Introduction
Maternal nutritional status is critical to fetal development, with nutritional status during pregnancy of particular importance.Reference Carlson and Aupperle1–Reference Yajnik and Deshmukh3 Nutrient intake during pregnancy reflects additional requirements of maternal and fetal systems. Several studies provide evidence of impaired macronutrient availability on future disease and highlight the tissue-specificity and timing of the nutritional insult on adult phenotype,Reference Symonds, Budge, Stephenson and Gardner4, Reference Yates, Tarling, Langley-Evans and Salter5 though less is known about the long-term effects of micronutrient deficiencies. Plasma copper (Cu) levels are increased during pregnancy, underscoring the importance of Cu availability for optimal fetal development.Reference Milne6 Delivery of maternal Cu to the fetus through placental transfer provides for the Cu requirements during the intrauterine period.Reference Alebic-Juretic and Frkovic7 The long-term effects of Cu deficiency during development on future disease are not well known.
Dietary Cu deficiency has numerous deleterious effects on the cardiovascular system that include structural changes in the heart and blood vessels, altered contractile and electrophysiological function, altered vascular signaling and blood pressure.Reference Saari and Schuschke8–Reference Uriu-Adams and Keen12 The effects of Cu deficiency on blood pressure are variable, and both hypertensionReference Klevay13, Reference Mederios14 and hypotensionReference Wu, Mederios, Lin and Thorn15 have been reported to occur, in rats fed with Cu-deficient diets. The differential effects of Cu deficiency on blood pressure may be due to differences in the strain of rats used in the experiments or to the differences in the ages of rats at the time dietary treatment was initiated. Cu deficiency initiated at a young age causes hypotensionReference Wu, Mederios, Lin and Thorn15 but leads to hypertension when initiated in older rats.Reference Klevay13, Reference Mederios14 Although reasons for the differential response of blood pressure to Cu deficiency are not clear, the blood pressure effects suggest that Cu deficiency perturbs the signaling mechanisms that regulate the contractile and dilator responses of vascular smooth muscle.
Direct effects of Cu deficiency on attenuation of endothelium-dependent relaxation by acetylcholine have been identified in a variety of animal models and vascular beds including the thoracic aorta,Reference Saari10 carotid arteriesReference Didion, Ryan and Didion16 and cremaster arterioles.Reference Schuschke, Falcone and Saari17 Findings indicate that severe Cu deficiency attenuates acetylcholine and sodium nitroprusside-induced vasodilation in the aorta and small arterioles, implicating reduced Cu, Zn-superoxide dismutase (CuZnSOD) activity and subsequent oxidative damage in the disruption of NO-mediated vascular smooth muscle relaxation.Reference Lynch, Frei and Morrow18–Reference Schuschke, Saari and Miller21 Studies of vascular responsiveness in models of Cu deficiency are restricted to dietary manipulation of Cu among male weanlings, resulting in severe Cu deficiency. The consequences of vascular functional alterations in mesenteric arteries resulting from exposure to the adverse environment of marginal maternal Cu deficiency during development have not been investigated. Accordingly, we examined the vascular functional responses to vasoconstrictors and vasorelaxants in mesenteric arteries from Cu-deficient dams and from offspring directly exposed to maternal Cu deficiency during in utero development and lactation. In addition, perpetuation of the effects of maternal Cu deficiency on vascular function was examined in a second generation of offspring.
