Introduction
The failure of seeds to germinate under conditions otherwise favourable for germination (of non-dormant seeds) is termed seed dormancy (Baskin and Baskin, Reference Baskin and Baskin1998, Reference Baskin and Baskin2004). Several scientists have attempted to classify seed dormancy by considering different factors responsible for it (e.g. Crocker, Reference Crocker1916; Harper, Reference Harper1957). However, Nikolaeva (Reference Nikolaeva1969, Reference Nikolaeva and Khan1977) developed the most comprehensive seed dormancy classification system, which is based on causes of seed dormancy. According to this system, as modified by Baskin and Baskin (Reference Baskin and Baskin1998, Reference Baskin and Baskin2004), the kinds of dormancy are divided into a hierarchy of classes, levels and types. Five classes of seed dormancy are recognized. Physical dormancy (PY) is caused by a water-impermeable seed or fruit coat, physiological dormancy (PD) by low growth potential of the embryo, combinational dormancy (PY+PD) by a water-impermeable seed coat and low growth potential of the embryo, morphological dormancy (MD) by an underdeveloped embryo that needs time to grow (the dormancy period) within the seed before the radicle emerges and morphophysiological dormancy (MPD) by an underdeveloped embryo that has low growth potential. PD is divided into three levels of dormancy (deep, intermediate and non-deep) and non-deep PD into five types. MPD is divided into nine levels of dormancy: non-deep simple, intermediate simple, deep simple, deep simple epicotyl, non-deep simple epicotyl, deep simple double, non-deep complex, intermediate complex and deep complex (Baskin and Baskin, Reference Baskin and Baskin1998, Reference Baskin and Baskin2004; Baskin et al., Reference Baskin, Chien, Chen and Baskin2008).
All five classes of dormancy occur in seeds of angiosperms, but only PD, MD and MPD occur in those of gymnosperms (Baskin and Baskin, Reference Baskin and Baskin1998). Within the gymnosperms, underdeveloped embryos, and thus MD and/or MPD, occur in Cycadales, Ginkgoales and some conifers. However, not all families of conifers have been studied in detail, e.g. the monogeneric family Cephalotaxaceae sensu stricto, which is the subject of this study. From a phylogenetic and taxonomic point of view, some studies (e.g. Chaw et al., Reference Chaw, Parkinson, Cheng, Vincent and Palmer2000; Cheng et al., Reference Cheng, Nicolson, Tripp and Chaw2000; Hao et al., Reference Hao, Xiao, Huang, Ge and Yang2008) provide evidence that Cephalotaxaceae and Taxaceae are separate families, while results from other studies (Quinn et al., Reference Quinn, Price and Gader2002; Rai et al., Reference Rai, Reeves, Peakall, Olmstead and Graham2008) suggest that Cephalotaxus is a member of the Taxaceae.
Cephalotaxus is not included in the extensive compilations of the kinds of seed dormancy in plants by Nikolaeva et al. (Reference Nikolaeva, Rasumova, Gladkova and Danilova1985), Nikolaeva (Reference Nikolaeva1990, Reference Nikolaeva1999) or Baskin and Baskin (Reference Baskin and Baskin1998). In Seeds of woody plants in China, Huang (Reference Huang2000) reported that germination in Cephalotaxus fortunei can extend for more than 1 year after sowing the seeds, which required a winter cold stratification period to germinate. More recently, Jiao et al. (Reference Jiao, Zhou, Jin and Li2007) reported that seeds of C. fortunei wet-stratified in sand at 5°C for 30 d germinated to 31% after incubation. Considering propagation of Cephalotaxus for horticultural purposes, Dirr (Reference Dirr2009) briefly mentioned that seeds of C. fortunei and C. harringtonia var. drupacea germinated to 33% after a 3-month cold stratification treatment and germination increased to over 50% when the 3-month cold-stratified seeds were given another warm (summer) plus cold (winter) stratification. However, none of these studies took into consideration the fact that seeds of Cephalotaxus have a small embryo (Forbis et al., Reference Forbis, Floyd and de Queiroz2002; Kirkbride et al., Reference Kirkbride, Gunn and Dallwitz2006) nor did they attempt to classify the kind of dormancy in seeds of this genus or to put their results into a phylogenetic context.
