INTRODUCTION
Tropical moist forests might allocate up to 50% of their annual net primary production (NPP) into fine roots (diameter ≤ 2 mm) (Gill & Jackson Reference GILL and JACKSON2000, Vogt et al. Reference VOGT, VOGT, PALMIOTTO, BOON, O'HARA and ASBJORNSEN1996), and organic-matter inputs to soil from dead roots can equal or surpass the return from leaf litter (Röderstein et al. Reference RÖDERSTEIN, HERTEL and LEUSCHNER2005). Thus, fine-root production and turnover serve as an important pathway of organic carbon input into soil, which stores the largest pool of terrestrial carbon (Jobbágy & Jackson Reference JOBBÁGY and JACKSON2000, Matamala et al. Reference MATAMALA, GONZÁLEZ-MELER, JASTROW, NORBY and SCHLESINGER2003).
Nitrogen (N) availability is one of the major controls on fine-root dynamics (Vogt et al. Reference VOGT, VOGT, PALMIOTTO, BOON, O'HARA and ASBJORNSEN1996). A decrease in fine-root biomass is frequently observed as N availability increases (Hendricks et al. Reference HENDRICKS, HENDRICK, WILSON, MITCHELL, PECOT and GUO2006, Nadelhoffer Reference NADELHOFFER2000). The ‘differential allocation hypothesis’ maintains that this decrease in fine-root biomass with an increase in N availability is due to a reduction in the proportion of biomass allocated to fine-root production (Hendricks et al. Reference HENDRICKS, HENDRICK, WILSON, MITCHELL, PECOT and GUO2006). For temperate forest ecosystems, a number of studies suggest that fine-root production decreases along natural (Tateno et al. Reference TATENO, HISHI and TAKEDA2004) and experimental N-availability gradients (Albough et al. Reference ALBOUGH, ALLEN, DOUGHERTY, KRESS and KING1998, Gower et al. Reference GOWER, VOGT and GRIER1992). In tropical montane forests below-ground biomass increases with altitude, while above-ground biomass decreases leading to an increase in root:shoot ratio (Girardin et al. Reference GIRARDIN, MALHI, ARAGÃO, MAMANI, HUARACA HUASCO, DURAND, FEELEY, RAPP, SILVA-ESPEJO, SILMAN, SALINAS and WHITTAKER2010, Leuschner et al. Reference LEUSCHNER, MOSER, BERTSCH, RÖDERSTEIN and HERTEL2007). If the increase in fine-root biomass results from an increase in fine-root production, this would suggest a below-ground shift in biomass allocation. Leuschner et al. (Reference LEUSCHNER, MOSER, BERTSCH, RÖDERSTEIN and HERTEL2007) hypothesized that an increasing nutrient limitation to tree growth is the cause for this shift. However, patterns of fine-root dynamics with changing N availability have rarely been investigated experimentally in tropical forests. In addition, although effects of altered nutrient availability on biomass allocation between below- and above-ground parts of plants have received a lot of attention in past studies (Reich Reference REICH, Waisel, Eshel and Kafkafi2002, Reynolds & D'Antonio Reference REYNOLDS and D'ANTONIO1996 and references therein), changes in fine-root redistribution with soil depth in response to altered nutrient supply have so far received little attention. Large uncertainty exists about the possible effects of elevated N input on fine-root biomass, production and redistribution at different soil depths, which may affect soil carbon balance in forests (Gower & Vitousek Reference GOWER and VITOUSEK1989, Nadelhoffer Reference NADELHOFFER2000, Norby & Jackson Reference NORBY and JACKSON2000).