Materials and methods
Animals and breeding
Approval for the study was obtained from the Animal Care and Use Committee of the Grand Forks Human Nutrition Research Center. Animals were maintained in accordance with the National Research Council Guidelines for the care and use of laboratory rats. Adult female Sprague-Dawley rats (Charles River, Wilmington, MA) weighing 145–150 g were housed in an environmentally controlled vivarium maintained at 22 ± 2°C and 50 ± 10% humidity with a 12 h/12 h light/dark cycle. Rats were divided into two groups (n = 20/group) and fed with AIN-93 G dietReference Reeves, Nielsen and Fahey22 formulated with CuSO4·5H2O to contain either 1 mg Cu/kg (Cu-deficient (CuD) diet ) or 6 mg Cu/kg (Cu-adequate (CuA) diet). The analyzed Cu concentrations were 1.11 mg Cu/kg diet in the CuD diet and 6.24 mg Cu/kg diet for the CuA diet. After 3 weeks of dietary treatment, female rats were mated with male Sprague-Dawley rats fed on commercial rat chow containing adequate amounts of Cu. Mating of CuA F0 and CuD F0 dams dyads was timed so that dams would give birth within hours of each other. Pregnant dams (F0) were maintained on their respective diets for the duration of gestation (∼22 days) and lactation (21 days). On the day following birth, litter sizes were adjusted to eight pups. Pup gender was determined and pups were randomly selected to achieve four male and four female pups per litter. Offspring from the resulting 40 litters were either cross-fostered or retained with the birth dam in equal proportions as follows: offspring from 10 litters of CuD F0 dams were cross-fostered to CuA F0 dams; offspring from 10 litters of CuA F0 dams were cross-fostered to CuD F0 dams; offspring from 10 litters each of CuA and CuD F0 dams were retained with the birth dams. The experimental design is summarized in Table 1. The resulting four groups of first generation (F1) offspring (n = 10/group) were designated as: CuA/CuA F1, offspring of dams fed with CuA diet suckled by the birth dam; CuA/CuD F1, offspring of dams fed with CuA diet suckled by dams fed with CuD diet; CuD/CuD F1, offspring of dams fed with CuD diet suckled by the birth dam; CuD/CuA F1, offspring of dams fed with CuD diet suckled by dams fed with CuA diet. After weaning on postnatal day 21 (PND21), F1 pups were transitioned to CuA diet.
Table 1 Experimental design. Within each litter of eight F1 offspring, half were crossed to a foster mother and half remained with the birth mother during lactation, equally divided by gender. F2 offspring groups were the result of mating between non-sibling pairs within F1 groups
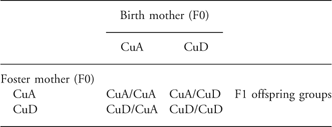
CuA, Cu adequate diet; CuD, Cu deficient diet.
At reproductive maturity (12–14 weeks), five non-sibling pairs from each of four F1 offspring groups were mated within groups, producing second generation (F2) offspring (n = 40). Litters from each of the four F1 mating groups were adjusted to eight pups that were subsequently suckled by their birth dams. The resulting four groups of F2 offspring (n = 10/group) were designated as: CuA/CuA F2; CuA/CuD F2; CuD/CuD F2 and CuD/CuA F2.
Cu status determination
Liver from one F1 male and female pups from each litter were harvested on PND 21 and analyzed by atomic absorption spectroscopy for Cu and Fe content.Reference Johnson and Kramer23 Hepatic Cu and Fe concentrations, hematocrit, hemoglobin concentration and plasma ceruloplasmin activity were determined in dams as previously describedReference Johnson and Anderson24 at the time their offspring were weaned. After the F1 offspring had been fed CuA diet for 6 weeks, Cu status of a F1 male and female from each litter was assessed by using hepatic Cu and Fe concentrations and plasma ceruloplasmin activities.
Isometric force measurement
Functional responses were determined in second order mesenteric arteries from F0 dams (7–8 per group) on PND 21 and F1 (5 per group) and F2 (5–7 per group) male and female offspring at 9 weeks of age. At time of euthanasia by isoflurane anesthesia followed by cardiac transection, the mesentery was removed and immediately placed in cold physiologic saline solution (PSS) containing: 119 mM NaCl, 4.7 mM potassium chloride (KCl), 2.5 mM CaCl2, 1.2 mM MgSO4, 25 mM NaHCO3, 1.2 mM NaH2PO4, 0.03 mM EDTA and 5.5 mM glucose adjusted to pH 7.4. Mesenteric arteries (200 μm) were excised and dissected free of surrounding tissues. Two arterial segments of 2 mm in length were mounted onto two 40 μm tungsten wires on a small artery wire myograph for measurement of isometric force and tension in a 5 ml bath of PSS, as originally described by Mulvany and Halpern.Reference Mulvany and Halpern25 Vessels were slowly warmed to 37°C with constant exposure to 95% O2/5% CO2 and normalized to achieve optimized internal circumference for development of tension. Normalized mesenteric arteries were set at 0.9× internal circumference when internal pressure reached 100 mmHg (L1 = 0.9 L100).Reference Anderson, Lopez, Zhang, Pavlish and Benoit26 Functional integrity of arteries was determined by challenge with phenylephrine (10 μM) following an equilibration period of 30 min, for a minimum of two consecutive priming doses, followed by washing with warmed PSS between doses. Functional integrity was assured when arteries reached a tension of 6 mN/mm in response to phenylephrine (PE) priming doses.
