Introduction
Parathalassiine flies are small (body length 1–3mm) nonmetallic greyish to dark-coloured dolichopodid flies that are generally found along sea coasts (Fig. 1) and river banks. They are distinguished from other dolichopodids by the following combination of features: antenna with single articled arista-like stylus, wing with cell dm usually present and emitting three veins (M1, M2, M4), crossvein bm-m nearly complete but not usually joining M1, and male terminalia with the left epandrial lamella bearing a ventral process. Seven extant genera are currently recognized in the subfamily Parathalassiinae, with only Parathalassius Mik and Microphorella Becker having been reported from the New World (Yang et al. Reference Yang, Zhang, Yao and Zhang2007; Cumming and Sinclair Reference Cumming, Sinclair, Brown, Borkent, Cumming, Wood and Zumbado2009). Here we record three additional genera, Thalassophorus Saigusa, Eothalassius Shamshev and Grootaert, and Chimerothalassius Shamshev and Grootaert, from the region, plus an additional undescribed genus. In this study a new species is described in each of Thalassophorus and Eothalassius and a key is given to the six genera occurring in the New World.

Fig. 1. Collection localities and habitats of Thalassophorus and Eothalassius. (A) Thalassophorus spinipennis Saigusa resting on intertidal rock on the coast of Rishiri Island, Japan (photograph by Masahiko Satô); (B–E) Collection localities of Thalassophorus arnaudi Brooks and Cumming sp. nov.: Ridley Island, British Columbia (B); Nellies Cove, Oregon (C); close-up of rocky substrate at Nellies Cove, Oregon (D); and Enderts Beach, California (E). (F) Collection locality of Eothalassius borkenti Cumming and Brooks sp. nov. at Cabo Blanco, Costa Rica, showing rock-shelf shoreline.
Study of the Parathalassiinae, as well as the other basal dolichopodid subfamily, Microphorinae, will ultimately increase our understanding of homologies throughout the remainder of the family, allowing for more rigorous phylogenetic analyses of the Dolichopodidae s.s. at the currently confused subfamily/tribe level (see discussion in Sinclair and Cumming Reference Cumming and Brooks2006). Generic limits within the Parathalassiinae and potential paraphyly of some of the included genera are presently being investigated by us (Cumming and Brooks Reference Cumming and Brooks2006; Brooks and Cumming Reference Brooks and Cumming2010). A detailed phylogenetic analysis of the entire subfamily will eventually be required to determine the number of valid genera that should be included. As we progress with our revisionary work on parathalassiines, we are compiling a large matrix of informative characters scored for numerous exemplars throughout the subfamily. This study represents the first of several revisions of New World Parathalassinae.
Material and methods
Specimens examined in this study are deposited in the Biosystematics Laboratory, Kyushu University, Fukuoka, Japan (BLKU), the Natural History Museum, London, United Kingdom (BMNH), the California Academy of Sciences, San Francisco, United States of America (CAS), the Canadian National Collection of Insects, Ottawa, Canada (CNC), the University of Guelph Insect Collection, Guelph, Canada, (DEBU), the Instituto Nacional de Biodiversidad, Santo Domingo de Heredia, Costa Rica (INBC), the United States National Museum of Natural History, Washington, D.C., United States of America (USNM), and the Zoologisches Forschungsmuseum Alexander Koenig, Bonn, Germany (ZFMK).
Terms used for adult structures primarily follow those of McAlpine (1981) and Cumming and Wood (Reference Cumming, Wood, Brown, Borkent, Cumming, Wood and Zumbado2009), except for the antenna and wing venation, where the terms of Stuckenberg (1999) and Saigusa (2006), respectively, are used. In the system outlined by Saigusa (2006), the dipteran wing vein A1 (as used in McAlpine Reference McAlpine1981) is homologized with the mecopteran CuP, and consequently CuA1 (of McAlpine) is termed M4, whereas CuA2 is CuA, the anal cell is cell cua, and the anal vein (A1+CuA2) is CuP +CuA. The wing-vein homologies as they relate to the Parathalassiinae are shown in Figure 2. Homologies of the male terminalia follow those of Sinclair and Cumming (Reference Cumming and Brooks2006).
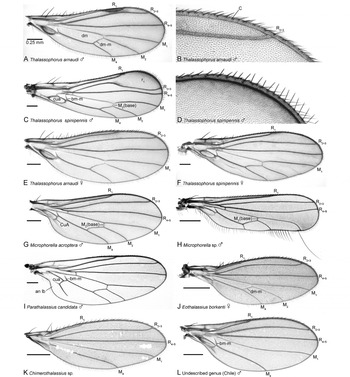
Fig. 2. Wings. (A) Thalassophorus arnaudi Brooks and Cumming sp. nov., male; (B) T. arnaudi Brooks and Cumming sp. nov., male, close-up of anterodistal portion, showing costal setae; (C) T. spinipennis Saigusa, male; (D) T. spinipennis Saigusa, male, close-up of anterodistal portion showing costal setae; (E) T. arnaudi Brooks and Cumming sp. nov., female; (F) T. spinipennis Saigusa, female; (G) Microphorella acroptera Melander, male; (H) Microphorella sp. (Yukon), male; (I) Parathalassius candidata Melander, male; (J) Eothalassius borkenti Cumming and Brooks sp. nov., female; (K) Chimerothalassius sp. (Dominica), sex unknown; (L) undescribed genus (Chile), male of undescribed species 1 (an lb, anal lobe; bm-m, basal medial crossvein; C, costal vein; cua, anterior cubital (=anal) cell; CuA, anterior branch of cubital vein; dm, discal medial cell; dm-m, discal medial crossvein; M1, 1st medial vein; M2, 2nd medial vein; M4, 4th medial vein; r1, 1st radial cell; R1, 1st radial vein; R2+3, 2nd+3rd radial vein; R4+5, 4th+5th radial vein).
Wing length is measured from the basicosta to the wing apex. Male and female terminalia were macerated in 85% lactic acid heated in a microwave oven. Figures of the male genitalia are oriented with the anatomically dorsal parts directed towards the top of the page and the anatomically ventral parts directed towards the bottom of the page, following Sinclair and Cumming (2006, figs. 347–350). In lateral views of the male genitalia, hatched areas delimit aspects of the medial surface of the opposing side that project beyond the limits of the facing side.
Systematics
The previous keys to the genera of Parathalassiinae from the Nearctic Region (Steyskal and Knutson Reference Steyskal and Knutson1981) and Central America (Cumming and Sinclair Reference Cumming, Sinclair, Brown, Borkent, Cumming, Wood and Zumbado2009) include only Parathalassius and Microphorella. With three additional genera, Thalassophorus, Eothalassius, and Chimerothalassius, now known from the New World, we present here a key to the parathalassiine genera of the entire region. We also include in the key a new undescribed genus that is currently known to us from very limited material of two undescribed species from the rocky coastline of the Valparaiso Region of Chile (specimens are deposited in DEBU).
