Published online by Cambridge University Press: 12 November 2004
Ticks are obligatory blood-feeding arthropods that secrete various immunomodulatory molecules to antagonize host inflammatory and immune responses. Cytokines play an important role in regulating these responses. We investigated the extent to which ticks interact with the sophisticated cytokine network by comparing the effect of salivary gland extracts (SGE) of 3 ixodid tick species, Dermacentor reticulatus, Amblyomma variegatum and Ixodes ricinus, all of which are important vectors of tick-borne pathogens. Using specific ELISAs, anti-cytokine activity was demonstrated with 7 cytokines: IL-8, MCP-1, MIP-1α, RANTES, eotaxin, IL-2 and IL-4. The results varied between species, and between adult males and females of the same species. Relatively high activity levels were detected in saliva of female D. reticulatus, confirming that the observed anti-cytokine activities are an integral part of tick saliva secreted into the host. Results with fractionated SGE indicated that from 2 to 6 putative cytokine binding molecules are produced, depending on species and sex. Binding ability of SGE molecules was verified by cross-linking with radio-isotope labelled MIP-1α. By targeting different cytokines, ixodid ticks can manipulate the cytokine network, which will greatly facilitate blood-feeding and provide a gateway for tick-borne pathogens that helps explain why ticks are such efficient and effective disease vectors.
Ixodid ticks (Acarina: Ixodidae) transmit the greatest variety of pathogens of any blood-feeding arthropod (Sonenshine, 1991). The long duration of their blood feeding, unique among ectoparasites, provokes specific host haemostatic, inflammatory, and immune responses. To feed successfully, ticks counterattack with anti-haemostatic, anti-inflammatory and immunomodulatory substances secreted in their saliva (Wikel, 1999). The resulting feeding site represents a favourable ecological niche for transmitted pathogens. Increasing evidence supports the hypothesis that many pathogens, including tick-borne viruses, exploit activities associated with tick saliva (so-called saliva-activated transmission, SAT) for successful transmission and replication (Nuttall, 1998). The ability of tick salivary gland extracts (SGE) to promote virus growth has been demonstrated in vitro (Hajnická et al. 1998), and their supporting activity to establish virus infection in the host has been confirmed (Labuda et al. 1993). In addition, several activities beneficial to viruses, such as suppression of natural killer (NK) cell activity (Kubeš et al. 1994), inhibition of the anti-viral effect of interferon α/β (Hajnická et al. 2000), and inhibition of production of some cytokines (Fuchsberger et al. 1995; Leboulle et al. 2002), have been described. However, it is not clear whether these observed activities are sufficient to form a ‘virus friendly’ environment in the host skin. Although it is likely that several activities are involved in the SAT phenomenon, the key mechanism of SAT remains unresolved.
A common strategy adopted by parasites to attack their hosts is the parasites' ability to interact with host cytokines. For example, large DNA viruses (such as pox- and herpesviruses) have evolved unique strategies to manipulate the host cytokine network (Alcami, 2003; Alcami & Koszinowski, 2000). These viruses encode immunomodulators that are homologues of cytokines (hence named virokines) and cytokine receptors (called viroceptors) (Alcami, 2003; Alcami & Koszinowski, 2000; Seet et al. 2001). In both cases, genetic material has been pirated from host cells in the course of virus-host co-evolution. Besides these, an expanding group of chemokine binding proteins of unknown origin have been described (Seet et al. 2001). For viruses, manipulation of the host cytokine network is a highly efficient and effective strategy for survival. Within the network, the family of small cytokines called chemokines act specifically against viruses (Lusso, 2000; Lalani, Barrett & McFadden, 2000); blocking their activity is a comparatively easy route for viruses to protect themselves against many aggressive immunological host reactions. Manipulation of the cytokine network is likely to benefit many other parasites, including ticks.