After an additional 30 min equilibration period, vessels were exposed to increasing, cumulative concentrations of each of the following: PE (Sigma) 10−9 to 10−3.5 M; KCl (Sigma) 4.7, 30, 60 and 120 mM; acetylcholine chloride (ACh, Sigma) 10−10 to 10−6 M; and sodium nitroprusside (SNP, Sigma) 10−10 to 10−6 M. Precontraction of vessels with PE to comparable tension (EC80) was completed before determination of relaxation responses. After thorough washing of vessels with PSS, the vessels were allowed to equilibrate for 30 min before each subsequent dose response. Concentration effect curves for each constrictor and relaxant were generated to determine the vascular responsiveness.
Statistical analyses
Unless otherwise indicated, data are expressed as mean ± s.e.m. Significance of the effects of dietary Cu treatment on the Cu status of dams was determined by Student t-test. Data from the offspring were analyzed separately for each gender by two-way ANOVA to determine the significance of effects for Cu status of the birth mother and postnatal mother and their interactions (SAS Version 9.1.3). When interactions were significant, differences between individual means were tested for significance with Tukey’s multiple comparison test. Concentration-effect curves generated from each vasoactive agent were displayed graphically to determine isometric tension. Drug concentrations eliciting a response of 50% (EC50) were derived from the concentration-effect curves and used for comparison between experimental and control dams and offspring groups. Differences in tension (mN/mm) and percent relaxation at each point across multiple concentrations were determined by repeated measures ANOVA with Sidak contrasts. P values of <0.05 were considered significant.
Results
Effect of diet on the Cu status of dams
Dams that consumed CuD diet beginning 3 weeks before conception and throughout pregnancy and lactation showed signs of poor Cu status 21 days after parturition. Hepatic Cu concentration in dams fed with CuD diet was 5.9 ± 1.8 μg/g dry liver compared with 10.4 ± 1.0 μg/g dry liver in dams fed with CuA diet (P < 0.05). Plasma ceruloplasmin activities were 15 ± 16 U/L and 84 ± 15 U/L in dams fed with CuD and CuA diets, respectively (P < 0.05). Although they were not anemic, the dams fed with CuD had lower (P < 0.05) hematocrits (43 ± 2%) and hemoglobin concentrations (14.6 ± 0.2 g/dL) than the dams fed with CuA diet (45 ± 1%, 15.4 ± 1 g/dL). However, hepatic Fe concentrations (235 ± 33 μg/g dry liver) were not different (P > 0.05) than the hepatic Fe concentrations (248 ± 56 μg/g dry liver) in dams fed with CuA diet.
Effect of cross-fostering on hepatic Cu concentrations in F1 offspring
Hepatic Cu concentrations in male and female offspring were affected by the Cu status of the birth mother (P < 0.0001 for the effect of birth mother, Table 2). The pooled mean ± s.e.m. for CuD/CuD F1 and CuD/CuA F1 male offspring was 9.0 ± 3.8 μg Cu/g dry liver compared with 61.8 ± 3.6 μg Cu/g dry liver for CuA/CuA F1 and CuA/CuD F1 male offspring. The pooled mean ± s.e.m for CuD/CuD F1 and CuD/CuA F1 female offspring was 10.0 ± 3.1 μg Cu/g dry liver compared with 58.5 ± 3.0 μg Cu/g dry liver for CuA/CuA F1 and CuA/CuD F1 female offspring. These data indicate that low hepatic Cu concentrations in the offspring of dams fed CuD were established prenatally and were not readily reversed by allowing the offspring to suckle dams that were fed with CuA diet. However, prenatal maternal Cu status did not affect hepatic Cu concentrations in 9-week-old offspring after they were fed with CuA diet for 6 weeks post-weaning (Table 2). Thus, the effect of low-maternal Cu intake on hepatic Cu concentrations was eliminated by weaning the offspring to diets providing adequate Cu. No differences were observed in plasma ceruloplasmin activity between any of the 9-week-old offspring (data not shown).