Key to New World genera of the subfamily Parathalassiinae
1. Wing cell dm present, with veins M2 and dm-m (Figs. 2A, 2C, 2E–2J)………2
— Wing cell dm absent, without veins M2 and dm-m (Figs. 2K, 2L)………5
2. CuA rounded, cell cua convex apically to entirely ovoid; anal lobe not developed (Figs. 2A, 2C, 2E–2H, 2J); scutellum with 1 pair of strong bristles………3
— CuA straight, cell cua truncate apically; anal lobe partially developed (Fig. 2I); scutellum with 2–3 pairs of strong bristles………Parathalassius Mik
3. Gena well-developed and projected below eye; palpus triangular, tapered to pointed tip (Fig. 3A); basal portion of costa with 2 strong spine-like bristles near crossvein h and 2 weaker spine-like bristles level with base of R2+3 (Figs. 2A, 2C, 2E, 2F); female cercus terminating as a slender cuticular projection (Figs. 5D, 5E)………Thalassophorus Saigusa
— Gena weakly developed, scarcely projected below eye; palpus broadly or narrowly rounded apically, not triangular (cf. Fig. 3B); basal portion of costa with at most one spine-like bristle (Figs. 2G, 2H, 2J); female cercus terminating with a long seta (Figs. 11A, 11C) or as a blunt point (Fig. 11D)………4
4. R1 reaching costa before middle of wing (or before base of M2) (Fig. 2J); arista-like stylus lengthened, at least 5 times length of postpedicel (Fig. 3C); palpus prominent, broadly rounded apically (cf. Fig. 3B); male terminalia with right epandrial lamella partially fused with hypandrium (Figs. 8B, 9B, 10B); female terminalia with acanthophorite setae (Figs. 11A, 11C, tg 10)………Eothalassius Shamshev and Grootaert
— R1 reaching costa beyond middle of wing (or beyond base of M2) (Figs. 2G, 2H); arista-like stylus not lengthened, at most 2 times length of postpedicel (cf. Figs. 3A, 3B); palpus small, narrowly rounded apically; male terminalia with right epandrial lamella not fused with hypandrium (cf. Figs. 4C, 6B); female terminalia with acanthophorite spines (Fig. 11D, tg 10)………Microphorella Becker
5. Gena scarcely projected below eye; mouthparts directed ventrally (cf. Fig. 3A) with broad fleshy labellum; palpus elongate and narrow, with long ventral setae; fore coxa with strong basal seta, anterior surface without field of short stout spine-like setae; female terminalia with acanthophorite setae (cf. Figs. 11A, 11C, tg 10)………Chimerothalassius Shamshev and Grootaert
— Gena distinctly projected below eye (Fig. 3B); mouthparts directed posteriorly with narrowed labellum (Fig. 3B); palpus broad, without long ventral setae (Fig. 3B); fore coxa without strong basal seta, anterior surface with field of short stout spine-like setae; female terminalia with acanthophorite spines (cf. Fig. 11D, tg 10)……undescribed genus (Chile)
Genus Thalassophorus Saigusa
Thalassophorus Saigusa, Reference Saigusa1986: 106.
Type species: Thalassophorus spinipennis Saigusa, by original designation.
Included species
The genus currently includes Thalassophorus spinipennis Saigusa and Thalassophorus arnaudi Brooks and Cumming sp. nov.

Fig. 3. Heads and antennae. (A) Thalassophorus arnaudi Brooks and Cumming sp. nov., lateral view of male head; (B) undescribed genus (Chile), lateral view of male head of undescribed species 1; (C) Eothalassius borkenti Cumming and Brooks sp. nov., lateral view of male antenna (ar styl, arista-like stylus; gn, gena; plp, palpus; pped, postpedicel).
Diagnosis
Thalassophorus is distinguished from other parathalassiine genera by the following characters: head (Fig. 3A) with gena well-developed and projected below eye, mouthparts directed ventrally with fleshy labellum, epipharyngeal carina lengthened and projecting vertically into head, palpus large triangular and tapered to pointed tip; thorax with prosternum fused to proepisternum forming precoxal bridge, scutellum with 1 pair of strong dorsally directed bristles near apex; wing (Figs. 2A, 2C, 2E, 2F) with costa bearing double row of short spine-like setae along anterior margin, basal portion of costa bearing 4 dorsally inclined spine-like bristles (2 strong bristles above crossvein h and 2 weaker bristles above base of R2+3), R1 reaching costa beyond middle of wing (or beyond base of M2), crossvein bm-m complete (T. spinipennis) or incomplete (T. arnaudi), cell dm present with veins M2 and dm-m, CuA rounded, cell cua ovoid, anal lobe not developed; male abdominal sternite 5 with large ventral prolongation (Fig. 4A); hypopygium (Figs. 4B, 4C, 6A, 6B) with right and left epandrial lamellae apparently separated from hypandrium, left epandrial lamella with weakly articulated ventral process, asymmetrical medial hypandrial process arising from posterior end with left side truncate and right side rounded in posterior view, postgonites cradling base of phallus with left and right lobes protruding from between dorsal and ventral surstylar lobes, phallus with basal flange, apex bifurcate, phallic plate present, hypoproct projected as undivided medial lobe bearing apical setae, cerci narrow and nearly symmetrical (Figs. 5C, 6C); female abdomen with apical segments retracted into segment 6 (T. spinipennis) or partially retracted into segment 5 (T. arnaudi), tergite and sternite 6 with row of well-developed posteromarginal setae, terminalia with tergite 10 bearing acanthophorite setae, cercus terminating as a slender cuticular projection (Figs. 5D, 5E).
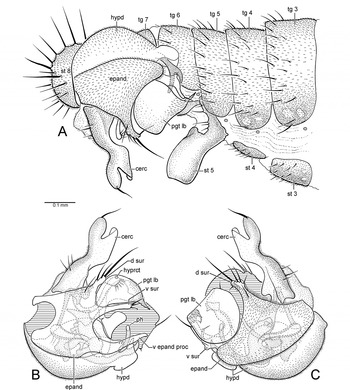
Fig. 4. Thalassophorus arnaudi Brooks and Cumming sp. nov., male. (A) Posterior portion of abdomen, right lateral view; (B) hypopygium, left lateral view; (C) hypopygium, right lateral view (cerc, cercus; d sur, dorsal lobe of surstylus; epand, epandrium; hypd, hypandrium; hyprct, hypoproct; pgt lb, postgonite lobe; ph, phallus; st, sternite; tg, tergite; v epand proc, ventral epandrial process; v sur, ventral lobe of surstylus).