Although several studies have reported effects of tick feeding or tick salivary gland products on cytokine expression or activities (Fuchsberger et al. 1995; Ferreira & Silva, 1999; Macaluso & Wikel, 2001), the first evidence of a specific cytokine inhibitor was that reported for Ixodes scapularis. Saliva of this Lyme disease vector was shown to contain a protein that binds human and murine IL-2, providing a mechanism for suppressing T cell proliferation and other IL-2 stimulated immune responses (Gillespie et al. 2001). The nature of the IL-2 binder was not reported and activity in other tick species was not examined. However, IL-8 binding activity has been described recently for 5 ixodid tick species (Hajnická et al. 2001). The activity is sufficiently potent to out-compete binding of the chemokine to its cell receptor and inhibit IL-8 induced chemotaxis of neutrophils. Thus it appears that ixodid ticks secrete cytokine binders to aid blood feeding. It seems unlikely that anti-IL-8 and IL-2 activities are uniquely adopted by ticks while other cytokines are untouched. Here we report anti-cytokine activity in saliva and/or SGE of 3 ixodid tick species against 7 of 10 cytokines selected on the basis that their actions are likely to have an adverse effect on tick blood feeding. Attempts were made to determine whether the ticks produce one or more anti-cytokines by testing individual tick SGE fractions prepared by liquid chromatography.
Representatives of 3 ixodid tick genera were used: Dermacentor reticulatus, the vector of Francisella tularensis; Ixodes ricinus, a vector of many pathogens including the Lyme disease spirochaete (Borrelia burgdorferi sensu lato) and tick-borne encephalitis virus; and Amblyomma variegatum, the primary vector of Ehrlichia (formerly Cowdria) ruminantium, the aetiological agent of heartwater disease of ruminants, and Rickettsia africae, a prevalent human pathogen. Because of the difficulties in obtaining sufficient quantities of tick saliva for testing anti-cytokine activities, SGE was used in place of saliva. Previous studies showed that the kinetics of SAT activity of SGE matches that of the saliva, indicating that SGE can be used as a surrogate for saliva (Jones, Kaufman & Nuttall, 1992). D. reticulatus and I. ricinus ticks were collected by flagging the vegetation in selected locations of western Slovakia known to be free of tick-borne encephalitis virus. A. variegatum ticks were used from a colony maintained at the Institute of Zoology (Bratislava). Adult ticks of D. reticulatus and I. ricinus were allowed to feed on ICR mice and were collected at day 5 of feeding; A. variegatum ticks were fed for 10–12 days on rabbits. The duration of feeding was selected to coincide with the estimated peak of immunomodulatory activity. SGE was prepared by modifying the method of Slovák et al. (2000). Briefly, feeding ticks were gently removed from the laboratory animals and their salivary glands dissected out in ice cold sterile 0·15 M NaCl (0·9%), washed 3 times with the same solution and pooled in Eppendorf tubes containing 10 μl of 0·15 M NaCl in deionized water. Salivary glands were then homogenized and centrifuged at 10000 g for 30 min at 4 °C. Protein concentration of SGE supernates was determined using the Bradford method (Bradford, 1976). Supernatant fluids were dried using a Speed-Vac, stored at 4 °C, and reconstituted in PBS.
Feeding D. reticulatus ticks removed from their hosts were rinsed in water to remove any superficial blood contamination and immobilized by dorsal attachment to double-sided tape. To induce secretion, 2 mM 3-hydroxytyramine hydrochloride (dopamine; Sigma) was inoculated into the coxal plate of the second pair of legs by using prepared glass microcapillaries. The mouthparts (hypostome and chelicerae) were then inserted into a capillary tube (Kwik-Fil Glass Capillaries, W-P Instruments, USA) with internal hole diameter appropriate to their size. Secretion usually takes place within 1min after inoculation of dopamine; extra stimulation was applied by weak pressure on the ventral side of the tick's opisthosoma. Secretion ceases within about 30 min (after which the tick may withdraw some or all of the fluid). Saliva was collected in Eppendorf tubes containing 10 μl of 0·15 M NaCl and then handled according to procedures described for SGE preparation, including protein measurement.