Table 2 Hepatic Cu concentrations in the first generation offspring and cross-fostered offspring of dams fed CuA or CuD diets throughout pregnancy and lactation

CuA, Cu adequate diet; CuD, Cu deficient diet.
Hepatic Cu concentrations were measured in 21 day-old offspring and 9 week-old first generation offspring following 6 weeks of Cu repletion. The offspring are designated as: CuA/CuA, offspring of dams fed Cu-adequate diet; CuA/CuD offspring of dams fed Cu-adequate diet cross-fostered to dams fed Cu-deficient diet; CuD/CuD, offspring of dams fed Cu-deficient diet; CuD/CuA, offspring of dams fed Cu-deficient diet cross-fostered to dams fed Cu-adequate diet. Values are means ± s.e.m for the number of animals shown in parentheses.
Effect of diet on mesenteric artery function in dams
Figure 1a and 1b represent receptor-mediated vasoconstriction induced by PE and non-receptor-mediated vasoconstriction induced by KCl, respectively. The EC50 between CuD and CuA F0 dams was not significantly different in vasoconstrictor responses to PE or KCl. There were no significant differences in endothelium-dependent relaxation responses to ACh or endothelium-independent relaxation responses to SNP in CuD F0 dams compared with CuA F0 dams (Fig. 1c and 1d). There were no significant differences in normalized internal vessel diameters between CuD and CuA animal groups. These findings suggest that marginal Cu deficiency in adult dams does not alter vasoconstrictor or relaxant responses in mesenteric arteries.

Fig. 1 Mesenteric artery responsiveness in dams. Arterial responses to phenylephrine (PE) (a), potassium chloride (KCl) (b), acetylcholine (ACh) (c) and sodium nitroprusside (SNP) (d) were not significantly different between Cu adequate (CuA) (n = 7) and Cu deficient (CuD) (n = 8) F0 dams.
Effect of maternal diet on mesenteric artery function in F1 offspring
The direct influence of in utero and postnatal exposure to marginal Cu deficiency on mesenteric artery contractile function was determined in F1 male and female offspring (Table 3). There were no significant differences in mesenteric artery contractile responsiveness to PE among F1 offspring (Figs 2a and 3a). Contractile responses to KCl (Fig. 2b) were increased between F1 CuD/CuA and CuD/CuD males when deficiency occurred during intrauterine development (EC50, −1.18 ± 0.13 v. −1.78 ± 0.13 for CuA and CuD, respectively; P = 0.01). There were no significant differences in contractile response to KCl among female offspring (Fig. 3b). These data suggest that the prenatal period represents a critical developmental window during which exposure to Cu deficiency alters vascular contractile function in male offspring.
Table 3 Summary of significant results of vascular function and related developmental period of copper deficiency in offspring groups

PE, phenylephrine; KCl, potassium chloride; ACh, acetylcholine chloride; SNP, sodium nitroprusside; CuA, Cu adequate diet; CuD, Cu deficient diet.

Fig. 2 Mesenteric artery responsiveness in F1 male offspring. There were no significant differences in responsiveness to phenylephrine (PE) (a), acetylcholine (ACh) (c) or sodium nitroprusside (SNP) (d). Contractile responses were increased in response to potassium chloride (KCl) (b) among Cu deficient (CuD)/CuD and Cu adequate (CuA)/CuD indicating the effect of maternal copper deficiency during in utero development (EC50, *P < 0.05; n = 5/group).