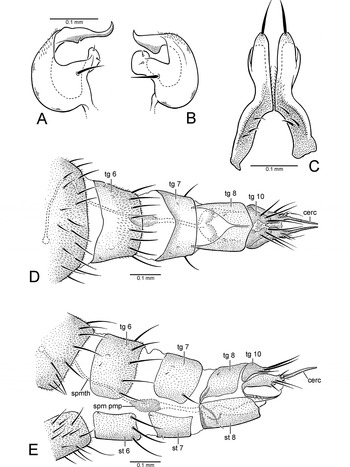
Fig. 5. Thalassophorus arnaudi Brooks and Cumming sp. nov. (A) Right postgonite lobe of male hypopygium, dorsal view; (B) left postgonite lobe of male hypopygium, dorsal view; (C) male cerci, dorsal view; (D) female terminalia, dorsal view; (E) female terminalia, left lateral view (cerc, cercus; spm pmp, sperm pump; spmth, spermatheca; st, sternite; tg, tergite).
Distribution
Thalassophorus is currently known from seacoasts of Rishiri Island, Hokkaido Prefecture, Japan (T. spinipennis; Fig. 7A), and western North America from British Columbia south to northern California (T. arnaudi; Fig. 7B).

Fig. 6. Thalassophorus spinipennis Saigusa, male paratype. (A) Hypopygium, left lateral view; (B) hypopygium, right lateral view; (C) cerci, dorsal view (cerc, cercus; d sur, dorsal lobe of surstylus; epand, epandrium; hypd, hypandrium; hyprct, hypoproct; pgt lb, postgonite lobe; ph, phallus; v epand proc, ventral epandrial process; v sur, ventral lobe of surstylus).
Remarks
Males of both species of Thalassophorus possess a large broad ventral prolongation of male abdominal sternite 5 (Fig. 4A). We have observed a very similar modification of sternite 5 in a southern European species of Microphorella that appears to be conspecific with Microphorella curtipes (Becker) (but see Shamshev and Grootaert Reference Shamshev and Grootaert2004, table 1). Pregenitalic modifications of sternite 5 (and sternite 6) have also been recorded in some species of Microphorella from Southeast Asia and New Guinea (Shamshev and Grootaert Reference Shamshev and Grootaert2004).
Species of Thalassophorus are restricted to rocky or stony marine shores, where adults occur on wet stones and rocks in the intertidal zone (Figs. 1A–1E). Immature stages are presently unknown.
Thalassophorus arnaudi Brooks and Cumming sp. nov.
(Figs. 1B–1E, 2A, 2B, 2E, 3A, 4, 5, 7B)
Type material
Holotype ♂, labelled: “CAN: BC: Prince Rupert/ Ridley Island, N54°14.13′/ W130°19.80′, 16.viii.2008/ shore/beach with rocks, logs/ & boulders S.E. Brooks”; “HOLOTYPE/ Thalassophorus arnaudi/ Brooks & Cumming” [red label] (CNC).
Paratypes: CANADA: British Columbia: 2 ♂♂, 5 ♀♀, same data as holotype; 1 ♂, 1 ♀, same data except, 15.viii.2008; 6 ♀♀, same data except 17.viii.2008; 1 ♂, 2 ♀♀, same data except 17.viii.2008, J.M. Cumming; 1 ♀, Prince Rupert, Ridley Island, 7.viii.1996, P.H. Arnaud, Jr., M.M. Arnaud, beach of rocks, boulders, and logs (USNM); UNITED STATES OF AMERICA: Oregon: 11 ♂♂, 9 ♀♀, Curry Co., Port Orford, Nellies Cove, 42°44′16″N, 124°30′28″W, 30.v.2009, rocky seashore, S.E. Brooks (CNC); 7 ♂♂, 4 ♀♀, same data except J.M. Cumming (CNC); California: 23 ♂♂, 14 ♀♀, Del Norte Co., Crescent City, Enderts Beach, 41°41′59″N, 124°08′31″W, 1.vi.2009, ex. seashore rocks, S.E. Brooks (CNC); 1 ♀, same data (USNM); 1 ♀, same data (CAS); 1 ♂, 1 ♀, same data (BMNH); 1 ♂, same data (ZFMK); 1 ♂, 1 ♀, same data (BLKU); 7 ♂♂, 1♀, same data except J.M. Cumming (CNC); 1 ♀, same data (USNM); 1 ♂, same data (CAS); 1 ♀, same data (ZFMK); 1 ♀, same data except, 3.vi.2009, tidal rocks, B.J. Sinclair (CNC).
Diagnosis
Males of T. arnaudi are distinguished from those of T. spinipennis by the following features: costa with swelling at end of R1 (Fig. 2A) (absent in T. spinipennis; Fig. 2C); cell r1 narrow beyond R1, ending well before wing apex (Fig. 2A) (expanded and extending to wing apex in T. spinipennis; Fig. 2C); apical costal section between R1 and M1 lacking erect spine-like setae (Fig. 2B) (setae present in T. spinipennis; Fig. 2D); R2+3 and R4+5 widely separated in apical part of wing (Fig. 2A) (closely approximated in T. spinipennis; Fig. 2C); bm-m incomplete (Figs. 2A, 2E) (complete in T. spinipennis; Figs. 2C, 2F); right postgonite lobe broad (Fig. 4C) (narrow in T. spinipennis; Fig. 6B); phallus with non-serrated claw-like process (Figs. 4B, 4C) (process serrated and fin-like in T. spinipennis; Figs. 6A, 6B); cerci with apices straight in dorsal view (Fig. 5C) (divergent in T. spinipennis; Fig. 6C); cercus constricted at middle in lateral view (Figs. 4A–4C) (not constricted in T. spinipennis; Figs. 6A, 6B). Females of T. arnaudi and T. spinipennis are distinguished by the following features: costal section between R1 and R2+3 about 2 times length of costal section between R2+3 and R4+5 (Fig. 2E) (about 3.5 times length in T. spinipennis; Fig. 2F); R2+3 and M1 divergent (Fig. 2E) (subparallel in T. spinipennis; Fig. 2F); abdominal tergite 8 narrowly divided medially (Fig. 5D) (broadly divided in T. spinipennis).

Fig. 7. Known distribution of Thalassophorus Saigusa. (A) Thalassophorus spinipennis Saigusa; (B) T. arnaudi Brooks and Cumming sp. nov.