An underdeveloped embryo is small relative to the size of the seed [i.e. embryo length (E):seed length (S) ratio is low] and must grow inside the seed before the radicle emerges (i.e. seed germinates). However, seeds of some taxa have a low E:S ratio, yet they do not grow before the seed germinates (Baskin and Baskin, Reference Baskin and Baskin2007). Thus, one objective of our study was to determine if the embryo in seeds of Cephalotaxus wilsoniana Hayata grows prior to emergence of the radicle. That is, is the embryo in fresh seeds underdeveloped or merely small and does not grow before the seed germinates? If embryo growth is a prerequisite for germination, seeds would have MD or one of the nine levels of MPD. If the embryo does not grow before the radicle emerges, seeds would be non-dormant or have PD. Warm, cold and warm plus cold stratification are known to play an important role in breaking MD, MPD or PD in seeds of both angiosperms and gymnosperms (Bewley and Black, Reference Bewley and Black1994; Baskin and Baskin, Reference Baskin and Baskin1998). Thus, embryo growth was monitored in seeds incubated at warm, warm plus cold and warm plus cold plus warm temperatures.
Assuming that the embryo in C. wilsoniana seeds is underdeveloped, our second objective was to determine if the seeds have MD or MPD; and if MPD, which of the nine levels (see above) within this dormancy class they have. Seeds with MD usually germinate in about 30 d or less, while those with MPD may require several months to germinate. The gibberellins GA3 (gibberellic acid) and GA4 promote seed germination in many species, and they can sometimes partially or completely replace cold stratification for promotion of seed germination (Kucera et al., Reference Kucera, Cohn and Leubner-Metzger2005; Chen et al., Reference Chen, Chien, Chung, Yang and Kuo2007, Reference Chen, Kuo and Chien2008). Further, GA has also been used in seed dormancy classification to help distinguish between the deep, intermediate and non-deep levels of MPD and of PD (Baskin and Baskin, Reference Baskin and Baskin2004). Thus, we tested the effect of GA3 and GA4 on germination. This study increases considerably our knowledge of the whole-seed physiology of Cephalotaxus and provides information that allows us to place seed dormancy in Cephalotaxaceae into a phylogenetic context within the gymnosperms.
Cephalotaxus species grow in shady to semi-shady sites but tolerate full sun and are resistant to diseases and insect attack, making them desirable as ornamentals. The genus was introduced to Europe, North America and Australia in the 1800s for use in landscaping (von Siebold and Zuccarini, 1835–Reference von Siebold and Zuccarini1870; Dirr, Reference Dirr1992; Tripp, Reference Tripp1994). In nurseries, most Cephalotaxus plants are propagated by the stem-cutting technique. Rooted cuttings from the top of the tree grow upward (orthotropic), but those from lateral shoots grow prostrate (plagiotropic) (Dirr and Heuser, Reference Dirr and Heuser1987). Cephalotaxus species are difficult to propagate by tissue culture (Janick et al., Reference Janick, Whipkey, Kitto and Frett1994). Another reason for wanting to propagate Cephalotaxus is that the leaves, twigs and heartwood contain a number of antitumour alkaloids, cephalotaxine, harringtonine, homoharringtonine, wilsonine and others (Mikolajczak et al., Reference Mikolajczak, Powell and Smith1972; Powell et al., Reference Powell, Mikolajczak, Weisleder and Smith1972; Takano et al., Reference Takano, Yasuda and Nishijima1996; Kuo et al., Reference Kuo, Hwang, Yang Kuo, Lee, Li and Shen2002; Wang et al., Reference Wang, Su, Yang, Won and Lin2004). Homoharringtonine and its derivatives have been used in clinical anti-cancer tests (O'Dwyer et al., Reference O'Dwyer, King, Hoth, Suffness and Leyland-Jones1986; Kantarjian et al., Reference Kantarjian, Talpaz, Santini, Murgo, Cheson and O'Brien2001). Thus, the propagation of large numbers of plants from seeds is desirable; however, lack of information on dormancy breaking and germination requirements has been a deterrent. This paper provides information that can be used to propagate plants of Cephalotaxus from seeds for future benefit from an economic as well as conservation perspective.