Fig. 3 Mesenteric artery responsiveness in F1 female offspring. Contractile responses to phenylephrine (PE) (a) and potassium chloride (KCl) (b) were not significantly different. Relaxation responses to acetylcholine (ACh) (c) were increased between F1 Cu deficient (CuD)/CuD and Cu adequate (CuA)/CuD female offspring (n = 5/group), the effect of F0 marginal copper deficiency during the postnatal period (EC50, **P < 0.01; *P < 0.05 at single concentrations). Reduced relaxation responses across increasing concentrations of sodium nitroprusside (SNP) (d) in F1 CuD/CuA compared with F1 CuD/CuD offspring were due to copper deficiency in the F0 birth dam (***P < 0.001 at single concentrations).
Among F1 male offspring, maternal Cu deficiency did not induce significant differences in endothelium-dependent relaxation (Fig. 2c). Endothelium-dependent relaxation responses in F1 offspring were increased in CuD/CuD and CuA/CuD female offspring, influenced by Cu status of the postnatal mothers (EC50, −7.85 ± 0.12 v. −8.38 ± 0.12 for CuA and CuD, respectively; P < 0.01). Maternal Cu deficiency in the postnatal period enhanced endothelium relaxation across multiple concentrations of ACh in female offspring (log −10, P < 0.05; log −9, P < 0.001; log −8, P < 0.001; Fig. 3c). There were no significant effects of the birth mother Cu status. These findings suggest that adequate maternal Cu status in the postnatal period is critical to functional endothelium-dependent relaxation responses in female offspring.
Relaxation responses to SNP among male offspring were not significantly different based upon maternal Cu status (Fig. 2d). Mesenteric arteries from female F1 CuD/CuA compared with CuD/CuD offspring had reduced endothelium-independent relaxation across increasing concentrations of SNP (log −8, P < 0.05; log −7, P < 0.05), demonstrating the in utero effect of maternal Cu deficiency. Although there were no significant differences in EC50, differences at increasing concentrations of SNP suggest a significant interaction between Cu status of both birth and postnatal mothers. The mismatch in Cu exposure from deficiency in utero to adequacy during postnatal development reduces relaxation responses in an endothelium independent manner.
Perpetuation of the effects of maternal Cu deficiency on mesenteric artery function in F2 progeny
As F1 dams and sires were Cu replete at the time of conception between 13 and 15 weeks of age and F2 offspring were not subjected to dietary intervention, alterations in F2 vascular function represent the influence of the persistence of F1 exposure to prenatal and/or postnatal F0 maternal Cu deficiency (Table 3). Among F2 male offspring, contractile responses to PE and KCl were not influenced by parental Cu status (Fig. 4a and 4b). Relaxation responses are shown in Fig. 4c and 4d. Across the concentrations of ACh, the mean endothelium-dependent relaxation responses were significantly reduced among F2 male offspring born to F1 CuD/CuA and CuD/CuD dams and sires (P < 0.05), pointing to the residual effects of Cu deficiency in F0 birth mother. In contrast, mesenteric arteries from male offspring born to F1 CuA/CuD and CuD/CuD dams and sires had increased endothelium-independent relaxation responses, perpetuating the effects of F0 Cu deficiency during lactation (EC50, log −7.04 ± 0.19 v. log −7.61 ± 0.18, P = 0.045 for postnatal F0 CuA and CuD, respectively). Parental Cu status did not result in significant differences in constrictor, endothelium-dependent or independent relaxation responses among female F2 offspring (Fig. 5).

Fig. 4 Mesenteric artery responsiveness in F2 male offspring. Contractile responses to phenylephrine (PE) (a) and potassium chloride (KCl) (b) were not significantly different. Mean endothelium-dependent relaxation responses to acetylcholine (ACh) (c) were decreased in F2 males born to F1 Cu adequate (CuA)/CuD and Cu deficient (CuD)/CuD dams and sires exposed to maternal copper deficiency during in utero development (*P < 0.05). In response to sodium nitroprusside (SNP) (d), postnatal copper deficiency in F0 dams increased endothelium independent relaxation responses in F2 CuA/CuD and CuD/CuD male offspring (EC50, *P < 0.05). Offspring totaled 5–6 per group.