We set up an N fertilization experiment in a lower montane rain forest in western Panama. N fertilization increased total fine litterfall and above-ground NPP in the first year and leaf litterfall in the first and second year of the study (Adamek et al. Reference ADAMEK, CORRE and HÖLSCHER2009). Gross and net rates of soil N mineralization and nitrification also increased during the first year of N addition compared with the control (Corre et al. Reference CORRE, VELDKAMP, ARNOLD and WRIGHT2010, Koehler et al. Reference KOEHLER, CORRE, VELDKAMP, WULLAERT and WRIGHT2009). The spatial distribution of fine-root biomass density in the organic layer of the control plots was positively correlated with extractable total N (Hölscher et al. Reference HÖLSCHER, DUNKER, HARBUSCH and CORRE2009) which can be seen as an indirect indication of N limitation in a stand. Our aim was to investigate the stand-scale response of fine-root dynamics to N fertilization. We hypothesized that fine-root biomass and production will decrease with increasing N availability because N addition would lessen the need for biomass investment into fine roots foraging for N. We assessed fine-root responses to N fertilization at three soil depths (organic layer, 0–10 cm and 10–20 cm in the mineral soil) that represented 71% of the total fine-root biomass within 1 m depth (Hölscher et al. Reference HÖLSCHER, DUNKER, HARBUSCH and CORRE2009). Our objectives were (1) to determine whether fine-root biomass and necromass in undisturbed soil and in ingrowth cores change under elevated N availability and at which soil depths these changes occur, and (2) to estimate fine-root production from undisturbed soil at the three depths.
METHODS
Site description and experimental design
The study area is situated in the western Panamanian province of Chiriquí. It lies within the Fortuna Forest Reserve which forms part of the La Amistad Biosphere Reserve. The Fortuna watershed forms a high valley in the Talamanca range. The study site is located at 1200–1300 m asl in the Quebrada Honda area (8°45′N, 82°15′W) at the north-western side of the Fortuna lake. The vegetation is a mature lower montane rain forest (sensu Grubb Reference GRUBB1977, Holdridge et al. Reference HOLDRIDGE, GRENKE, HATHEWAY, LIANG and TOSI1971). The estimated number of tree species from two 1-ha plots in the Quebrada Honda valley is approximately 90 (J. Dalling, pers. comm.). The most abundant species are Oreomunnea mexicana (Standl.) Leroy (Juglandaceae), Eschweilera panamensis Pittier (Lecythidaceae), Vochysia guatemalensis Donn. Sm. (Vochysiaceae), Cassipourea elliptica (Sw.) Poir. (Rhizophoraceae), Hedyosmum bonplandianum Mart. (Chloranthaceae) and Guarea glabra Vahl (Meliaceae). Also common is Colpothrinax aphanopetala R. Evans (Arecaceae). Mean annual precipitation was 5545 ± 308 mm (1997–2007) without a clear dry season (no month < 100 mm precipitation); mean annual temperature was 20.0 °C ± 0.1 °C (1999–2007). Soil characteristics were determined in January 2006 prior to treatment application (see Koehler et al. Reference KOEHLER, CORRE, VELDKAMP, WULLAERT and WRIGHT2009 for details on soil sampling and chemical analyses). These characteristics (Table 1) did not differ between plots which were later randomly assigned as control and N-fertilized. The soil in the study site has developed on volcanic ash deposits and is classified as Aluandic Andosol (FAO classification) or Alic Hapludand (U.S. Soil Taxonomy). The mineral soil has a sandy loam texture and was covered by an organic layer with a mean thickness of 4.2 ± 0.4 cm.
Table 1. Soil characteristics (mean ± SE, n = 8 plots) of the study site determined in January 2006 prior to N manipulation.

The experiment was set up in a paired-plots design with four replicates. Control and N-fertilized treatments were randomly assigned to each pair of plots. Each plot was 40 × 40 m, and plots were separated by at least 40 m. Plots lacked streams or swampy areas, gaps and slopes steeper than 15°. Measurements of side lengths were corrected for inclination (Condit Reference CONDIT1998). The N-fertilized plots received 125 kg urea-N ha−1 y−1 divided into four applications per year (February 2006, May 2006, July 2006, October 2006, February 2007, June 2007, August 2007). We chose urea for a practical reason: NH4NO3 is not sold in Panama due to security concerns. Urea was applied manually, walking back and forth across each subplot and changing directions (east–west and north–south) in subsequent applications.
Fine-root analyses
We assessed fine-root dynamics in response to N fertilization with two different approaches: sequential coring and ingrowth cores. For each method, six sampling points were located in the inner 20 × 20-m area of each plot. Roots were sampled with a root corer and soil cores were divided into three depths: organic layer, 0–10 cm and 10–20 cm in the mineral soil. Roots were washed by hand over a 1-mm mesh screen and categorized into live (hereafter fine-root biomass) and dead roots (hereafter fine-root necromass) by examination under the stereomicroscope based on colour, elasticity and degree of cohesion of cortex, periderm and stele (Leuschner et al. Reference LEUSCHNER, HERTEL, CONERS and BUTTNER2001, Persson Reference PERSSON1978). Roots were dried at 65 °C for 24 h. Fine-root biomass and necromass in the organic layer refer to the mean organic layer thickness of each plot.