Materials and methods
Study organism
The genus Cephalotaxus, which consists of six species, occurs in eastern Asia, including Japan, Korea, Taiwan and China; in the eastern Himalayas, including eastern India and northern Myanmar (Burma); and in northern Thailand, northern Laos and northern Vietnam (about 16–44°N, 92°31′–144°E) (Li and Keng, Reference Li and Keng1994; Mabberley, Reference Mabberley2008). Fossils of Cephalotaxus have been found in the Miocene in North America; in the Eocene, Oligocene, Miocene and Pliocene in Europe and as far back as the Eocene in Asia, where it is still extant (Manchester et al., Reference Manchester, Chen, Lu and Uemura2009). Our study species Cephalotaxus wilsoniana Hayata is endemic to Taiwan and widely but sparsely distributed in evergreen and deciduous broad-leaved and coniferous forests throughout the island at altitudes of 1400–2200 m (Li and Keng, Reference Li and Keng1994; personal observation).
Description, collection and handling of seeds
The seeds of Cephalotaxus are larger than those of Taxus; the former are completely surrounded by the seed coat and the latter by a cup-like (aril) coat. Cephalotaxus has been called plum yew because the seeds look like small plums. The seeds of C. wilsoniana consist of an embryo with two cotyledons (embryo length:seed length ratio = 0.31 ± 0.04, n = 10) surrounded by the megagametophyte, a thin endotesta, a stony sclerotesta and a fleshy outer seed coat. The embryo in mature seeds is differentiated and linear-shaped, and it occupies a small cavity in the megagametophyte; a suspensor links the embryo to the micropyle. Most of the interior of the seed is filled with the megagametophyte. Seeds without the fleshy coat were 16.71 ± 0.93 mm long, 8.81 ± 0.26 mm wide and 7.36 ± 0.32 mm thick (n = 20). There were 1220 seeds per litre and 1992 seeds per kg. Moisture content of fresh seeds was 35 ± 6% (fresh weight basis, n = 4 replicates of three seeds each) as determined by oven drying for 17 h at 103°C (International Seed Testing Association, 1999).
Mature seeds from six female trees were harvested from Meifeng (24°02′17″N, 121°8′43″E), Nanto County, central Taiwan, at an elevation of 2100 m on 24 October 2008. In the laboratory, the soft outer fleshy coat of seeds was macerated on a mesh (4.0 mm2) stainless-steel pan by hand, using gloves because of the sticky fleshly coat, and then seeds were flushed with water to remove the fleshy part of the coat. All cleaned seeds that sunk were air-dried at room temperature for 24 h and then used for dormancy-breaking and germination tests and for observations on embryo growth. We cut more than 15 freshly harvested C. wilsoniana seeds and found that all of them had a healthy-looking megagametophyte and embryo. Seeds of C. wilsoniana are brownish in colour when they mature in late October through November.
All seeds were mixed with moist sphagnum moss (cut into small pieces) and placed inside sealable polyethylene bags (0.04 mm in thickness). Water content of the sphagnum moss was about 400% of its dry mass. Seeds were incubated at various temperatures, depending on the experiment (see below). At each alternating temperature regime, the high and low temperatures were given for 12 h each day, and light was given during the high-temperature phase of the daily cycle (hereafter, light). The light source was cool white fluorescent bulbs, and photon irradiance at the germination substrate level was about 80–100 μmol m− 2 s− 1. Due to the coarse nature of the sphagnum, most seeds received some light, but at any given point in time a few may have been in darkness. However, at weekly intervals the contents of each bag were poured out on a table to facilitate examination of seeds for germination. After germination was monitored, seeds and sphagnum were returned to the bag, resulting in a re-shuffling of seeds with regard to their position in the sphagnum and thus the light they received. Consequently, all seeds were in light part (or all) of the time the lights were on in the incubator. Each treatment consisted of three replications of 25 seeds each. Seeds with a radicle ≥ 2 mm long were recorded weekly as germinated and removed from the bag. Results are expressed as mean ( ± 1 SE) germination percentage and as mean ( ± 1 SE) germination time (MGT) in days. MGT = (Σn it i)/N, where n i is the number of seeds germinated in t i days from the beginning of the test, and N is the total number of germinated seeds at the end of the test (Naylor, Reference Naylor1981). MGT is a measure of the rate of germination and of the sharpness of the germination peak.