Fig. 5 Mesenteric artery responsiveness in F2 female offspring. There were no significant differences in vascular responsiveness to phenylephrine (PE) (a), potassium chloride (KCl) (b), acetylcholine (ACh) (c) or sodium nitroprusside (SNP) (d). Offspring totaled 6–7 per group.
Discussion
The cardiovascular consequences of Cu deficiency first were identified almost one century ago,Reference Klevay27 though the mechanisms underlying vascular alterations have not been fully described. A significant body of knowledge on vascular responses induced by dietary Cu deficiency in male Sprague-Dawley rats exists. However, variations in the severity and timing of Cu deficiency have been associated with conflicting findings in vascular relaxation responses. For instance, Schuschke et al.Reference Schuschke, Percival and Saari28 determined that endothelium-dependent relaxation in the microcirculation was reduced by severe Cu deficiency but was unaffected by marginal Cu deficiency. Of particular importance is that studies investigating the effect of dietary Cu restriction on vascular function have been limited to the juvenile and adult periods, intervals during which the vasculature is developed. Other reports show that Cu deficiency leads to hypertensionReference Klevay13, Reference Mederios14 when initiated in older rats but to hypotension when initiated in younger rats.Reference Wu, Mederios, Lin and Thorn15 These findings suggest that Cu deficiency may influence the development of the vasculature in a manner that differentially affects vascular signaling pathways depending on the developmental stage of the rat. Our study design, which differed significantly from those reported in the literature in relation to animal sex, the severity and timing of Cu deficiency, and the vascular bed investigated, allowed us to expand knowledge regarding the effects of Cu deficiency on the vasculature by showing that Cu deficiency initiated prenatally alters vascular responses across two generations of offspring. To our knowledge, we are the first to identify the effects of marginal Cu deficiency during embryonic, fetal and postnatal development on subsequent vascular function during postnatal life in both male and female offspring.
The cross-fostered experimental design allowed for the identification of vulnerable periods during intrauterine and postnatal development (Table 3). Our results indicate the influence of low maternal Cu intake had both in utero and postnatal influences on vascular responses in F1 offspring depending on the nature of the response and gender of the offspring. For instance, the increase in KCl-induced vasoconstriction was observed only in F1 male offspring and depended on the Cu status of the birth mother. This finding indicates that the altered receptor independent response to KCl in the male offspring was a consequence of prenatal exposure to Cu deficiency. In contrast, maternal Cu intake affected both endothelium-dependent and independent vasorelaxation only in female F1 offspring. Endothelium-dependent vasorelaxation in response to ACh was affected by the Cu status of the postnatal mother while endothelium-independent vasorelaxation in response to SNP was influenced by in utero Cu exposure. These findings suggest that receptor and endothelium-independent vascular responses like KCl-induced vasoconstriction and SNP-induced vasorelaxation in F1 offspring are sensitive to Cu exposure during prenatal development while endothelium or receptor-mediated responses like ACh-induced vasorelaxation are sensitive to Cu exposure during postnatal development. The findings also indicate that the influence of maternal Cu intake affects male and female F1 offspring differently, with effects on vasoconstriction predominating in male offspring and effects on vasorelaxation predominating in female offspring. As a prenatal nutrient, Cu is not unique in producing differential effects between male and female offspring. A previous report showed that male offspring of Fe-deficient rat dams exhibit elevated systolic blood pressure at 6 weeks of age whereas female offspring exhibit reduced systolic blood pressure.Reference Gambling, Dunford and Wallace29 Others have shown that gender-specific changes in mesenteric artery function were the result of hypoxia.Reference Hemmings, Williams and Davidge30 Although we report that hemoglobin and hematocrit were slightly lower in CuD dams, the reduction likely did not cause hypoxia because the animals were not anemic. Therefore, it is unlikely that hypoxia had a major effect on vascular function in offspring of our CuD dams. However, the mechanisms underlying the gender dependence for the effect of maternal Cu intake on vascular responses in the F1 generation are not known.