SGE prepared from adult female D. reticulatus (2·5 mg proteins from 50 ticks), I. ricinus (5·0 mg proteins from 270 ticks) and A. variegatum (5·5 mg proteins from 30 ticks) was separated by fast phase liquid chromatography under native conditions at 5 °C using a Superose 12 HR10/30 column and Superdex 75 (Pharmacia, Sweden) with an equilibrium buffer of 0·02 M NH4HCO3, pH 8·0, flow rate of 0·4 ml/min. One fraction was collected per 1·5 min. Fractions were dried using the same protocol as that for SGE preparation and reconstituted in PBS prior to use.
Cytokine levels were measured using commercial ELISA kits and their cytokines (human or mouse) obtained from R&D Systems, Abingdon, UK, unless indicated otherwise: human macrophage inflammatory protein 1α (MIP-1α) DuoSet (Cat. No. DY270); human monocyte chemotactic protein 1 (MCP-1) DuoSet (Cat. No. DY279); human eotaxin ELISA development system (Cat. No. 320-EO, MAB320, BAF320); human RANTES DuoSet (Cat. No. DY278); human interleukin 4 (IL-4) DuoSet (Cat. No. DY204); human interleukin 2 (IL-2) DuoSet (Cat. No. DY202); human interleukin-12 (IL-12) Quantikine ELISA kit (Cat. No. D1200); human interleukin 8 (IL-8) Module Set (Cat. No. BMS204MST) Bender MedSystems Diagnostics, Vienna, Austria; mouse tumour necrosis factor (TNF-α; Factor-Test-X™, Genzyme); mouse interferon-γ (IFN-γ; INTERTEST-γ™, Genzyme). For each assay, 50 pg of the recombinant human or mouse cytokine in PBS supplemented with 10% bovine calf serum were mixed with 5 μl of tick saliva or SGE (diluted in PBS to the given amount of protein) or a given volume of SGE fractions to give a total volume of 105 μl in ELISA diluent containing BSA. Each mixture was incubated for 1·5 h at room temperature with gentle shaking and then applied to the ELISA plates (100 μl/well). Absorbance measurements were recorded in the linear range of the standard curves for each ELISA kit. Based on previous studies, a reduction in detectable levels of a particular cytokine, when compared with the control, was interpreted as evidence of putative cytokine binding (Gillespie et al. 2001; Hajnická et al. 2001). Dopamine (used to induce salivation) was not found to influence the ELISA results.
Ten μl samples of SGE fractions prepared from 10 days-fed A. variegatum female ticks were allowed to adsorb to pre-wetted Immobilon-P membranes (Millipore Corp., USA) for 45 min at 4 °C. Membranes were washed in TTBS (20 mM Tris-HCl, 500 mM NaCl, 0·05% Tween-20, pH 7·5) and blocked with 15 ml of TTBS+3% bovine serum albumin (BSA) for 1 h at room temperature. After 3 washes in TTBS, membranes were incubated in 10 ml of TTBS+3% BSA containing 50 μl of 125I-MIP-1α (925 kBq/0·5 ml, IM285, Amersham Pharmacia Biotech) for 90 min at room temperature with gentle shaking. Following 6 washes in TTBS, membranes were dried and exposed to X-ray film (Hyperfilm MP, Amersham Pharmacia Biotech).
Fraction 34 of SGE prepared from 5 days-fed Dermacentor reticulatus female ticks was used. Three μl of human recombinant 125I-MIP-1α (IM285, Amersham Pharmacia Biotech) were incubated with 50 μl of the fraction for 60 min at room temperature. After the addition of 3 μl of PBS containing 10 mg/ml DTSSP (3,3′-dithiobis[sulfosuccinimidyl propionate]), the mixture was incubated for 30 min and the chemical cross-link reaction was terminated with 3 μl of 1 M Tris-HCl (pH 7·2). Products were analysed by SDS-PAGE (7·5–12·5% acrylamide) under non-reducing conditions followed by autoradiography.
To test for anti-cytokine activity, the absorbance measurements for each dilution series were analysed in a nested general linear model. Readings from 2 replicate wells were averaged, and a line fitted for each of 2 dilution series for both male and female ticks, for each tick species – cytokine combination. For those for which no dose-response was detectable, this was for one of two reasons: (i) anti-cytokine activity of the dilution series was not significantly different from the control, or (ii) anti-cytokine activity was so high that a dose-response was not observed within the concentration range tested. To distinguish these two possibilities, a one-sample t-test was conducted between the average absorbance measurements for a dilution series and the relevant controls.