Effect of various temperature regimes on germination
To determine if seeds germinate without any pretreatments, they were incubated in light at 30/20, 25/15, 20/10, 15/6°C and 25°C for 98 weeks.
Effect of warm to cold and of cold stratification on radicle emergence
The purpose of this experiment was to determine if warm followed by cold stratification or only cold stratification promotes radicle emergence. For warm followed by cold stratification, seeds were warm-stratified in light at 15/6, 20/10 and 25/15°C for 12, 24, 36 and 52 weeks. Following each warm-stratification pretreatment, seeds were moved to 5°C in darkness and cold stratified for 8, 12, 16 or 24 weeks. After the various periods of cold stratification, seeds were incubated in light at their respective previous warm stratification temperatures and monitored weekly for 30 weeks. For cold stratification only, seeds were stratified at 5°C in darkness for 12, 24, 32, 40 and 52 weeks and then moved to light at 15/6 and 25/15°C for germination. Total length of the experiment including cold stratification and germination was 68 weeks.
Effect of cold to warm and warm to cold temperature sequences on radicle and shoot emergence
Since seeds of C. wilsoniana are dispersed in late November through December, the temperature treatment they receive in the field is cold stratification in winter, warm stratification in spring to summer and cold stratification in fall to winter. However, dormancy break of the seeds may occur at a certain season. The purpose of this experiment was to monitor seed germination at simulated seasonal temperature regimes at Meifeng near the seed collection site (Fig. 1). Seeds mixed with moist sphagnum moss were incubated in light in the following two temperature sequences except at 5°C, where seeds were in continuous darkness (arrow indicates seeds were moved from one temperature regime in the sequence to the next one in the sequence): (1) beginning with cold temperature, 5°C for 4 weeks → 15/6°C for 8 weeks → 20/10°C for 8 weeks → 25/15°C for 12 weeks → 20/10°C for 8 weeks → 15/6°C for 8 weeks → 5°C for 4 weeks, then continuing the sequence (i.e. 15/6 → 20/10 → 25/15°C, etc.) if all seeds had not germinated; and (2) beginning with warm temperature, 25/15°C for 12 weeks → 20/10°C for 8 weeks → 15/6°C for 8 weeks → 5°C for 4 weeks → 15/6°C for 8 weeks → 20/10°C for 8 weeks → 25/15°C for 12 weeks, then continuing the sequence (i.e. 20/10 → 15/6 → 5°C, etc.) if all seeds had not germinated. All seeds were monitored weekly for radicle emergence. In the first temperature sequence, seeds with an emerged radicle were incubated continuously at 20/10°C for observations on rate of shoot emergence.

Figure 1 Mean daily monthly mean temperature (○), mean daily monthly maximum temperature (●), mean daily monthly minimum temperature (▲) and mean daily monthly precipitation (black bars) at Meifeng, Taiwan (data purchased from Meifeng Highland Experimental Farm, National Taiwan University).
Effect of temperature on embryo growth
To determine the conditions required for embryo growth, embryo length was measured in fresh seeds of C. wilsoniana and at 4-week intervals in warm-stratified seeds, in warm to cold-stratified seeds, in seeds that were re-incubated at the original warm temperatures after warm to cold stratification and in seeds in the cold to warm temperature sequence. Embryos were dissected from seeds using a razor blade, and ten embryos each were measured under a dissecting microscope equipped with a calibrated micrometer. To determine the critical embryo length for radicle emergence, seeds for which the seed coat had split but none of the radicle had emerged were excised and measured, i.e. the embryo had grown to its maximum length, and the next phase of growth would be radicle emergence.