Description
Male: Body length 1.6–2.3mm, wing length 2.1–2.3mm. Dark brown ground colour, with greyish blue-green and rusty brown pruinosity, legs slightly paler. Head (Fig. 3A): Dark brown ground colour with dense greyish blue-green pruinosity, dorsally with faint rusty tinge. Broader than thorax in dorsal view; ovoid in lateral view, with lower margin truncate; about 1.3 times broader than high in anterior view. Neck inserted high on head. Ocellar triangle conspicuous. Occiput weakly concave on upper median part. Eyes dichoptic, entirely covered with ommatrichia, medial edge with weak emargination near antenna, ommatidia smaller anterodorsally. Frons greyish blue-green with faint rusty tinge, broadly widening above. Face greyish blue-green, narrowest at middle, with width less than diameter of anterior ocellus. Clypeus greyish blue-green, not separated from face, projecting and somewhat beak-like in lateral view when mouthparts exerted (flattened when mouthparts retracted), widening below, apical margin weakly pointed. Bristles of head well-differentiated; dorsal bristles strong and black: pair of inclinate fronto-orbitals well-separated from base of antenna, pair of lateroclinate anterior ocellars, 2–3 pairs of tiny proclinate posterior ocellars, pair of inclinate postocellars, 2–4 pairs of lateroclinate verticals (verticals variable in number and arrangement); postocular setae short and uniserial dorsally becoming longer and scattered below, uppermost postoculars dark, remainder pale; postgena with pale setae along edge of mouth opening. Antenna entirely dark brown, inserted above middle of head in profile; scape short, funnel-shaped; pedicel subequal in length to scape, spheroidal with subapical circlet of setulae; postpedicel about 1.8–2.1 times longer than wide, drop-shaped, clothed in moderately long fine hairs; stylus about 1.7 times length of postpedicel, with short hairs. Palpus dark brown, clothed with minute pile, apical half with several weak setae on outer surface. Epipharyngeal carina lengthened, extended to clypeal ridge, keel-shaped in lateral view; epipharyngeal blades narrow; labellum with 6 geminately sclerotized pseudotracheae. Gena strongly projected below eye. Thorax: Dark brown ground colour with dense greyish blue-green pruinosity, with rusty brown tinge in dorsal view. Mesoscutum moderately arched, prescutellar depression apparent. Proepisternum with small setula. Antepronotum narrow with 2 pale setulae per side. Postpronotal lobe distinct with 1–2 small setulae. Mesonotum shield-shaped in dorsal view, longer than wide, bristles well-differentiated and black. Acrostichal setae absent, pair of setulae on extreme anterior margin of mesoscutum; anterior mesoscutum with transverse row or group of 3–4 setulae per side anterolateral to 1st dorsocentral bristle; 5–6 dorsocentral bristles (2nd dorsocentral strongest, 1st, 3rd, and 4th sometimes reduced, 1–2 additional setulae sometimes present), 1 strong presutural supra-alar bristle, 1–3 postsutural supra-alar bristles, 2 notopleural bristles, and 1 post-alar bristle per side. Scutellum broadly subtriangular. Mesopleuron bare. Halter with light-brown stalk, knob pale brownish white. Legs: Dark brown, coxae and femora with blue-green tinged pruinosity (weaker on femora), femora and tibiae paler at knees, tarsi somewhat lighter on ventral surface; legs (except anterior surface of mid-femur) devoid of well-developed bristles, mostly covered with fine black setae, except for coxae, trochanters, and ventral surface of femora with pale setation; tarsomere 5 with apex broadly pointed in dorsal view; claws enlarged. Foreleg: Coxa with pale setae on anterior surface; femur somewhat thickened basally, slightly longer than tibia, basal third with field of short pale hairs ventrally; tibia slender; tarsus slightly longer than tibia; basitarsus as long as tarsomeres 2–4 combined, without spinose anterior tubercle at base; tarsomeres 2–4 decreasing in length apically; tarsomere 5 subequal in length to tarsomere 3. Midleg: Coxa with pale setae on anterior surface along lateral and apical margins; femur slender, slightly longer than tibia, basal third with field of short pale hairs ventrally, apical 2/3 with anterior row of 9–10 prominent bristles; tibia slender; tarsus slightly longer than tibia; basitarsus as long as tarsomeres 2–4 combined; tarsomeres 2–4 decreasing in length apically; tarsomere 5 slightly longer than tarsomere 4. Hindleg: Coxa with 3–4 pale setae on outer surface; femur slender, slightly longer than tibia, ventral margin with series of short pale setae becoming stronger and darker apically; tibia slender, weakly bowed, slightly enlarged apically, with dorsal setae prominent and somewhat erect; tarsus subequal in length to tibia; basitarsus as long as tarsomeres 2–3 combined, without spinose posteroventral tubercle at base; tarsomeres 2–4 decreasing in length apically; tarsomere 5 slightly longer than tarsomere 4. Wing (Figs. 2A, 2B): Hyaline, about 3 times longer than wide, veins dark brown. Pterostigma absent, membrane entirely covered with minute microtrichia, alula absent. Costa circumambient, with swelling present at end of R1. Extreme anterior base of costa with row of 4 setae, anterior margin bearing 2 rows of short spine-like setae (in addition to 4 dorsally inclined spine-like bristles), posterior margin with setae finer and longer. Costal section between crossvein h and R1 with setae prominent and widely spaced. Costal section between R1 and M1 lacking erect spine-like setae (Fig. 2B, cf. Fig. 2D). All longitudinal veins complete, reaching wing margin, Sc faint apically. R1 reaching wing margin at costal swelling. Cell r1 narrow, not expanded beyond R1, ending well before wing apex. R2+3 and R4+5 widely spaced and parallel in apical part of wing. R4+5 straight. R4+5 and M1 widely spaced and divergent in apical part of wing. M1, M2, and M4 nearly straight beyond cell dm. Base of Rs originating opposite humeral crossvein. Short r-m crossvein present (sometimes indistinct) in basal fourth of wing at base of R4+5. Crossvein bm-m incomplete. Cell dm extending to middle of wing. Cells br, bm, and cua in basal fourth of wing. Cell br slender, subequal in length to bm and cua, sometimes indistinct. Cells bm and cua broader than br. Cell cua closed, with ventral margin darkened and thick. Anal vein (CuP+CuA) absent. Calypter with fine pale setae. Abdomen (Fig. 4A): Dark brown ground colour with blue-green tinged pruinosity. Abdominal muscle plaques present. Tergites 1–6 with short posteromarginal setae; sternites 2–4 with scattered marginal setulae; sternite 5 with single row of posteromarginal setulae lateral to ventral prolongation, otherwise bare; segments 6 and 7 with few setulae, mainly bare. Segments 1–4 symmetrical, with simple tergites and sternites; segments 5–7 narrowed, somewhat more heavily sclerotized and laterally compressed to form cavity on right side for hypopygium. Sternites 6–7 simple, not contorted, lacking pregenitalic processes. Sternite 8 ovoid, setose, tergite 8 forming narrow sclerotized band. Hypopygium (Figs. 4A–4C): Dark brown, projecting apical components paler. Lateroflexed to right; inverted, with posterior end directed anteriorly; small and compact, about 1/3 length of abdomen; asymmetrical; foramen unformed. Epandrium divided into left and right lamellae. Left epandrial lamella (Fig. 4B) trifurcate in lateral view with rounded basal emargination, partially overlapping left side of hypandrium, ventral portion fused with hypandrium but epandrial margin distinct; ventral epandrial process (Fig. 4B) digitiform, weakly tapering apically, bearing 2 short, closely approximated setulae before middle. Left surstylus (Fig. 4B) bilobed, dorsal and ventral lobes separated by deep U-shaped cleft through which left postgonite lobe protrudes. Dorsal lobe of left surstylus shorter than ventral lobe, with several long apical setae and 1 lateral seta at base. Ventral lobe of left surstylus digitiform, length about 2 times that of dorsal lobe, apex with several long medially directed setae. Right epandrial lamella (Figs. 4A, 4C) broadly flask-shaped in lateral view, partially overlapping right side of hypandrium, not fused with hypandrium, lacking ventral process. Right surstylus (Fig. 4C) bilobed, dorsal and ventral lobes separated by broad shallow cleft through which right postgonite lobe protrudes. Dorsal lobe of right surstylus subequal in length to ventral lobe, with several long apical setae and 1 lateral seta at base. Ventral lobe of right surstylus complex, trifurcate, mostly hidden in lateral view by right postgonite lobe. Hypandrium (Figs. 4B, 4C) bowl-shaped, subequal in length to epandrium. Postgonite with internal portion arch-shaped in dorsal/ventral view with short anterolateral apodemes; left postgonite lobe (Figs. 4B, 5B) broad, dome-like, concave medially, with pointed, medially directed apical projection and thumb-like medial projection bearing 1 strong seta basally and 2 setulae near expanded apex, with auxiliary flap-like lobe situated below ventral lobe of surstylus; right postgonite lobe (Figs. 4A, 4C, 5A) similar to left lobe but larger, with apical margin truncate, pointed apical projection elongate, auxiliary lobe indistinct. Phallus with apical portion Y-shaped, with nonserrated claw-like process (Figs. 4B, 4C). Ejaculatory apodeme (Figs. 4B, 4C) keel-like, laterally flattened. Hypoproct (Figs. 4B, 4C) about half cercus length, slender with weak apical setae. Cercus (Figs. 4A–4C) constricted at middle in lateral view, apex straight in dorsal view (Fig. 5C) and bearing strong apical seta.
