hutchinson, Clague, Barrie and Conway2009). However, many areas on Vancouver Island exhibit a similar general pattern following deglaciation of rapid marine regression with relative sea level falling below the modern shoreline by ~11,500 BP (~13,300 cal BP) and reaching a lowstand soon thereafter. In contrast with this general pattern, coastal areas adjacent to northeast Vancouver Island show relatively little postglacial isostatic adjustment (Roe et al. Reference Roe, Doherty, Patterson and Milne2013; Shugar et al. Reference Shugar, Walker, Lian, Eamer, Neudorf, McLaren and Fedje2014). Isostatic uplift or crustal forebulge in front of the glacial margin north of Vancouver Island subaerially exposed Cook Bank as a coastal plain near north Vancouver Island at 10,470±75 BP (RIDDL-985; 12,600–12,085 cal BP) (Clague Reference Clague1983; Luternauer et al. Reference Luternauer, Clague, Conway, Barrie, Blaise and Mathews1989), after which relative sea level dropped to ~95 m below modern at that location. Postglacial relative sea-level lowstands are not known to have exposed land bridges between Vancouver Island and the mainland, where a deep marine channel sustained a water barrier from early postglacial times through the Holocene. Therefore, the timing of deglaciation and known relative sea-level changes suggest that terrestrial vertebrates were present at Arch-2 Cave on Vancouver Island when water barriers limited their immigration from the mainland.
Terrestrial vertebrates with varying dispersal capabilities were present on Vancouver Island as Late Pleistocene glacial ice retreated. Large mammals with terrestrial and aquatic dispersal capacities were present, such as brown bear at Arch-2 Cave by 12,370±35 BP (14,695–14,148 cal BP). Giant short-faced bear (Actodus simus), black bear (Ursus americanus), and mountain goat (Oreamnos americanus) on north Vancouver Island at between 12,400 and 11,000 BP (~14,500 and 12,900 cal BP) and bison (Bison bison) on south Vancouver Island at 11,750±110 BP (SFU-BG-1; 13,787–13,344 cal BP) (Hebda and Spalding Reference Hebda and Spalding1996; Wilson et al. Reference Wilson, Kenady and Schalk2009) may have crossed water barriers to the island, possibly during lower eustatic sea levels. Small mammals such as Peromyscus and Phenacomys that have limited over-water dispersal abilities were at Arch-2 Cave on north Vancouver Island as early as 11,960±45 BP (14,004–13,637 cal BP).
There are several possibilities for postglacial small vertebrate dispersals to Arch-2 Cave. Rafted ice and debris may have supported small vertebrates from the mainland to Vancouver Island across water barriers. Temporary ice bridges may have formed in ocean water with reduced salinity due to glacial meltwater influx, facilitating dispersal. Alternatively, small vertebrates may have dispersed to Arch-2 Cave from glacial refugia that have been proposed for this region such as at Cook Bank (Hetherington et al. Reference Hetherington, Barrie, Reid, MacLeod, Smith, James and Kung2003) and or in unglaciated nunataks on high mountain peaks (Heusser Reference Heusser1971). Assignment of large early postglacial Vancouver Island specimens to P. cf. keeni in this study is consistent with the latter hypothesis and with insular relictual gigantism in P. keeni (McTaggart-Cowen Reference McTaggart-Cowan1935).
Relictual Gigantism in Vancouver Island Peromyscus
Peromyscus keeni was initially characterized as a separate species from P. maniculatus based on the larger body size and shorter tail length of P. keeni (Sheppe Reference Sheppe1961; McTaggart-Cowan and Guiguet Reference McTaggart-Cowan and Guiguet1965). The taxonomy and distribution of P. keeni were later refined by Hogan et al. (Reference Hogan, Hedin, Koh, Davis and Greenbaum1993), who considered morphological, karyological, and genetic information (Osgood Reference Osgood1909; Gunn and Greenbaum Reference Gunn and Greenbaum1986; Allard et al. Reference Allard, Gunn and Greenbaum1987; Allard and Greenbaum Reference Allard and Greenbaum1988). More recently, genetic studies based on mitochondrial cytochrome-b in modern mice confirm the species designation (Zheng et al. Reference Zheng, Abrogast and Kenagy2003; Lucid and Cook Reference Lucid and Cook2004). These genetic studies favor an isolated source for P. keeni, consistent with geographic separation of this species from P. maniculatus in topographic or glacial refugia from the middle or Late Pleistocene (Zheng et al. Reference Zheng, Abrogast and Kenagy2003).