Effect of GA3 and GA4 on germination (radicle and shoot emergence)
The purpose of this experiment was to determine if gibberellins promote seed germination by substituting for cold (but not warm) stratification. Freshly harvested seeds and those warm-stratified in light at 25/15, 20/10 and 15/6°C for 12, 16 and 24 weeks were soaked in double-distilled water (ddH2O) and in solutions of 2500 μM GA3 (potassium salt, 95% purity, Sigma, St Louis, Missouri, USA) or GA4 (>90% purity, from Professor Lewis N. Mander, Australian National University) for 24 h at room temperature (about 25°C) prior to incubation. These treated seeds were then mixed with moist sphagnum moss and returned to light at their original temperatures of 25/15, 20/10 or 15/6°C. Seeds were monitored weekly for 52 weeks, and those with a radicle ≥ 2 mm long were recorded as germinated. Also, radicle-emerged seeds were treated with the same concentrations of GA3 and GA4 for 24 h at room temperature, and then shoot emergence was monitored in light at 25/15, 20/10 and 15/6°C. Each treatment consisted of three replications of 25 seeds each. Results are expressed as percentage of radicle or shoot emergence.
Statistical analysis
Radicle emergence data were converted to percentages based on the number of treated seeds, and means ( ± SE) of germination percentage and embryo length and MGT (three replications) were calculated. Percentage shoot emergence was calculated based on the number of radicle-emerged seeds. Mean percentage of radicle emergence and shoot emergence, MGT and embryo lengths were compared by analysis of variance (ANOVA) and by the least significant difference (LSD) test at the 5% level of significance using SAS (SAS Institute Inc., Cary, North Carolina, USA) and Microsoft Office Excel 2003. Percentage data were arcsine square-root transformed before analysis, but only non-transformed data are shown in tables and figures.
Results
Effect of various temperature regimes on germination
Regardless of incubation temperature, none of the (untreated) seeds had germinated after 50 weeks. Seeds at 15/6°C had begun to germinate after 52 weeks of incubation, and germination increased slowly thereafter. After incubation for 98 weeks, about 61% of the seeds had germinated (radicle emergence) at 15/6°C, but none had done so at 20/10, 25/15, 30/20 or 25°C (Fig. 2).

Figure 2 Cumulative germination percentages of Cephalotaxus wilsoniana seeds at various temperatures. Bars are ± 1 SE.
Effect of warm to cold and of cold stratification on radicle emergence
Warm stratification of seeds at 15/6, 20/10 or 25/15°C and then cold stratification at 5°C increased germination percentages and rates (decreased MGT) (Table 1). For example, seeds kept for 36 weeks at 15/6°C, 52 weeks at 20/10 and 52 weeks at 25/15°C followed by 16 weeks at 5°C germinated to 90, 92 and 84%, respectively, and MGTs were 62, 24 and 33 d, respectively. Warm stratification for < 36 weeks and/or cold stratification for < 8 weeks decreased seed germination percentages, and MGTs remained high. Cold stratification at 5°C for up to 52 weeks did not increase germination percentages at 15/6°C, but it decreased MGT (Table 2). Germination was 0% for seeds cold stratified at 5°C for ≤ 32 weeks and then incubated at 25/15°C. However, seeds stratified at 5°C for 40 and 52 weeks germinated to 8 and 21%, respectively, and MGT was 73 and 69 d, respectively.
Table 1 Effect of warm (15/6, 20/10 and 25/15°C) to cold (5°C) stratification on percentage and rate (MGT in days in parenthesis) of radicle emergence from Cephalotaxus wilsoniana seeds

Radicle emergence was recorded for 30 weeks after warm to cold stratification. Means (n = 3) ± SE in each warm to cold treatment for percentage of radicle emergence or for MGT followed by the same letter are not significantly different (LSD, P = 0.05). –, data not available.
Table 2 Effect of cold stratification at 5°C on mean (±SE, n=3) percentage and rate (MGT in days) of radicle emergence from Cephalotaxus wilsoniana seeds subsequently incubated in light at 15/6 and 25/15°C. Numbers in a row followed by the same letter are not significantly different (LSD, P=0.05)

Effect of cold to warm and of warm to cold temperature sequences on radicle and shoot emergence
No seeds had germinated after 1 year of incubation in the temperature sequence that began at 5°C (Fig. 3A). Germination began at 15/6°C in the second cycle of the sequence and increased rapidly to 70% at 20/10°C and to 75% at 25/15°C (Fig. 3A). In the temperature sequence that began at 25/15°C, seeds germinated to 30% during the second exposure to 20/10°C and to 79% during the fourth exposure to 20/10°C, after about 90 d of incubation (Fig. 3B).