For the sequential coring method (root corer diameter = 4 cm, length = 28 cm), fine roots were sampled in four sampling series at intervals of 3–6 mo (July 2006, November 2006, February 2007, August 2007). Soil cores were stored in a freezer until they were processed (1–4 mo). Fine-root biomass and necromass from the six sampling points per plot were averaged to represent each replicate plot.
For the ingrowth core method, three ingrowth cores were installed at six sampling points per plot to represent three sampling series (at 0.5, 1 and 1.5 y of incubation from February 2006 to September 2007). For the installation of the ingrowth cores, all visible roots were sorted out from the soil taken with a root corer (diameter = 8 cm, length = 28 cm). The root-free soil was back-filled into the hole in the same sequence of soil layers as was found in the undisturbed soil. In the case that there was not enough soil left after sorting out the roots, root-free soil from a neighbouring location within the same plot was used to fill up. Live roots that have grown into the root-free soil area were harvested with a root corer (diameter = 7 cm, length = 28 cm), and soil cores were stored in a refrigerator until they were processed (1–3 mo). Diameter of live roots in ingrowth cores did not exceed 3 mm so that not only dry mass of fine roots but of all roots was determined. Fine-root mass in ingrowth cores from the six sampling points per plot was averaged to represent each replicate plot.
We also attempted to estimate fine-root production. Although several approaches for the estimation of fine-root production are debated (Hendricks et al. Reference HENDRICKS, HENDRICK, WILSON, MITCHELL, PECOT and GUO2006, Hertel & Leuschner Reference HERTEL and LEUSCHNER2002, Vogt et al. Reference VOGT, VOGT and BLOOMFELD1998), a single valid method has not been established so far. Fine-root sampling by sequential coring is probably the most commonly used approach to estimate fine-root production in forest ecosystems (Hertel & Leuschner Reference HERTEL and LEUSCHNER2002, Vogt et al. Reference VOGT, VOGT and BLOOMFELD1998). Without the need for any installation prior to sampling, this method assesses fine-root mass in an undisturbed soil. Important shortcomings of this method are that missing seasonal minima and maxima of fine-root biomass and simultaneously occurring growth and decay of fine roots can result in an underestimation of fine-root production. The ingrowth core method can be used to compare relative growth rates of fine roots between experimental manipulations (Vogt et al. Reference VOGT, VOGT and BLOOMFELD1998). However, recolonization of a root-free soil core cannot be equated with fine-root production in undisturbed soil as fine-root growth may be delayed by recovery from the previous injury, may proceed at artificially low root density, and fine roots which may have died during the incubation period are not accounted for (Hertel & Leuschner Reference HERTEL and LEUSCHNER2002). Therefore, we applied the sequential coring method to estimate fine-root biomass, necromass and production and used the ingrowth core method to compare fine-root biomass redistribution with depth in response to N fertilization. Annual fine root-production was calculated for each sampling point and separately for the organic layer of the control plots and the mineral soil depths of both treatments with the minimum–maximum method (McClaugherty et al. Reference McCLAUGHERTY, ABER and MELILLO1982) across the four sampling series. For the organic layer of the N-fertilized plots, fine-root production could not be calculated because total fine-root mass decreased continuously throughout the measurement period.
Statistical analyses
Tests for normality using Kolmogorov–Smirnov D statistics and equality of variance using the Levene statistic (Sokal & Rohlf Reference SOKAL and ROHLF1981) were conducted for each parameter. For fine-root biomass and necromass by sequential coring, treatment effects were assessed using linear mixed-effects models (Crawley Reference CRAWLEY2002) in which treatment is considered as a fixed effect and spatial replication (experimental plots) nested in time (four sampling series) as random effect. Details are described in a related study conducted in our site (Koehler et al. Reference KOEHLER, CORRE, VELDKAMP, WULLAERT and WRIGHT2009); in short, the model includes (1) a variance function which allows different variances of the response variable per level of the fixed effect, and/or (2) a first-order temporal autoregressive process which assumes that the correlation between measurements decreases with increasing time difference. These analyses were conducted using R2.6.0. For fine-root production by sequential coring and fine-root mass in ingrowth cores, treatment differences for each depth were assessed using Mann–Whitney U test, while differences among depths for each treatment were assessed using Kruskal–Wallis H test followed by multiple comparison extension test. These analyses were conducted using SigmaStat 3.1 (Systat Software Inc., Chicago, USA). We conducted a power analysis (Sokal & Rohlf Reference SOKAL and ROHLF1981) for fine-root biomass, separately for each sampling period and depth, and for fine-root production for each depth to determine how large the treatment effect would be for statistical significance at P = 0.05. Mean and SE, determined from four replicate plots per treatment, are reported as measures of central tendency and dispersion.