SGE prepared from partially fed females and males of 3 tick species (D. reticulatus, A. variegatum and I. ricinus) was screened by ELISA for anti-cytokine activity. SGE of all 3 species demonstrated putative cytokine binding of at least 3 of the 7 cytokines, in a dose-dependent manner (Fig. 1). D. reticulatus female SGE reduced the detected levels of 6 cytokines; activity against RANTES was not statistically significant. D. reticulatus male SGE exhibited relatively high anti-cytokine activities with all the cytokines except IL-4 against which it had no detectable effect. There was no evidence of activity against TNF-α, IFN-γ, or IL-12 when SGE (or fractions of SGE) from 5 days-fed female D. reticulatus was tested by ELISA.

Fig. 1. Comparison of anti-cytokine activity of SGE obtained from 5 days-fed Dermacentor reticulatus female and male ticks, from 10 days-fed female and 12–13 days-fed male Amblyomma variegatum ticks, from 5 days-fed Ixodes ricinus female and male ticks. Various concentrations of SGE (47·6, 23·8, 9·5, 4·76, and 0·95 ng/μl or 5·0, 2·5, 1·0, 0·5 and 0·1 μg total protein; labelled 1 to 5, respectively) were pre-incubated with 50 pg of each cytokine (total volume 105 μl) for 90 min before addition to the ELISA plates. A reduction in optical density (OD) relative to the control indicates a decrease in the amount of cytokine detected by ELISA.
A. variegatum female SGE demonstrated markedly high activity against eotaxin (females t5=11·26, P<0·0005 for dilution series 1 and t5=10·54, P<0·0005 for series 2; males t5=102·8, P<0·0005 for series 1 and t5=45·0, P<0·0005 for series 2) and significant dose responses to IL-8, MCP-1, MIP-1α, and IL-4, and no anti-IL-2 activity (in sharp contrast to D. reticulatus females). The dose response against RANTES was not statistically significant (as observed for D. reticulatus females). Male ticks demonstrated similar activities although in all cases their SGE contained relatively higher anti-cytokine activity than conspecific females under the conditions used; activity against IL-2 was not statistically significant.
Anti-cytokine activities of I. ricinus female ticks presented a different picture to that observed with the other two tick species. Significant dose response curves were observed with IL-8, IL-2 and IL-4 only. In contrast to females, male I. ricinus SGE was active against all the cytokines although the dose response curve with MCP-1 was not significant (possibly because only one replicate was used due to a shortage of SGE).
To confirm that the observed anti-cytokine activities are an integral part of tick saliva secreted into the host, we selected D. reticulatus as the species with the most diverse anti-cytokine activity. D. reticulatus females were harvested at day 5 of feeding on ICR laboratory mice and their saliva was collected and tested by ELISA. There was a significant dose-dependent reduction in optical density for all cytokines (dose, nested within treatment and dilution series, F14,42=46·3, P<0·0005), including RANTES for which SGE activity was non-significant (Fig. 1). The highest relative activity appeared to be against IL-8 (Fig. 2). These results demonstrate that the range of anti-cytokine activities of SGE reflect the activities of tick saliva.

Fig. 2. Dermacentor reticulatus saliva prepared from 5 days-fed females inhibits detection of human recombinant cytokines. Various concentrations of saliva (95·2, 23·8, 9·5, 1·9, 0·95 ng/μl or 10·0, 2·5, 1·0, 0·2 and 0·1 μg total protein; labelled 1–5, respectively) were pre-incubated with 50 pg of each cytokine (total volume 105 μl) and analysed by ELISA as described for Fig. 1.