Female: Body length 1.8–2.6mm, wing length 2.4–2.7mm. Similar to male except face slightly wider; costa lacking swelling at end of R1. Terminalia (Figs. 5D, 5E) with tergite 8 narrowly divided medially (Fig. 5D), narrowly fused with sternite 8 anterolaterally; tergite 10 medially divided, with three long acanthophorite setae on each side; cercus elongate, with several setae anterior to slender cuticular apical projection; spermatheca an unsclerotized unpigmented tube with sperm pump at base.
Distribution
Unlike its Palaearctic congener T. spinipennis, which is presently known only from Rishiri Island, Japan (Fig. 7A), T. arnaudi appears to be more widely distributed in the Nearctic Region and occurs along the west coast from seashore localities in British Columbia, Oregon, and California (Figs. 1B–1E, 7B).
Remarks
The habitat of T. arnaudi ranges from primarily rocky shorelines comprised of boulders mixed with cobble and gravel (Figs. 1B–1D) to more sandy beaches with regular cobble-gravel deposits and emergent boulders (Fig. 1E). Adults were collected by sweeping over bare intertidal rocks.
Etymology
This new species is named in honour of dipterist and renowned collector Dr. Paul H. Arnaud, Jr., of the California Academy of Sciences. Paul and his wife, Madeline, collected the first known specimen from Ridley Island, British Columbia, in 1996.
Genus Eothalassius Shamshev and Grootaert
Eothalassius Shamshev and Grootaert, Reference Shamshev and Grootaert2005: 108.
Type species:Eothalassius platypalpus Shamshev and Grootaert, by original designation.
Included species
The genus currently includes Eothala-ssius platypalpus Shamshev and Grootaert, Eothalassius gracilis Shamshev and Grootaert, and Eothalassius borkenti Cumming and Brooks sp. nov.Microphorella merzi Gatt also appears to belong to Eothalassius (Shamshev and Grootaert Reference Shamshev and Grootaert2005, p. 117) (see Remarks below).
Diagnosis
Eothalassius is distinguished from other parathalassiine genera by the following characters: head with face narrow ventrally, gena scarcely projected below eye, antenna with arista-like stylus lengthened (at least 5 times length of postpedicel) (Fig. 3C), mouthparts directed ventrally with fleshy labellum, epipharyngeal carina lengthened and projecting vertically into head, palpus broad (particularly in males) with apex slightly pointed to widely rounded apically; thorax with prosternum fused to proepisternum forming precoxal bridge, scutellum with 1 pair of strong bristles near apex; wing (Fig. 2J) with costa bearing double row of short spine-like setae along anterior margin, R1 short reaching costa before middle of wing (or before base of M2), crossvein bm-m incomplete, cell dm present or if absent without vein dm-m, CuA rounded, cell cua convex apically, anal lobe not developed; male terminalia (Fig. 10) with epandrium partially fused with hypandrium, left epandrial lamella with apparently non-articulated ventral process, postgonites cradling base of phallus with left and right lobes protruding from between dorsal and ventral surstylar lobes, hypoproct projected as pair of non-setose asymmetrical lobes, cerci broad and asymmetrical (Figs. 9B, 10C); female abdomen with apical segments retracted into segment 6, terminalia with tergite 10 bearing acanthophorite setae, cercus slender and terminating with long seta (Figs. 11A, 11C).
Distribution
Eothalassius is known from the coasts of Southeast Asia and Papua New Guinea (E. gracilis, E. platypalpus) (Shamshev and Grootaert Reference Shamshev and Grootaert2005) (Fig. 12A) and the Pacific side of Costa Rica (E. borkenti) (Fig. 12B). Microphorella merzi, which is probably congeneric with Eothalassius, is known from the Mediterranean coast of Turkey, Cyprus, and Malta (Gatt Reference Gatt2003) (Fig. 12C).
Remarks
As reported by Gatt (2003), M. merzi has the hypopygium with the epandrium and hypandrium partially fused, the female terminalia with acanthophorite setae and a slender cercus terminating in a long apical seta, a head with a narrow ventral face and an enlarged male palpus. On the basis of these characters and the lack of cell dm with crossvein dm-m absent (similar to E. platypalpus), it is likely that M. merzi belongs to Eothalassius as suggested by Shamshev and Grootaert (2005, p. 117), although the antennal stylus is not as lengthened as in other species of the genus. However, formal assignment of this species within Eothalassius as a new combination should await a detailed examination of this species.
Shamshev and Grootaert (2005) indicate that the type series of E. gracilis was collected from the littoral and supralittoral zones of sandy coastal beaches, whereas E. platypalpus was collected along creeks exiting onto sandy coastal beaches. Microphorella merzi was also collected on sandy coastal beaches (Gatt Reference Gatt2003). Eothalassius borkenti appears to favour rocky seashores (Fig. 1F). Immature stages are presently unknown.
Eothalassius borkenti Cumming and Brooks sp. nov.