RESULTS
Sequential coring indicated that fine-root biomass and necromass across the four sampling periods did not differ between the control and N-fertilized plots at any depth (Figure 1a–d; Table 2). A power analysis showed that across the four sampling periods changes in fine-root biomass of 2–11 times (for the organic layer) and 2–50 times (for the mineral soil depths) larger than those we observed were needed for significant treatment differences at P = 0.05. In the control plots, the temporal pattern of fine-root biomass in the organic layer showed an initial increase followed by a decrease (Figure 1a) whereas the opposite trend was observed in the mineral soil depths of both treatments (Figure 1b, c); the latter was reflected in the overall (sum of all depths) pattern (Figure 1d). However, only the trend for fine-root biomass at 0–10 cm in the mineral soil of the control plots was significant across the sampling period (r2 = 0.998, P = 0.038). In the N-fertilized plots, fine-root biomass in the organic layer continuously declined across the sampling period (r2 = 0.951, P = 0.025; Figure 1a). This was due to a decrease in fine-root density from 6.3 ± 0.8 g dm−3 soil to 2.9 ± 0.8 g dm−3 soil (r2 = 0.908, P = 0.047).

Figure 1. Mean (± 1 SE, n = 4 plots) fine-root biomass (FRB) and fine-root necromass (FRN) across four sampling series from the sequential coring separately for the organic layer (a), at 0–10 cm (b) and 10–20 cm in the mineral soil (c), and over all three depths (d).
Table 2. Fine-root biomass, necromass and production in control and N-fertilized plots (starting February 2006) measured by sequential coring across four sampling series between July 2006–August 2007 (mean ± SE; n = 4 plots). Fine-root production in the organic layer of the N-fertilized plots was not determined (n.d.) as total fine-root mass decreased across the measurement period. There were no significant differences between treatments (Linear mixed-effects model at P ≤ 0.05 for fine-root biomass and necromass; Mann–Whitney U test at P ≤ 0.05 for fine-root production).

The continuous decrease of fine-root biomass in the organic layer of the N-fertilized plots violated the assumption of a cyclic course of fine-root biomass during the measurement period, which is essential for the estimation of fine-root production. The constant decrease of fine-root biomass in the N-fertilized organic layer implies that fine-root mortality occurred at higher rates than fine-root production. Hence, fine-root production could not be estimated for the N-fertilized organic layer and our estimate of overall fine-root production (from organic layer down to 20 cm in the mineral soil) for the N-fertilized plots might be low. The estimated fine-root production was higher at 0–10 cm than 10–20 cm in the mineral soil in the control (Kruskal–Wallis H-test, H = 7.42, P = 0.01) and N-fertilized plots (H = 7.73, P = 0.01; Table 2). During 1.5 y of N addition, overall fine-root production was not affected by N fertilization (324 ± 33 g m−2 y−1 and 416 ± 37 g m−2 y−1 in control and N-fertilized plots, respectively; Table 2). Our power analysis indicated that across the four sampling periods changes in fine-root production in the mineral soil of two-fold (for the 10–20-cm depth) to seven-fold (for the 0–10-cm depth) larger than those we measured were necessary for significant treatment differences at P = 0.05.
Fine-root biomass in ingrowth cores was still increasing after 1.5 y in the organic layer of both control and N-fertilized plots (Figure 2a). In both mineral soil depths fine-root biomass in ingrowth cores decreased after it reached a maximum at 1 y (Figure 2b, c). Fine-root biomass in ingrowth cores at 10–20 cm in the mineral soil was higher in the N-fertilized than in the control plots after 1.5 y (13 ± 2 g m−2 and 28 ± 4 g m−2 in control and N-fertilized plots, respectively; Mann–Whitney U-test, T = 10.0, P = 0.03; Figure 2c). Overall fine-root biomass in ingrowth cores (sum of three depths) did not increase beyond approximately 225 g m−2 after 1 y in both the control and N-fertilized plots (Figure 2d).