Figure 3 Cumulative germination percentages of Cephalotaxus wilsoniana seeds incubated in a cold to warm to cold to warm temperature sequence (A), or in a warm to cold to warm to cold to warm temperature sequence (B). Bars indicate ± 1 SE.
Effect of temperature on embryo growth
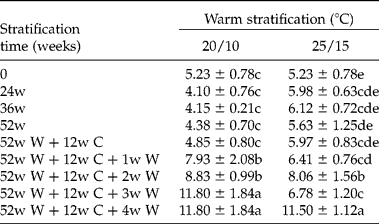
Embryo length in fresh seeds was 5.23 ± 0.78 mm (Table 3). Embryo length in seeds warm stratified at 25/15 or 20/10°C for 52 weeks did not change significantly. Embryos in seeds warm stratified for 52 weeks and then cold stratified for 12 weeks did not change significantly. However, embryos grew rapidly when seeds were moved from 5°C to either 20/10°C or 25/15°C, reaching the full length required for germination (11.65 ± 1.22 mm) in 3–4 weeks. Embryo growth in seeds in the cold to warm temperature sequence (Fig. 3A) occurred after 54–60 weeks, when seeds were at 15/6°C for the third time. Embryos grew to the full length required for radicle emergence, and the embryo length/seed length (E:S) ratio was 0.67 ± 0.09 (Table 3, Fig. 4). Although embryo length increased >120% before the radicle emerged, the cotyledons continued to grow inside the seed after radicle emergence (Fig. 4). The colour of some cotyledons changed from white to light green after 24 weeks of warm stratification.
Table 3 Embryo length (mm) in Cephalotaxus wilsoniana seeds warm-stratified (W) at two temperature regimes for 24, 36 or 52 weeks (w) followed by cold stratification (C) at 5°C for 12 weeks (w) and then incubated at original warm-stratified temperature for germination for 1, 2, 3 and 4 weeks
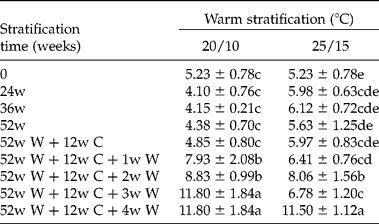
Embryo length of 52w W+12w C was measured after 12 weeks of cold stratification. Any means (n = 10) ± SE at 20/10°C or at 25/15°C in column for embryo length followed by the same letter are not significantly different from each other (LSD, P = 0.05).

Figure 4 Longitudinal sections of seeds of Cephalotaxus wilsoniana showing growth of the embryo in (A) fresh seed; (B) seed warm stratified at 20/10°C for 52 weeks; (C) seed warm stratified at 20/10°C for 52 weeks followed by cold stratification at 5°C for 12 weeks; (D) seed warm stratified at 20/10°C for 52 weeks followed by cold stratification at 5°C for 12 weeks, and then incubated at 20/10°C for 1 week; (E) seed warm stratified at 20/10°C for 52 weeks followed by cold stratification at 5°C for 12 weeks, and then incubated at 20/10°C for 3 weeks, showing a fully grown embryo at the time the seed coat splits. Seed with radical emerged is shown in (F). Cotyledons continued to elongate inside the seed after radicle emergence (F). (See online at http://journals.cambridge.org for a colour version of this figure.)
Effect of GA3 and GA4 on germination (radicle and shoot emergence)
The germination percentage (radicle emergence) of neither fresh seeds nor of those previously warm stratified at 25/15, 20/10 or 15/6°C for 12, 16 or 24 weeks was affected by a 2500 μM solution of either GA3 or GA4 at any of the incubation temperatures (data not shown). However, for radicle-emerged seeds, treatment with 2500 μM GA3 or GA4 increased the rate of shoot emergence. GA4 was more effective than GA3, i.e. 25/15°C+GA4>25/15°C+GA3>25/15°C, and rate of shoot emergence decreased with a decrease in temperature, i.e. 25/15°C>20/10°C>15/6°C. Shoot emergence was 4 weeks faster in the GA4 plus 25/15°C than in the GA4 plus 15/6°C treatment, 5 weeks faster than in the GA3 plus 15/6°C and 6 weeks faster than in the 15/6°C control (Fig. 5).