To estimate whether similar or distinct molecules are likely to be involved in tick anti-cytokine activity, we fractionated SGE of the partially fed female ticks using size exclusion chromatography and then tested each fraction against the 7 selected cytokines. Each ELISA was performed with a range of fraction volumes for each tick species to determine the optimal dose (the volume that gave a detectable optical density reading with a clearly visible activity peak). Results obtained with the optimal fraction volume are shown in Fig. 3. Optimal volumes ranged from 2 μl for D. reticulatus in IL-8 and MCP-1-specific ELISAs (relatively high activity) to 25 μl for D. reticulatus in the MIP-1α specific ELISA (comparatively low activity). Peak activities were in the MW range 15–40 kDa estimated from the elution of standards. This broad range possibly reflects dimerization of some of the active molecules. Peak activity fractions were not delineated in the 280 nm absorbance trace of the SGE preparations and attempts to isolate the active molecules indicate they are of low abundance (data not shown).

Fig. 3. Anti-cytokine activity of SGE fractions from Dermacentor reticulatus, Amblyomma variegatum and Ixodes ricinus female ticks demonstrated by specific ELISA. For ELISA assays pre-selected volumes of fractions indicated in the upper left corner of each graph were used. A reduction in optical density (OD) relative to the control indicates a decrease in the amount of cytokine detected. Arrows indicate the elution positions of molecular weight standards (from left to right): bovine serum albumin (67 kDa), ovalbumin (43 kDa), chymotrypsinogen A (25 kDa), and RNase A (13·7 kDa). The upper traces show the 280 nm absorbance profiles of each SGE size exclusion chromatogram.
Anti-cytokine activities of D. reticulatus female SGE fractions were demonstrated for each cytokine to different degrees (Fig. 3), consistent with the results obtained using unfractionated SGE and saliva (Figs 1 and 2). The activities of D. reticulatus fractions generally appeared greater than those of the other two tick species based on the comparative fraction volumes (2–10 μl) that gave optimal results. The exception was with MIP-1α for which the optimal volume was 25 ml for D. reticulatus compared with only 5 μl for A. variegatum.
Peaks for individual anti-cytokine activities were associated with different fractions. For D. reticulatus, the level of MCP-1, eotaxin, IL-2 and IL-4 was markedly reduced compared with the respective control when assayed in the presence of SGE fractions 34–35, MIP-1α with fraction 36–37, RANTES in the presence of fraction 40, while the highest anti-IL-8 activity was associated with fractions 42–43 and to a lesser degree with fraction 38. The diversity of fractions giving maximal detected activity indicates that several tick molecules are involved.
Fractions of A. variegatum SGE showed markedly different anti-cytokine profiles compared with D. reticulatus. Double peaks of anti-cytokine activity were characteristic for most of the tested cytokines, possibly reflecting dimerization of the active molecule typical of some other tick saliva molecules (Paesen et al. 1999 and unpublished data). Again, peak activities were detected in different fractions: anti-IL-4 activity in fraction 35, anti-IL-8 in fractions 36–37, anti-MIP 1α, anti-MCP-1 and -eotaxin in fraction 37–38, and anti-RANTES activity in fraction 40. As with unfractionated female SGE, no anti-IL-2 was observed in any of the tested fractions. Fractionation indicated 2 distinct peaks of activity against RANTES whereas the dose response curve of SGE with RANTES was non-significant.
Anti-cytokine activities in I. ricinus female SGE fractions were observed against only 3 cytokines (IL-8, IL-2 and IL-4), consistent with the results obtained with unfractionated female SGE (Fig. 1). Highest levels of activity were obtained against IL-4, with the peak associated with fractions 29 and 30. Comparatively lower activity levels were observed against IL-2 and IL-8, both associated with fraction 34 (Fig. 3).
The binding ability of tick salivary molecules with chemokine MIP-1α was verified by cross-linking using 125I-MIP-1α and analysing the resulting products by SDS-PAGE and autoradiography. Using fraction 34 of SGE derived from D. reticulatus adult females fed for 5 days, a radio-isotope-labelled band cross-linked to 125I-MIP-1α was detected with a molecular mass of approximately 40 kDa (Fig. 4A, lane 1).