Figure 2. Mean (± 1 SE, n = 4 plots) fine-root biomass (FRB) and fine-root necromass (FRN) across three sampling series from the ingrowth core approach separately for the organic layer (a), at 0–10 cm (b) and 10–20 cm in the mineral soil (c) and over all three depths (d). The samples taken in August 2006, February–March 2007 and August–September 2007 represented 0.5 y, 1 y and 1.5 y, respectively, after ingrowth core installation. At 10–20 cm in the mineral soil, fine-root biomass with different letters indicates a significant difference between treatments at 1.5 y (Mann–Whitney U-test at P ≤ 0.05).
DISCUSSION
Fine-root mass and production in the control plots
The mean fine-root biomass of our site (458 ± 21 g m−2) was higher than the mean (357 ± 51 g m−2) for tropical lower montane rain forests, whereas our mean fine-root necromass (101 ± 9 g m−2) was lower than the mean for this forest type (480 ± 72 g m−2) (Hertel & Leuschner, in press). Total fine-root mass (biomass + necromass; 559 ± 23 g m−2) was lower than the mean (804 ± 75 g m−2; Hertel & Leuschner, in press) for tropical lower montane rain forests but was higher than that measured by Cavelier (Reference CAVELIER1992) in the Fortuna area (400 g m−2 from the organic layer down to 25-cm depth including mineral soil). Also, our measured total fine-root mass was comparable to that reported by Hölscher et al. (Reference HÖLSCHER, DUNKER, HARBUSCH and CORRE2009) (484 g m−2 from the organic layer down to 10 cm in the mineral soil) from the same control plots. Comparing our values with those from tropical lowland forests in Central America, fine-root biomass and total fine-root mass were lower from a Fluvaquentic Hapludoll soil, an Oxic Dystrandept soil and Typic Haploperox soils in La Selva, Costa Rica (Espeleta & Clark Reference ESPELETA and CLARK2007, Gower Reference GOWER1987, Powers et al. Reference POWERS, TRESEDER and LERDAU2005), and from Inceptisol, Oxisol and Alfisol soils (Cavelier Reference CAVELIER1992, Cavelier et al. Reference CAVELIER, WRIGHT and SANTAMARÍA1999, Powers et al. Reference POWERS, TRESEDER and LERDAU2005, Yavitt & Wright Reference YAVITT and WRIGHT2001) on Barro Colorado Island, Panama. However, fine-root biomass values from an Ultisol soil in La Selva, Costa Rica (Denslow et al. Reference DENSLOW, ELLISON and SANFORD1998), were higher than ours. The decrease in fine-root biomass with depth in our site is paralleled by a decrease in gross and net rates of soil N mineralization and nitrification from the organic layer to 0–5 cm in the mineral soil (Corre et al. Reference CORRE, VELDKAMP, ARNOLD and WRIGHT2010, Koehler et al. Reference KOEHLER, CORRE, VELDKAMP, WULLAERT and WRIGHT2009).
Fine-root biomass in ingrowth cores showed an opposite temporal pattern to fine-root biomass in undisturbed soil. Furthermore, fine-root biomass in ingrowth cores reached considerably lower maximum levels than were found in undisturbed soil. Hence, we opted for the sequential coring method for the estimation of fine-root production. Fine-root production in our site lies below the mean for tropical lower montane rain forests (512 ± 91 g m−2 y−1 from 12 studies reviewed by Hertel & Leuschner, in press); this mean, however, was influenced by an extraordinarily high value from a Venezuelan forest growing on an extremely acidic Oxisol with low Ca availability (Priess et al. Reference PRIESS, THEN and FÖLSTER1999), and excluding this value rendered a mean (457 ± 76 g m−2 y−1) comparable to our estimates (324 ± 33 g m−2 y−1). Thus, our estimate of fine-root production based on the sequential coring approach approximated those established for lower montane rain forests. However, if turnover of the smallest fraction of fine roots occurred during our sampling intervals our method may have led to a conservative estimate of fine-root production.