Our finding that exposure to maternal Cu deficiency during intrauterine development was associated with reduced endothelium-independent relaxation in female F1 offspring is consistent with reports of reduced relaxation across increasing concentrations of SNP in severe Cu deficiency.Reference Schuschke, Falcone and Saari17, Reference Lynch, Frei and Morrow18 In the case of severe Cu deficiency, reduced relaxation may reflect the diversion of NO away from signaling and into lipid peroxidation pathways through the formation of peroxynitrite. However, we suggest that the alterations in vascular function resulting from maternal Cu deficiency represent the persistent effects of developmental Cu deficiency rather than Cu status of the offspring, as all F1 offspring were Cu replete at the time of investigation.
Investigation of a second generation of offspring revealed evidence of an indirect effect of F0 Cu deficiency, in contrast to the effects of direct exposure seen in the F1 offspring. Impaired vascular functional responses in F2 offspring were identified in male offspring, though patterns were inconsistent with those identified in their F1 dams and sires. The impaired vascular responses were manifest as enhanced responsiveness to SNP in male F2 offspring of parents that were initially exposed to postnatal Cu deficiency (i.e., F1 CuA/CuD and CuD/CuD dams and sires) and reduced responsiveness to ACh in male F2 offspring of parents initially exposed to prenatal Cu deficiency (i.e., F1 CuD/CuA and CuD/CuD dams and sires). These findings indicate that altered vascular function in the F2 generation is perpetuated in the absence of sustained Cu deficiency in the F1 generation and is suggestive that nutritional insults resulting from intrauterine exposure to Cu deficiency leads to heritable functional outcomes in vascular responsiveness. Furthermore, the perpetuation of the nutritional insult of marginal Cu deficiency during in utero and postnatal developmental periods suggests epigenetic alterations as the mode of inheritance, as DNA methylation patterns are determined during these critical developmental windows.Reference Waterland and Michels31, Reference Gicquel, El-Osta and Le Bouc32
Nutritional modulation during the vulnerable periods of in utero development and lactation leads to developmental plasticity and risk for subsequent development of disease.Reference Gluckman, Hanson, Cooper and Thornburg33 The importance of Cu to the developing fetus is evidenced by increased Cu levels during pregnancy, important for placental transfer of Cu from the mother to the fetus for metabolic needs during postnatal life.Reference Alebic-Juretic and Frkovic7 Although the mechanisms underlying the effects of maternal Cu deficiency on vascular responsiveness that we observed in offspring require further investigation, the known biological roles of Cu point to several possible mechanisms. Cu deficiency may serve as an anti-angiogenic, impairing vascular development during critical periods.Reference Gartner, Griffith and Pan34 Cu serves as a stimulus for vascular endothelial growth factor (VEGF) production, which is central to the process of angiogenesis.Reference Zhou, Jiang and Kang35 Restriction of available Cu during fetal development may alter the developing vasculature via reduced VEGF, impairing vascular smooth muscle functional responses, favoring enhanced endothelium-independent relaxation. Another plausible explanation for altered vascular function may be related to reduce the activity of lysyl oxidase, a Cu-dependent enzyme critical to the cross-linking of collagen and elastin.Reference Smith-Mungo and Kagan36 Developmental alteration of the architecture of elastic fibers in resistance arteries may favor relaxation in response to mechanical forces in the offspring. Earlier reports have shown low-Cu,ZnSOD activity results in oxidative damage that contributes to developmental defects in rat and mouse embryos of CuD dams.Reference Hawk, Lanoue and Keen37,38 Thus, the defect in vascular signaling we observed in the offspring of CuD dams may be a direct outcome of increased oxidative damage during windows that are critical for normal development of the vasculature. Even though the mechanisms related to our findings are not clear, this study provides further support for the importance of adequate maternal dietary Cu intake during critical periods of development for normal cardiovascular function in offspring across generations. Thus, our findings have implications for Cu status among childbearing women and the development of cardiovascular disease spanning generations.
Acknowledgement
The authors would like to thank Nathan Swentko, Steve Dufault and Linda Kibot for their technical assistance and LuAnn Johnson for the statistical analysis.
Statement of Interest
None