Fig. 4. Binding of MIP-1α to fractions of tick SGE. (A) Cross-linking of radio-isotope-labelled MIP-1α to active compounds in fraction 34 of SGE prepared from 5 days-fed Dermacentor reticulatus female ticks. Chemical cross-linking reactions were performed with DTSSP and subjected to SDS-PAGE under non-reducing conditions. Sample of fraction 34 (within the peak of anti-MIP-1α activity detected by ELISA; see Fig. 3) was incubated with 125I-MIP-1α (A, lane 1). Lane A2 contains only 125I-MIP-1α (7·8 kD) as a control. Arrow indicates position of tick compound bound to MIP-1α (~40 kDa). (B) Binding of 125I-MIP-1α to fractions 30 (1), 33 (2), 35 (3) and 38 (4) of SGE prepared from 10 days-fed Amblyommavariegatum female ticks (as in Fig. 3) spotted onto a PVDF membrane. Scanned radiograms.
Selected fractions of A. variegatum were tested for their ability to bind isotope-labelled MIP-1α (Fig. 4B). Fractions were spotted onto a PVDF membrane and probed with 125I-MIP-1α. The resulting binding imitated anti-cytokine activity detected by ELISA. Comparatively strong binding was obtained with fraction 38 and to a lesser degree with fraction 30 (corresponding to the 2 peaks of activity detected by ELISA, see Fig. 3), and weak binding with fractions 33 and 35.
Our findings demonstrate a large variety of anti-cytokine activities associated with tick salivary products. These products most likely bind to the cytokine molecules as demonstrated by the cross-linking and dot blot experiments with MIP-1α, and earlier reports of binding with IL-2 and IL-8 (Gillespie et al. 2001; Hajnická et al. 2001). Binding to cytokines presumably prevents specific monoclonal antibodies accessing their target epitopes, hence reducing the detectable cytokine levels determined by specific ELISAs. Previous studies indicate the potential significance of these observations, showing that the tick molecules can neutralize the biological activity of cytokines, at least in vitro. The IL-2 binder provides a possible mechanism for the T cell inhibitory activity of tick saliva (Gillespie et al. 2001; Mejri, Rutti & Brossard, 2002) while the IL-8 binder inhibits IL-8 induced chemotaxis of neutrophils (Hajnická et al. 2001). Furthermore, peak activity fractions detected by IL-8 specific ELISA corresponded with maximum activity in receptor-binding inhibition assays, indicating that the IL-8 binder effectively out competes IL-8 receptors on neutrophils for IL-8 (Kocáková et al. 2003). Our failure to detect activity against TNFα, IFNγ, and IL-12 may reflect the absence of binding molecules specific for them. If this is the case, ticks may use alternative strategies to control these important pro-inflammatory cytokines, e.g. by the inhibition of their production (Ramachandra & Wikel, 1992; Zeidner et al. 1997).
The diversity of anti-cytokine activities is associated with various tick molecules and most probably with more than one molecule per tick. Adult females of D. reticulatus may produce a different cytokine binder for each tested cytokine if all the observed differences in peak activity fractions reflect different binding molecules. Alternatively, some molecules may be active against more than one cytokine. For example, for both D. reticulatus and A. variegatum, the peak activity fraction for anti-MCP-1 activity was the same as for eotaxin. MCP-1 and eotaxin bind different cell receptors and yet they have significant protein sequence homology of 66% (Charo et al. 1994; Ponath et al. 1996). However, peak activity of female I. ricinus for IL-8 and IL-2 was also the same (fraction 34) and yet the chemokine, IL-8, and the non-chemotactic cytokine, IL-2, are unrelated (Robb et al. 1984; Clark-Lewis et al. 1991). Thus fraction 34 of I. ricinus may contain 2 different anti-cytokines. Clearly, much more work is required to characterize the tick anti-cytokines. Whatever their mode of action, their activity against human cytokines (and mouse IL-2; Gillespie et al. 2001) indicates they have overcome species-specific differences in cytokine molecules since humans are not significant hosts of any of the tick species examined (although they will feed successfully on humans).