Figure 5 Effect of GA3 and GA4 on shoot emergence in radicle-emerged seeds at three temperature regimes. Treated seeds were soaked in a 2500 μM solution of GA3 or GA4 for 24 h before incubation.
Discussion
None of the freshly harvested seeds of C. wilsoniana incubated at 15/6, 20/10, 25/15, 30/20 or 25°C had germinated after 50 weeks, indicating that they were highly dormant. Beginning at week 52, a few seeds had germinated at 15/6°C, and more germinated through week 98, at which time the experiment was terminated. The slow germination of seeds during 98 weeks at 15/6°C suggests that the warm stratification requirements for germination were being fulfilled at 15°C and the cold stratification requirements at 6°C (Nikolaeva, Reference Nikolaeva and Khan1977). Since (1) the underdeveloped linear embryo in C. wilsoniana seeds increased in length by 120% before radicle emergence occurred, and (2) many months of exposure to simulated habitat temperature regimes were required for germination, we conclude that seeds have both morphological and physiological dormancy, i.e. morphophysiological dormancy (MPD).
Which one of the nine levels of MPD do seeds of C. wilsoniana have? To answer this question, we first need to know the temperature requirements for embryo growth. If embryos grow at temperatures suitable for cold stratification (0–10°C), seeds have one of the complex levels of MPD. On the other hand, if embryos grow at high temperatures ( ≥ 15°C) they have one of the simple levels of MPD. Embryos grew in seeds of C. wilsoniana incubated at 20/10 and 25/15°C, with 20/10°C being optimal (Table 3). However, the embryo did not grow in seeds incubated continuously at these two temperatures for 52 weeks. Embryos only grew at 20/10°C and 25/15°C if seeds previously had received a long period of warm stratification followed by cold stratification and then moved to warm again.
Seeds of C. wilsoniana do not have epicotyl MPD because at the simulated spring temperature regime (20/10°C) there was only a short delay of 2 weeks between time of radicle and shoot emergence. Neither warm stratification alone nor gibberellns (GA3, GA4) broke dormancy, suggesting that seeds do not have either non-deep or intermediate simple MPD. The only sequence of temperature regimes that resulted in embryo growth and germination was warm → cold → warm, leading to the conclusion that the seeds have deep simple MPD. In deep simple MPD, the breaking of physiological dormancy (PD) occurs in two steps, which we can designate as PD-1 and PD-2. PD-1 is broken by warm stratification and PD-2 by cold stratification. Using the dormancy symbols of Nikolaeva (see Baskin and Baskin, Reference Baskin and Baskin2008), the formula for dormancy in seeds of C. wilsoniana can be written as C1b-C3-B1b, where C is PD, the first phase of which is non-deep (C1) and broken by a period of warm stratification (subscript 1b); the second phase is deep PD (C3) and is broken by a period of cold stratification; and then the underdeveloped embryo (B) grows during a second period of warm stratification (subscript 1b).
In seeds with deep simple MPD, embryo growth occurs at non-cold-stratifying temperatures. However, there is variation among species with regard to timing of embryo growth, germination and the breaking of PD-1 and PD-2 (Table 4). In Taxus mairei, embryo growth occurs while PD-1 is being broken; in Jeffersonia diphylla, embryo growth occurs after PD-1 is broken; and in C. wilsoniana, embryo growth does not occur until after both PD-1 and PD-2 are broken.
Table 4 Comparison of deep simple MPD in seeds Taxus mairei, Jeffersonia diphylla and Cephalotaxus wilsoniana showing when PD-1 and PD-2 are broken and when embryo growth (EG) and germination (G) occur

PD-1, Breaking first part of physiological dormancy; PD-2, breaking second part of physiological dormancy.