(Figs. 1F, 2J, 3C, 8, 9, 10, 11A–11C, 12B)
Type material
Holotype ♂, labelled: “CR: Herradura/ 16.XII.1993/ A. Borkent/ CD 1709”; “ex. tidal rocks/ at low tide”; “HOLOTYPE/ Eothalassius borkenti/ Cumming and Brooks” [red label] (CNC). Paratypes: COSTA RICA: 1 ♂, 1 ♀, same data as holotype (CNC); 1 ♀, 1 partial specimen (sex unknown), same data except, 25.x.1993, CD 1629, ex. tidal pools (CNC); 1 ♂, Manuel Antonio National Pk., 17.xi.1993, A. Borkent, CD 1670, sweeping intertidal zone near entrance of park (flowing freshwater stream nearby) (INBC); 1 ♀, Cabo Blanco, 19.i.1994, A. Borkent, CD 1734, tidal rocks (CNC); 1 ♀, Puntarenas, 2km S Cabuya, 10.xi.2000, A. Borkent CD 5098*, swp tidal rocks (CNC); 1 ♀, same data (INBC); 1 ♂, Puntarenas, Corcovado National Pk., San Pedrillo, 1–50m, 8°37′15N, 83°44′6E, 14.viii.2001, M. Buck, rocky seashore sweeping, debu00176474 (DEBU); 1 ♀, same data except debu00176399 (DEBU); 1 partial specimen (sex unknown), same data except debu00176410 (DEBU); 3 ♀♀, same data except 11.viii.2001, S.A. Marshall, beach sweep, debu00187966, debu00187988, debu00187989 (DEBU); 1 ♀, Osa Peninsula, Corcovado National Pk., nr. San Pedrillo Ranger Stn, 8°37′N, 83°44′E, 12–15.viii.2001, J.M. Cumming, intertidal zone (CNC).
Diagnosis
The only species of Eothalassius known from the New World, E. borkenti is most easily distinguished from other congeners (i.e., E. platypalpus, E. gracilis, and M. merzi) by the presence of relatively long setae on the basal portion of the costa, including a single erect spine-like costal bristle near the base of R2+3 (Fig. 2J). Eothalassius borkenti has the wing with cell dm (and crossvein dm-m) present, unlike E. platypalpus and M. merzi but not E. gracilis. In addition, males of E. borkenti have the hypopygium characterized by large broad asymmetrical cerci and distinctively shaped asymmetrical hypoproct lobes (Figs. 10A–10C), and females have each acanthophorite of the terminalia narrowed anteriorly (Figs. 11A, 11C, tg 10).
Description
Male: Body length 1.2–1.3mm, wing length 1.1–1.2mm. Dark brown ground colour with greyish green and rusty brown pruinosity, legs slightly paler. Head: Dark brown ground colour with greyish green pruinosity. Broader than thorax in dorsal view; ovoid in lateral view; about 1.3 times broader than high in anterior view. Neck inserted high on head. Ocellar triangle conspicuous. Occiput weakly concave on upper median part. Eyes dichoptic, entirely covered with ommatrichia, medial edge with very weak emargination near antenna, ommatidia smaller anterodorsally. Frons greyish green, broadly widening above. Face narrowest at middle, width less than diameter of anterior ocellus. Clypeus greyish, small, not separated from face. Bristles of head well-differentiated; strong and dark brown: pair of reclinate fronto-orbitals above antennal base, pair of lateroclinate anterior ocellars, 2 pairs of tiny proclinate posterior ocellars, pair of inclinate postocellars, 2 pairs of lateroclinate verticals; postocular setae short and uniserial, pale; postgena with pale setae along edge of mouth opening. Antenna (Fig. 3C) brown, inserted near middle of head in profile; scape short, funnel-shaped; pedicel slightly longer than scape, spheroidal with subapical circlet of setulae; postpedicel about as long as wide, drop-shaped, clothed in moderately long fine hairs; stylus at least 5 times length of postpedicel, with short hairs. Palpus brown, clothed with minute pile and several weak setae on outer surface; nearly as wide as long, broadly rounded to nearly truncate apically. Epipharyngeal carina lengthened, extended to and overlapped by clypeal ridge; epipharyngeal blades narrow; labellum apparently with 6 inconspicuous geminately sclerotized pseudotracheae. Gena narrow. Thorax: Dark brown ground colour with dense greyish green pruinosity, with rusty brown tinge in dorsal view. Mesoscutum moderately arched, prescutellar depression barely apparent. Proepisternum and antepronotum without small setulae. Postpronotal lobe distinct with 1 small setula. Mesonotum shield-shaped in dorsal view, longer than wide, bristles well-differentiated. Acrostichal setae absent; 4 dorsocentral bristles (1st weakest, 3rd sometimes absent), 1 presutural supra-alar bristle (close to upper notopleural bristle), 1 postsutural supra-alar bristle, 2 notopleural bristles (upper notopleural close to presutural supra-alar bristle), and 1 small post-alar bristle per side. Scutellum broadly subtriangular; scutellar bristles usually directed dorsally. Mesopleuron bare. Halter with light-brown stalk, knob pale brownish white. Legs: Brown, tibiae and tarsi slightly darker; legs (except anterior surface of mid-femur) devoid of well-developed bristles, mostly covered with fine pale-brown setae; tarsomere 5 with broad apical process in dorsal view. Foreleg: Coxa with pale setae on anterior surface; femur somewhat thickened basally, slightly longer than tibia; tibia slender; tarsus slightly longer than tibia; basitarsus as long as tarsomeres 2–4 combined, without spinose anterior tubercle at base; tarsomeres 2–4 decreasing in length apically; tarsomere 5 subequal in length to tarsomere 3. Midleg: Coxa with few pale apical setae; femur slender, slightly longer than tibia, apical 1/2 with anterior row of 5–6 prominent bristles; tibia slender; tarsus slightly longer than tibia; basitarsus as long as tarsomeres 2–4 combined; tarsomeres 2–4 decreasing in length apically; tarsomere 5 twice as long as tarsomere 4. Hindleg: Coxa with few pale apical setae; femur slender, slightly longer than tibia, weakly bowed; tibia slender, slightly enlarged apically; tarsus subequal in length to tibia; basitarsus nearly as long as tarsomeres 2–3 combined, without spinose posteroventral tubercle at base; tarsomeres 2–4 decreasing in length apically; tarsomere 5 slightly longer than tarsomere 4. Wing (Fig. 2J): Hyaline, about 3 times longer than wide, veins light brown. Pterostigma absent, membrane entirely covered with minute microtrichia, alula absent. Costa circumambient, with single erect spine-like costal bristle near base of R2+3. Anterior costal margin with double row