Presumably, the diversity of anti-cytokine activities reflects the ability of ticks to eliminate or diminish the action of the cytokine and chemokine network in the host at least locally where ticks are feeding. To date, only the effects on IL-2 and IL-4 production have been studied in vivo. Lymphocytes collected from draining lymph nodes of BALB/c mice infested with I. ricinus larvae, nymphs or adults produced depressed levels of IL-2 but elevated levels of IL-4 (Ganapamo, Rutti & Brossard, 1995, 1996; Mejri et al. 2001). After 2 infestations of BALB/c mice with D. andersoni nymphs, production of IL-2 was decreased while production of IL-4 was significantly enhanced (Macaluso & Wikel, 2001). IL-4 elevation corresponds with polarization towards a Th2 response following tick infestations (Zeidner et al. 1997; Ferreira & Silva, 1999; Schoeler, Manweiler & Wikel, 2000). This apparent conflict with evidence reported here of an IL-4 binding protein that reduces detectable levels of the cytokine may reflect differences between immunomodulatory effects at the feeding site compared with systemic effects.
Adult female I. ricinus appears to have a limited repertoire of anti-cytokine activity compared to other species and conspecific males. However, it is possible that binding is directed against regions on the cytokines that do not interfere with the binding sites of monoclonal antibodies used in the ELISA kits. This would explain why, in preliminary studies, SGE of female I. ricinus showed binding with 125I-MIP-1α (data not shown) although there was no anti-MIP-1α activity detected by ELISA. Alternatively, ticks that do not display anti-cytokine activity detectable by ELISA may have adopted a different strategy of control, possibly targeting other untested cytokines having a knock-on effect. Further studies are needed to determine the anti-cytokine activity of I. ricinus adult females and, in particular, of nymphs as they are important vectors of the pathogens causing tick-borne encephalitis and Lyme disease.
What can ticks gain from targeting the action of cytokines? Each of the 7 tested cytokines (including 5 chemokines) has a variety of immunological functions and they overlap with each other in their activities. Thus IL-2 promotes the proliferation and maturation of activated T cells; induces secretion of interferon-γ and tumour necrosis factors-α and -β; activates neutrophils; stimulates proliferation and maturation of activated helper T cells; and stimulates proliferation of activated NK cells and enhances the ability of these cells to kill target cells. IL-4 is a pleiotropic cytokine produced by activated T cells, mast cells, and basophils. It is an important regulator for the CD4+ subset (Th1-like vs. Th2-like) development. IL-8 is a potent chemoattractant for neutrophils and is also chemotactic for basophils, T cells and eosinophils. Eotaxin is a potent eosinophil chemoattractant, but does not affect mononuclear cells or neutrophils. MCP-1 is a chemoattractant for monocytes and basophils. MIP-1α chemoattracts CD8+ and CD4+ T cells; it also attracts B cells and eosinophils. RANTES (acronym for Regulation upon Activation, Normal T cell Expressed and presumably Secreted) is a monocyte chemoattractant that can also attract T cells and eosinophils (Gale & McColl, 1999; Spellberg & Edwards, 2001).
Obviously, elimination of the action of just one of these cytokines would have no or very little effect in helping ticks to feed. Rather, ticks need to eliminate the whole cytokine network to be successful in feeding. How do they face the plethora of cytokine actions? Two basic strategies exist to manipulate the cytokine network. One possibility is to inhibit the production of cytokines (Fuchsberger et al. 1995) whereas the alternative is to inhibit their action (Hajnická et al. 2001). The latter may occur either by occupying the receptor or by direct binding of the cytokine. An alternative strategy is to increase production of IL-10, an immune suppressor, although evidence that ticks do this is contradictory (Kopecký et al. 1999; Hannier et al. 2003). Irrespective of which mechanism they adopt, the most important strategy for ticks is to counterattack several cytokines and chemokines simultaneously to obtain the desired effect. This cluster bomb strategy is essential because of the redundancy in the cytokine network (Mantovani, 1999). Targeting the cytokine rather than the receptor also circumvents redundancy as most cytokines have more than one receptor. Thus ticks appear to have adopted a strategy similar to that of certain viruses, by analogy producing acarokines (cytokine-binding molecules of ticks, a name coined by the late Dr Norbert Fuchsberger).