The ecological significance of deep simple MPD in seeds of C. wilsoniana is that seeds matured and dispersed in late November through December can not germinate the following spring. To germinate in the field, seeds must receive warm stratification (summer), cold stratification (winter) and warm stratification (spring). For seeds in the warm → cold → warm temperature sequence, embryo growth occurred in the final warm incubation, and then seeds germinated soon after the embryo grew. For another collection of C. wilsoniana seeds harvested at the same location in 2007 and incubated at 20/10, 25/15 and 30/20°C, < 5% of the seeds had germinated after 2 years. However, when these seeds were moist cold-stratified at 5°C for 12 weeks and then re-incubated at 20/10, 25/15 and 30/20°C, 88, 83 and 13% of them germinated, respectively (C.T. Chien, unpublished data). Thus, germination of seeds naturally dispersal at maturity in autumn will not germinate until the second spring.
Our study has shown that seeds of C. wilsoniana have an underdeveloped embryo and deep simple MPD. Thus, conifer families now known to have MPD include Podocarpaceae, Taxaceae and Cephalotaxaceae. MPD is not known in Pinaceae, Araucariaceae, Cupressaceae or Sciadopityaceae. These four families have seeds with fully developed embryos, and thus the seeds are either non-dormant or have PD. Cephalotaxus and Taxus belong to the same clade, but Podocarpus belongs to another clade within the conifers (Chaw et al., Reference Chaw, Parkinson, Cheng, Vincent and Palmer2000; Quinn et al., Reference Quinn, Price and Gader2002). Further, Cephalotaxus is basal in the taxad clade, which includes Taxus, Pseudotaxus, Austrotaxus, Torreya, Amentotaxus and Cephalotaxus (Cheng et al., Reference Cheng, Nicolson, Tripp and Chaw2000). Within the taxad clade, at least Amentotaxus (Li, Reference Li2000), Torreya (Martin, Reference Martin1946; Weng, Reference Weng2000) and Taxus (Chien et al., Reference Chien, Kuo-Huang and Lin1998; Wang, Reference Wang2000), along with Cephalotaxus, have underdeveloped embryos and thus either MD or MPD. Within the taxad clade, the level of MPD is known in Taxus baccata (Devillez, Reference Devillez1978), T. brevifolia, T. cuspidata (Nikolaeva et al., Reference Nikolaeva, Rasumova, Gladkova and Danilova1985) and T. mairei (Chien et al., Reference Chien, Kuo-Huang and Lin1998), and all of them have deep simple MPD.
In addition, according to Nikolaeva et al. (Reference Nikolaeva, Rasumova, Gladkova and Danilova1985) seeds of Torreya californica and T. grandis have a combination of dormancy types, the former species mechanical dormancy combined with non-deep simple MPD and the later species mechanical dormancy combined with MD. Baskin and Baskin (Reference Baskin and Baskin1998) have argued that mechanical dormancy is a part of PD, and Nikolaeva (Reference Nikolaeva2004) agreed. In which case, T. californica would have non-deep simple MPD and T. grandis some unidentified level of MPD. Further studies are needed in the taxad clade to work out the relationships between deep simple MPD in the basal genus Cephalotaxus and the kind of dormancy in the higher taxa within the clade.
From a propagation perspective, knowledge of the warm → cold → warm dormancy breaking and germination requirement of seeds of C. wilsoniana will allow people to speed up the rate of seedling production; that is, instead of allowing seeds to be exposed to low winter temperatures immediately after dispersal, they could be given warm stratification followed by cold stratification and then sown on benches for germination in the nursery, thereby decreasing the time to seedling production by about 15 weeks.
Acknowledgements
The authors thank Der-Ming Yeh (Meifeng Highland Experimental Farm, National Taiwan University) for allowing us to collect seeds of Cephalotaxus wilsoniana. We thank Chang-Yen Chen, Wen-Yu Hsu, Yen-Wei Chang, Ta-Yuan Chien and Kai-Chun Yang, Taiwan Forestry Research Institute, and Tzu-Tong Kao, National Taiwan University, for technical assistance. The paper is dedicated to our good colleague the late Dr Yu-Pin Cheng, who helped collect seeds of Cephalotaxus wilsoniana. This research was supported by a grant (99AS-8.1.2-F1-G1) from the Council of Agriculture, the Executive Yuan, Taiwan.