Effects of N fertilization on fine-root biomass, production and biomass redistribution
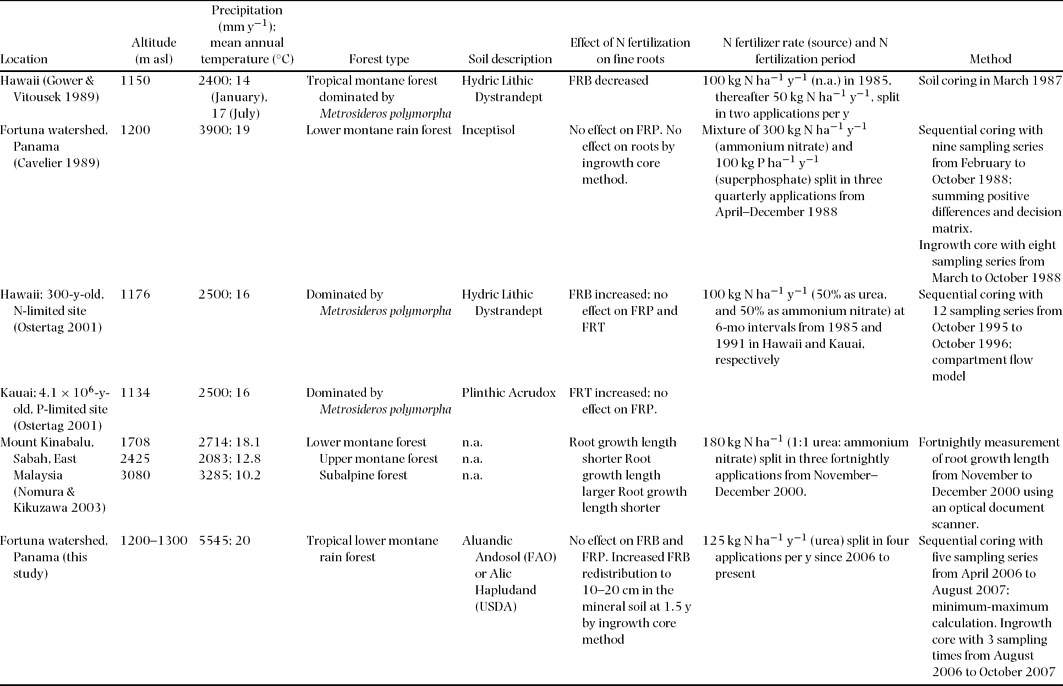
From the sequential coring approach, we did not observe effects of N fertilization on fine-root biomass and production over 1.5 y. In a forest in the Fortuna watershed, fine-root production was also unaffected by 9 mo of combined N and P fertilization (Cavelier Reference CAVELIER1989; Appendix 1). In a Hawaiian montane forest, despite N limitation to different components of above-ground productivity (e.g. stem diameter growth, leaf litter production and foliar N concentration) (Vitousek & Farrington Reference VITOUSEK and FARRINGTON1997, Vitousek et al. Reference VITOUSEK, WALKER, WHITEAKER and MATSON1993), 10 y of N fertilization of an N-limited 300-y-old site (on Hydric Lithic Dystrandept soil) had no significant effect on fine-root production, turnover rates and standing stock of fine-root necromass, with the only exception being a small increase in standing fine-root biomass (Ostertag Reference OSTERTAG2001; Appendix 1). At the same site, Gower & Vitousek (Reference GOWER and VITOUSEK1989; Appendix 1) found reduced fine-root biomass after 1.5 y of N fertilization. Thus, short- and long-term effects of N fertilization on fine roots may be divergent. Comparison with the Hawaiian sites is difficult because the ecosystem is largely dominated by one tree species (Metrosideros polymorpha); hence, all trees at such sites can be expected to react in the same way to N fertilization. However, species in a mixed-species ecosystem may respond differently to the same exogenous stimulus, because the overall effect is influenced by each species’ response to the change in N availability of the ecosystem.