of spine-like setae longer, more prominent, and widely spaced towards base. Posterior margin of costa with setae finer and longer. All longitudinal veins complete, reaching wing margin, Sc faint apically. R1 reaching wing margin before middle of wing. R2+3, R4+5, and M1 equally spaced, nearly parallel in middle of wing and divergent in apical part of wing. M1, M2, and M4 slightly curved beyond cell dm. Base of Rs originating opposite humeral crossvein. Short r-m crossvein indistinct in basal fourth of wing at base of R4+5. Crossvein bm-m incomplete. Cell dm present, extending to middle of wing, with vein dm-m. Cells br, bm, and cua in basal fifth of wing. Cell br indistinct. Cells bm and cua subequal in length and width, both slightly broader than br. Cell cua closed, with distal margin nearly abutting costa. Anal vein (CuP+CuA) absent. Calypter with fine setae.Abdomen (Figs. 8, 9): Brown ground colour with rusty brown pruinosity. Abdominal muscle plaques present. Tergites 1–6 with short posteromarginal setae; sternites 2–4 with scattered setulae; sternites 5–6 with 2 setulae; tergite 7 bare; sternite 7 bare. Segments 1–4 symmetrical with simple tergites and sternites; at least segments 6–7 narrowed and laterally compressed to form cavity on right side for hypopygium. Sternites 6–7 simple, not contorted, lacking pregenitalic processes. Sternite 8 quadrate, with several setae posteriorly, tergite 8 atrophied. Hypopygium (Figs. 8, 9, 10): Lateroflexed to right; inverted with posterior end directed anteriorly; small, less than half length of abdomen; asymmetrical; foramen unformed. Epandrium divided into left and right lamellae. Left epandrial lamella partially overlapping left side of hypandrium, trifurcate in lateral view (Fig. 10A); ventral epandrial process present (Figs. 10A, 10C), apparently non-articulated at base, bearing 2 short setulae near midpoint, with spatulate apex. Left surstylus (Figs. 10A, 10C) bilobed, dorsal and ventral lobes separated by broad cleft through which left postgonite lobe protrudes. Dorsal lobe of left surstylus with 2 small apical processes bearing a long bristle. Ventral lobe of left surstylus blunt, with 3 short apical setae. Right epandrial lamella (Figs. 8B, 10B) broadly subrectangular in lateral view, partially fused with hypandrium, lacking ventral process. Right surstylus (Figs. 10B, 10C) bilobed, dorsal and ventral lobes separated by shallow cleft through which right postgonite lobe protrudes. Dorsal lobe of right surstylus short, bearing 2 long apical bristles. Ventral lobe of right surstylus flap-like, articulated at base, bearing 1 long curved ventral bristle and several apical setae. Hypandrium short and deep in lateral view (Figs. 8B, 10A, 10B), without medial process arising from posterior end (Figs. 8A, 10C). Postgonite with internal portion arch-shaped in dorsal/ventral view with short anterolateral apodemes, projecting dorsomedially as pointed process; left postgonite lobe (Figs. 9B, 10A, 10C) multilobate, with rounded dorsal projection bearing 1 strong seta above slender medial projection and pointed elongate ventral projection; right postgonite lobe (Figs. 9B, 10B, 10C) similar to left lobe but flap-like and shallowly trifurcate along medial margin, with thick strong seta plus short seta on dorsal margin. Phallus tubular, J-shaped in lateral view, bent upwards (Figs. 10A–10C). Ejaculatory apodeme short, rod-like. Hypoproct projected as pair of overlapping asymmetrical lobes; left lobe broad with flange-like apex, right lobe digitiform with blunt apex (Fig. 10C). Cerci broad, asymmetrical (Figs. 8B, 9A, 9B, 10), left cercus partially divided into basal and apical components.
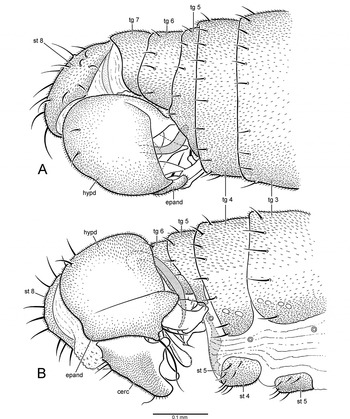
Fig. 8. Eothalassius borkenti Cumming and Brooks sp. nov., male. (A) Posterior portion of abdomen, dorsal view; (B) posterior portion of abdomen, right lateral view (cerc, cercus; epand, epandrium; hypd, hypandrium; st, sternite; tg, tergite).

Fig. 9. Eothalassius borkenti Cumming and Brooks sp. nov., male. (A) Posterior portion of abdomen, left lateral view; (B) posterior portion of abdomen, ventral view (cerc, cercus; epand, epandrium; hypd, hypandrium; st, sternite; tg, tergite).

Fig. 10. Eothalassius borkenti Cumming and Brooks sp. nov., male. (A) Hypopygium, left lateral view; (B) hypopygium, right lateral view; (C) hypopygium, posterior view (cerc, cercus; d sur, dorsal lobe of surstylus; epand, epandrium; hypd, hypandrium; hyprct, hypoproct; pgt lb, postgonite lobe; ph, phallus; v epand proc, ventral epandrial process; v sur, ventral lobe of surstylus).
Female: Body length 1.2–1.5mm, wing length 1.1–1.3mm. Similar to male. Terminalia (Figs. 11A–11C) with tergite 8 medially divided, slender anteriorly, narrowly fused with sternite 8 anterolaterally; tergite 10 medially divided and narrowed anteriorly, with three acanthophorite setae on each side; cercus slender, terminating in long apical seta; spermatheca an unsclerotized unpigmented tube with sperm pump at base.
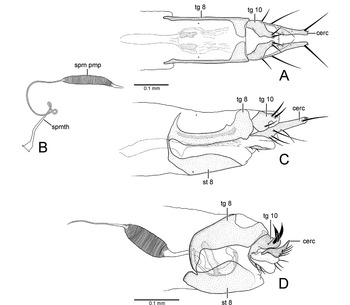
Fig. 11. Female terminalia. (A) Eothalassius borkenti Cumming and Brooks sp. nov., dorsal view; (B) E. borkenti Cumming and Brooks sp. nov., spermatheca; (C) E. borkenti Cumming and Brooks sp. nov., left lateral view; (D) Microphorella sp. (Yukon), left lateral view (cerc, cercus; spm pmp, sperm pump; spmth, spermatheca; st, sternite; tg, tergite).
Remarks
Eothalassius borkenti was referred to as an undescribed species of Microphorella in Cumming and Sinclair (Reference Cumming, Sinclair, Brown, Borkent, Cumming, Wood and Zumbado2009, p. 668). This species was collected on rocky seashores (Fig. 1F).
Etymology
This species is named in honour of Dr. Art Borkent of Salmon Arm, British Columbia, who collected the first known specimens of the type series.
Genus Chimerothalassius Shamshev and Grootaert
Chimerothalassius Shamshev and Grootaert, Reference Shamshev and Grootaert2002: 131.
Type species: Chimerothalassius ismayi Shamshev and Grootaert, by original designation.
Included species
The genus currently includes Chimerothalassius ismayi Shamshev and Grootaert and Chimerothalassius sp. (Dominica).