This study used M1 size as a proxy for body size to compare Late Pleistocene, early Holocene, and modern Peromyscus from Vancouver Island. Results indicate that modern P. maniculatus and P. keeni have a significantly smaller body size than ancient Peromyscus. Linear discriminant analysis was used to assign ancient Peromyscus specimens to P. cf. keeni, and although species-level identifications of these ancient specimens remains tentative, and results do not exclude the possibility that more than one Peromyscus species lived on Vancouver Island in early postglacial times, the results of this study are consistent with relictual gigantism in P. keeni. However, the original hypothesis presented by McTaggart-Cowan (Reference McTaggart-Cowan1935) did not directly address post-Pleistocene body-size changes in Peromyscus such as are observed in this study. Gigantism in small island vertebrates is one expectation that derives from the more comprehensive island rule hypothesis, which suggests that after mainland vertebrates colonize islands, small-bodied species tend to evolve larger bodies and large-bodied species tend to become smaller bodied (Foster Reference Foster1964; Case Reference Case1978; Lomolino Reference Lomolino2005). Large size in Late Pleistocene and early Holocene Peromyscus on Vancouver Island appears consistent with this premise, yet the results presented here indicate that diminution occurred in island Peromyscus during the Holocene.
Several factors may have contributed to body-size variation in Vancouver Island Peromyscus. Morphological changes in insular mammals are often adaptive and can result from selective pressures specific to island settings. Relatively small Peromyscus populations and few competitors in the insular Pleistocene environment could have facilitated food availability and encouraged larger body size. Similarly, it is possible that selective pressures on Holocene Vancouver Island, such as shifts in the numbers of predators or competitors, resulted in Peromyscus body-size decline. It is also possible, however, that region-wide factors such as shifts in the biotic environment due to climatic fluctuations and amelioration during the Holocene promoted body-size changes in island and mainland Peromyscus. Genetic mixing with smaller Peromyscus arriving from the mainland during the Holocene—if immigration increased with human traffic to and from the mainland—is another possible explanation for Holocene diminution in island Peromyscus. Body-size variation in Peromyscus on Vancouver Island in this study highlights the importance of a regionally informed approach to understanding body-size changes in insular vertebrates through time. Additional studies of island and mainland Peromyscus in the Pacific Northwest are needed to further evaluate body-size trends as well as their ecological and evolutionary underpinnings.
Conclusion
The faunal assemblage from Arch-2 Cave on north Vancouver Island contains large and small vertebrate faunas, including endemic Soricidae, Vespertilionidae, Tamiasciurus, Peromyscus, and Aves, as well as Ursus arctos and Phenacomys that are now locally extirpated. Radiocarbon age estimates of 12,370±35 BP (14,695–14,148 cal BP) on brown bear and 11,960±45 BP (14,004–13,637 cal BP) on Cricetidae bones indicate these faunas were present on the island as glacial ice receded from this region at the end of the Pleistocene and as relative sea level fell from a postglacial highstand, suggesting local source areas for these terrestrial vertebrates. The high relative abundance of Peromyscus in the postglacial Late Pleistocene Arch-2 Cave assemblage is consistent with broad environmental tolerance in these pioneer species.
This study compared the size of ancient and modern Peromyscus to test the relictual gigantism hypothesis initially presented by McTaggart-Cowan (Reference McTaggart-Cowan1935). Using M1 lengths and widths as proxies for body size, modern P. maniculatus and P. keeni were compared with Late Pleistocene and early Holocene Peromyscus specimens from Arch-2 and Pellucidar caves. Large fossil specimens were assigned statistically to P. cf. keeni. These results suggest that large body size in Keen’s mouse is a relictual character and diminution occurred in Vancouver Island Peromyscus during the Holocene.
Acknowledgments
I wish to thank the Royal BC Museum for funding and John Southon, University of California, Irvine, Keck-CCAMS facility, for radiocarbon dating.
Supplementary Material
Supplemental material deposited at Dryad: doi: 10.5061/dryad.313hd
Appendix
A list of modern Peromyscus specimens measured in this study (RBCM: Royal BC Museum, Victoria, British Columbia, Canada).
Woss, B.C.—Family Cricetidae, Peromyscus maniculatus: RBCM 17667, 17669, 17670, 17682, 17689, 17690, 17691, 17695, 17696, 17697, 17698, 17699, 17700, 17701, 17702, 17703, 17711, 17712, 17714. Peromyscus keeni: 17673, 17676, 17677, 17678, 17681, 17684, 17687, 17688, 17693, 17694, 17707, 17708, 17709, 17710, 17713.
Brooks Peninsula, B.C.—Family Cricetidae, Peromyscus maniculatus: RBCM 10727, 10728, 10730, 10731, 10734, 10735, 10738, 10744, 10745, 10746, 10747, 10748, 10750, 10751, 10753, 10754, 10755, 10756, 11498. Peromyscus keeni: 10741, 10742, 10759, 10766, 10780, 10788, 11391.