Changes of fine-root distribution after experimental manipulation were observed by Sayer et al. (Reference SAYER, TANNER and CHEESMAN2006) who doubled the monthly litter input in an old-growth lowland forest in Panama over a period of 1.75 y and measured fine-root biomass that had grown into the litter layer, at 0–5 cm and at 5–10 cm in the mineral soil. Higher fine-root biomass in the litter layer was found to be related to a decreased fine-root biomass in the mineral soil at 5–10 cm and was suggested to be promoted by the more easily obtainable nutrients in the doubled litter layer (Sayer et al. Reference SAYER, TANNER and CHEESMAN2006). In our study, the N-fertilized plots showed increased fine-root biomass at 10–20 cm in the mineral soil by ingrowth cores and also exhibited a constant decrease in fine-root biomass density in the organic layer by sequential coring. This trend suggests that fine roots were redistributed to the deeper mineral soil. N fertilization in our site has changed the magnitude of available N and the vertical distribution of mineral N, as indicated by increases in gross and net rates of soil N mineralization and nitrification in the organic layer and 0–5-cm depth of the mineral soil combined (Corre et al. Reference CORRE, VELDKAMP, ARNOLD and WRIGHT2010, Koehler et al. Reference KOEHLER, CORRE, VELDKAMP, WULLAERT and WRIGHT2009) and in nitrate concentrations of soil solutions at 1.5 m depth during the 2006–2007 measurements (Pame-Baldos Reference PAME-BALDOS2009). Furthermore, the increased available N in N-fertilized plots might have induced a change in fine-root redistribution to permit a more extensive exploration of the mineral soil for other nutrients without being constrained by the otherwise low N availability of the unamended soil. In addition, the increasing total P stocks from the organic layer to the mineral soil in our site (Table 1) suggest that the stronger fine-root redistribution to the mineral soil might be caused by fine roots foraging for P, which may cause additional limitation to above-ground production once N limitation is alleviated. In our forest site, we observed an increase in leaf-litter production, total litter production and above-ground NPP already in the first year after N fertilization began (Adamek et al. Reference ADAMEK, CORRE and HÖLSCHER2009). In a recent review, Benner et al. (in press) stated that tropical montane forests are usually not only N-limited but also often P-limited. This was also shown by the increased production of phosphatase enzymes by roots in N-fertilized Hawaiian montane forests (Olander & Vitousek Reference OLANDER and VITOUSEK2000, Treseder & Vitousek Reference TRESEDER and VITOUSEK2001) despite no change in fine-root production (Ostertag Reference OSTERTAG2001). In a comparison of three Amazonian forest types, fine roots were less concentrated in the organic layers of two relatively P-rich caatinga and bana forests growing on Spodosols than in a relatively N-rich tierra firme forest on an Oxisol (Sanford Reference SANFORD1989). Wood et al. (Reference WOOD, LAWRENCE and CLARK2006) reported that trees in the tropical lowland rain forest of La Selva, Costa Rica, exploit deeper nutrient pools for P than for other nutrients. If this also holds true for montane forests, it seems reasonable to infer that in our N-fertilized plots fine roots were redistributed to deeper soil depths to forage for P after limitation by N is alleviated.
In conclusion, N fertilization for 1.5 y did not affect fine-root biomass, necromass and production. Instead, fine-root biomass redistribution at 10–20 cm in the mineral soil increased at 1.5 y of N fertilization. The increased available N in the N-fertilized plots may have favoured the change in fine-root distribution to explore the deeper mineral soil for other nutrients which may cause additional limitation to above-ground production once N limitation is alleviated. Finally, this study was conducted during the early stage of N manipulation, and the short-term fine-root responses to N addition may be different from the long-term response under this continuing N manipulation experiment.
ACKNOWLEDGEMENTS
The Smithsonian Tropical Research Institute (STRI) provided administrative and technical support. We highly appreciate the help of the research assistants Arturo Morris, Rodolfo Rojas, Evidelio Garcia and Ignacio Delcid. We are grateful to Catharina Meinen and Marieke Harteveld for advice on root sampling, processing and production calculation. Dr James Dalling provided background information and assisted in the site selection. This study is under the NITROF project funded by the Robert Bosch Foundation (Germany) and awarded to M. D. Corre as independent research group leader. Permission to conduct research in the Fortuna Forest Reserve was kindly granted by the Panamanian National Environmental Authority (ANAM), ENEL Fortuna and STRI.
Appendix 1. Effects of N fertilization on fine roots (diameter ≤ 2 mm) in tropical montane forests. FRB = fine-root biomass, FRP = fine-root production, FRT = fine-root turnover, n.a. = not available.