Diagnosis
Chimerothalassius is distinguished from other parathalassiine genera by the following characters: head with gena scarcely projected below eye, mouthparts directed ventrally with fleshy labellum, palpus elongate and narrow with long ventral setae; thorax with prosternum fused to proepisternum forming precoxal bridge, scutellum with 1 pair of strong dorsally directed bristles near apex; legs with fore coxa bearing strong basal seta, fore femur with 2 rows of ventral setae, tarsomere 5 of each leg with apical finger-like process; wing (Fig. 2K) with costa bearing double row of short spine-like setae along anterior margin, R1 short reaching costa before middle of wing, crossvein bm-m complete, cell dm absent without veins M2 and dm-m, CuA rounded, cell cua convex apically, anal lobe not developed; male terminalia (known only for C. ismayi) with right and left epandrial lamellae apparently separated from hypandrium, left epandrial lamella with apparently non-articulated ventral process, postgonites cradling base of phallus with left and right lobes protruding from between dorsal and ventral surstylar lobes, cerci short and nearly symmetrical; female abdomen with apical segments retracted into segment 5, terminalia with tergite 10 bearing acanthophorite setae, cercus narrowly rounded apically.
Distribution
Chimerothalassius is known from coastal localities on the Caribbean island of Dominica (Chimerothalassius sp.) (Fig. 12B) and South Island, New Zealand (C. ismayi) (Fig. 12A). Figure 12A includes the type locality of C. ismayi from South Birdlings Flat listed in Shamshev and Grootaert (2002), as well as additional new localities from Cable Bay (female specimen deposited in USNM) and Port Levy on Banks Peninsula (male specimen deposited in DEBU).

Fig. 12. Known distributions of Eothalassius Shamshev and Grootaert and Chimerothalassius Shamshev and Grootaert. (A) Eothalassius gracilis Shamshev and Grootaert, E. platypalpus Shamshev and Grootaert, and C. ismayi Shamshev and Grootaert; (B) Chimerothalassius sp. from Dominica and E. borkenti Cumming and Brooks sp. nov.; (C) “Microphorella” merzi Gatt.
Remarks
Shamshev and Grootaert (2002) indicated that the type series of C. ismayi was swept from a stony beach at South Birdlings Flat. The Port Levy specimen of C. ismayi was collected with a pan trap set along a sea streambed. Label data indicate that one of the two specimens of Chimerothalassius sp. from Dominica (Layou River mouth) was collected along the seashore. Immature stages are presently unknown.
Chimerothalassius sp.
Material examined
DOMINICA: 1 ♀, Layou River mouth, 9.i.1965, W.W. Wirth, seashore, Bredin-Archbold, Smithsonian Biological Survey, Dominica (USNM); 1 partial specimen (slide-mounted wing), Rodney's Rock, 5.ii.1964, H. Robinson (USNM).
Diagnosis
Chimerothalassius sp. is distinguished from C. ismayi by the body being brown rather than grey; the basal portion of the costa bearing group of 3–4 spine-like bristles near the base of R2+3 (Fig. 2K) (absent in C. ismayi); the costa with a prominent double row of spine-like setae along the anterior margin (less prominent in C. ismayi); a longer arista-like stylus, about 4 times longer than the postpedicel (as opposed to 2 times longer in C. ismayi); and the anepisternum without a dorsal row of 4 short setae (present in C. ismayi). The male is unknown.
Distribution
At present, Chimerothalassius sp. is known only from coastal localities on the Caribbean island of Dominica (Fig. 12B).
Remarks
With the exception of the slightly damaged slide-mounted wing (Fig. 2K), the remainder of the specimen from Rodney's Rock has apparently been lost or inadvertently destroyed. The wing of the Rodney's Rock specimen is nearly identical with that of the female specimen from the mouth of the Layou River (approximately 2km north of Rodney's Rock), and is presumably conspecific. Additional specimens, including males, will need to be collected before this new species can be described.
Discussion
Phylogenetic relationships of the parathalassiine genera are at present unclear. The South African genera Plesiothalassius Ulrich and Amphithalassius Ulrich appear to be the most generalized extant representatives of the subfamily (Ulrich Reference Ulrich1991) and are probably the stem or sister group to the other extant genera (Cumming and Brooks Reference Cumming and Brooks2006; Brooks and Cumming Reference Brooks and Cumming2010). Excluding these two genera, the Holarctic genus Parathalassius may be the sister group to the remaining genera, based primarily on wing shape and venation (Cumming and Brooks Reference Cumming and Brooks2002). Problems persist with the definition of Microphorella, a widely distributed genus that appears to be paraphyletic with respect to at least Thalassophorus and Eothalassius (Cumming and Brooks Reference Cumming and Brooks2006). If Thalassophorus and Eothalassius are retained at the generic level, then subdividing Microphorella into several additional genera may be required. The relationships of Chimerothalassius and the undescribed genus from Chile are also presently unclear, although together they might be the sister lineage to the previously mentioned Microphorella group (Brooks and Cumming Reference Brooks and Cumming2010).
A detailed phylogenetic analysis of the entire Parathalassiinae is needed to resolve relationships, as well as to determine the number and ranking of generic groups within the subfamily. This will hopefully help explain some of the interesting zoogeographic patterns that appear to be emerging in the Parathalassiinae, with a possibly basal South African component, a number of Southern Hemisphere taxa with additional New World connections, and less restricted generic geographic distributions than previously documented.
Given the small body size of the Parathalassiinae and their frequent association with inadequately sampled coastal marine habitats, distributional patterns of these flies are undoubtedly poorly known. However, the two known species of Thalassophorus appear to represent a standard Asio-Nearctic Tertiary disjunct distribution, which is well known in the Empidoidea (see Sinclair and Saigusa Reference Sinclair and Saigusa2002; Sinclair et al. 2012). The widespread Old World – New World connections now found within Eothalassius and Chimerothalassius may represent even older relict distributional patterns known in groups such as ditomyid fungus gnats (see Cranston Reference Cranston, Yeates and Wiegmann2005), or may be the result of more recent anemochore dispersal through oceanic drift (see Kirk-Spriggs and McGregor Reference Kirk-Spriggs and McGregor2008). Increased sampling of small agile Diptera from intertidal, estuarine, and other seashore habitats throughout the world will certainly uncover numerous additional parathalassiine taxa that when described and analyzed phylogenetically will increase our evolutionary understanding of this extremely interesting basal lineage of Dolichopodidae.
Acknowledgements
We thank Masahiko Satô for the photograph of T. spinipennis in the field, and for the gift of three specimens that have been deposited in CNC. Art Borkent graciously supplied the photograph of the shoreline at Cabo Blanco, Costa Rica. Norm Woodley (USNM), Steve Marshall (DEBU), Toyohei Saigusa (Fukuoka), and Hans Ulrich (ZFMK) are acknowledged for the loan of specimens used in this study. Barry Flahey (CNC) skilfully inked Figures 3–6, 8, and 9. We thank Brad Sinclair (CNC) for useful discussions on homology and character-state distributions within the Microphorinae and Parathalassiinae. Frederic Beaulieu and Serge Laplante kindly provided the French translation of the abstract.