In contrast to large DNA viruses such as poxviruses and herpesviruses, the comparatively small RNA containing tick-borne viruses have limited capacity to manipulate host cytokines. However, at least 8 different tick-borne viruses (members of 3 virus families: Bunyaviridae, Flaviviridae, and Orthomyxoviridae) show evidence of exploiting the immunomodulatory activities of their vector ticks, so-called SAT (Nuttall et al. 2000). Similar observations have been made for other tick-borne pathogens, including Borrelia burgdorferi sl and Francisella tularensis (Pechová et al. 2002; Zeidner et al. 2002; Kročová et al. 2003). Although the mechanism of SAT has not been determined, manipulation of the cytokine network seems a likely factor. Inoculation of C3H/HeJ mice with a mixture of TNF-α, IFN-γ and IL-2 at the time of tick feeding suppressed B. burgdorferi transmission by I. scapularis, showing that cytokine manipulation affects borrelia transmission (Zeidner et al. 1996). By binding numerous cytokines and suppressing their activity at the feeding site, ticks are providing a potential gateway to the host for tick-borne pathogens.
This work was supported by Slovak VEGA grants 2/4028/04 and 2/3111/23, by Science and Technology Assistance Agency under contract No. APVT-51-004702, National Program of Research and Development No. SP 51/028 08 00/028 08 03, by the Natural Environment Research Council (support for P.A.N.), and by Evolutec Ltd, Oxford, UK.

Fig. 1. Comparison of anti-cytokine activity of SGE obtained from 5 days-fed Dermacentor reticulatus female and male ticks, from 10 days-fed female and 12–13 days-fed male Amblyomma variegatum ticks, from 5 days-fed Ixodes ricinus female and male ticks. Various concentrations of SGE (47·6, 23·8, 9·5, 4·76, and 0·95 ng/μl or 5·0, 2·5, 1·0, 0·5 and 0·1 μg total protein; labelled 1 to 5, respectively) were pre-incubated with 50 pg of each cytokine (total volume 105 μl) for 90 min before addition to the ELISA plates. A reduction in optical density (OD) relative to the control indicates a decrease in the amount of cytokine detected by ELISA.

Fig. 2. Dermacentor reticulatus saliva prepared from 5 days-fed females inhibits detection of human recombinant cytokines. Various concentrations of saliva (95·2, 23·8, 9·5, 1·9, 0·95 ng/μl or 10·0, 2·5, 1·0, 0·2 and 0·1 μg total protein; labelled 1–5, respectively) were pre-incubated with 50 pg of each cytokine (total volume 105 μl) and analysed by ELISA as described for Fig. 1.

Fig. 3. Anti-cytokine activity of SGE fractions from Dermacentor reticulatus, Amblyomma variegatum and Ixodes ricinus female ticks demonstrated by specific ELISA. For ELISA assays pre-selected volumes of fractions indicated in the upper left corner of each graph were used. A reduction in optical density (OD) relative to the control indicates a decrease in the amount of cytokine detected. Arrows indicate the elution positions of molecular weight standards (from left to right): bovine serum albumin (67 kDa), ovalbumin (43 kDa), chymotrypsinogen A (25 kDa), and RNase A (13·7 kDa). The upper traces show the 280 nm absorbance profiles of each SGE size exclusion chromatogram.
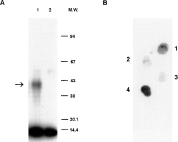
Fig. 4. Binding of MIP-1α to fractions of tick SGE. (A) Cross-linking of radio-isotope-labelled MIP-1α to active compounds in fraction 34 of SGE prepared from 5 days-fed Dermacentor reticulatus female ticks. Chemical cross-linking reactions were performed with DTSSP and subjected to SDS-PAGE under non-reducing conditions. Sample of fraction 34 (within the peak of anti-MIP-1α activity detected by ELISA; see Fig. 3) was incubated with 125I-MIP-1α (A, lane 1). Lane A2 contains only 125I-MIP-1α (7·8 kD) as a control. Arrow indicates position of tick compound bound to MIP-1α (~40 kDa). (B) Binding of 125I-MIP-1α to fractions 30 (1), 33 (2), 35 (3) and 38 (4) of SGE prepared from 10 days-fed Amblyommavariegatum female ticks (as in Fig. 3) spotted onto a PVDF membrane. Scanned radiograms.