Introduction
The Upper Cretaceous (Coniacian–Campanian) Niobrara Chalk of Kansas is a famed source of well-preserved vertebrate fossils, and is likely the “most-diverse and best-known Mesozoic fish assemblage in North America” (Wilson and Bruner, Reference Wilson and Bruner2004, p. 583; Shimada and Fielitz, Reference Shimada and Fielitz2006). As the Farallon Plate was subducted under the North American Plate, in the channel known as the Western Interior Seaway, the Niobrara Chalk was deposited as part of the Niobrara cyclothem; it represents the farthest extent of Western Interior Seaway depositional events. The Western Interior Seaway served as a throughway for marine organisms, resulting in a diverse fossil fauna. Abundant macroinvertebrates (cephalopods, bivalves, ammonoids, and crinoids) are present in the Niobrara Chalk, as are invertebrate trace fossils (Frey, Reference Frey1972). Vertebrate diversity of the Niobrara Chalk is comprised of bony fishes, (Stewart, Reference Stewart1999; Shimada and Fielitz, Reference Shimada and Fielitz2006) cartilaginous fishes (Stewart, Reference Stewart1978; Shimada,Reference Shimada1996), and tetrapods (sea turtles [Matzke, Reference Matzke2007], plesiosaurs, mosasaurs [Everhart, Reference Everhart2001, Reference Everhart2002], pterosaurs [Bennett, Reference Bennett2000], and avian and non-avian dinosaurs [Carpenter et al., Reference Carpenter, Dilkes and Weishampel1995]). The fish fauna includes isolated teeth, denticles, and body fossils of numerous taxa (Shimada and Fielitz, Reference Shimada and Fielitz2006), including holocephalans (Edaphodon, Callorhynchidae), elasmobranchs (Ptychodontidae, Mitsikurinidae, Odontaspidae, Cretoxyrhinidae, Anacoracidae), batoids (Cretomanta, Rhinobatidae), and bony fishes (actinopterygians [Pycnodontiformes, Semionotiformes, and many members of the teleost stem and crown], as well as sarcopterygians [Coelacanthiformes]).
The depositional environment of the layers that yielded Platylithophycus may have been hyposaline, and likely represented a circalittoral zone, as is the case with most marine chalks (Frey, Reference Frey1972). The depositional environment of the Smoky Hill Member of the Niobrara Chalk has been well reviewed, but the formation is otherwise not thoroughly catalogued or integrated, partly because it is mostly exposed as a series of discontinuities (Hattin, Reference Hattin1982). Hattin (Reference Hattin1982) described a depositional environment with poorly oxygenated benthic zones, and a paleoenvironment in which epibenthic communities were highly diverse and nearly all benthic invertebrate taxa were suspension feeders.
Platylithophycus has been ascribed to two different phyla over the past 70 years, first deemed a green alga, and later re-described as a cephalopod. Johnson and Howell (Reference Johnson and Howell1948) were the first to describe Platylithophycus and compared the texture of the slab with that of calcareous green algae, such as Codium. They described two parts of a “plant”: (1) surfaces covered with hexagonal plates, and (2) supposedly calcium carbonate-covered thread-like filaments (Johnson and Howell, Reference Johnson and Howell1948, fig. 1). They struggled to determine how these two parts were related to one another—they proposed the hexagonal, tessellated structures might have been protoplasmic objects produced inside cells, rather than representing an external surface of the “plant.” They called the tessellated surfaces “fronds,” and described filaments so dense that they lay matted both beneath and on top of the fronds (Johnson and Howell, Reference Johnson and Howell1948).
The focus of Miller and Walker’s (Reference Miller and Walker1968) work was to describe two new teuthid cephalopods from the Niobrara Formation, but they also included a revision of Platylithophycus. Their experience with cephalopod fossils led them to compare Platylithophycus with a sepiid (cuttlefish), based primarily on its textural similarities to cuttlebone. However, they did not confirm the fossil’s chemical composition to support their assertion that it was composed of aragonite. Moreover, their diagnosis ignored the “fronds” described by Johnson and Howell, and only discussed the “filaments” (Johnson and Howell, Reference Johnson and Howell1948; Miller and Walker, Reference Miller and Walker1968). Despite describing a “septate ventral pad,” and “porous pen structure,” (not figured in their paper), Miller and Walker (Reference Miller and Walker1968, p. 183) were unable to assign Platylithophycus at the family level. They refuted Dr. Maxim K. Elias’ unpublished observations and re-assignment to the genus Trachyteuthis, instead designating the original genus Platylithophycus to the order Sepiida, Zittel Reference Zittel1895. This description made Platylithophycus the oldest sepiid squid then on record (Miller and Walker, Reference Miller and Walker1968).
Importantly, in all these earlier studies, the hard tissue in Platylithophycus was assumed to be composed of calcium carbonate, although a simple test such as the application of a dilute organic (e.g., formic, acetic) acid would easily have falsified that interpretation, because these acids attack calcium carbonate but not calcium phosphate (a property that forms the basis of a widely used preparation technique in vertebrate paleontology; Toombs, Reference Toombs1948). When we performed this test, the fossilized tissue was unaffected.
Materials and methods
Materials
Well-preserved gill arches and cartilage fragments (UNSM IP16868). Three small chips of the holotype (previously numbers 26071, 26072, and 26073 in Princeton University’s geology collection) had been given to the American Museum of Natural History in 1982 with a note to rejoin the holotype (then residing at the Carnegie Museum). These fragments are now reunited with UNSM IP 16868.
Locality information
No coordinates were provided at the time of this specimen’s collection, so its exact position is unknown beyond the following: “Upper Cretaceous Niobrara Formation, three miles northeast of Monument Rocks, Cove County, Kansas” (Johnson and Howell, Reference Johnson and Howell1948, p. 632).
Methods
Specimen was examined using a dissecting microscope, and photographed using a DinoLite handheld microscope (AnMo Electronics Corporation), and photographed under UV light (by Mike Eklund, ThinkLabz), which produced fluorescence consistent with use of some kind of sealant or epoxy treatment (not necessarily consistent with biological or geological fluorescence). Morphology of UNSM IP16868 was compared with figures and descriptions from the work of Johnson and Howell (Reference Johnson and Howell1948) and Miller and Walker (Reference Miller and Walker1968). The fragments previously known as Princeton specimens 26072 and 26073 (now part of UNSM IP 16868) were imaged using scanning electron microscopy, with a Zeiss EVO 60 Variable Pressure SEM.
Repository and institutional abbreviation
Specimen housed at the University of Nebraska State Museum (UNSM).
Systematic note
The original species name has been emended here to agree with the gender of the genus name (Latin phycus, masculine noun III declension; cretaceus, adjective I class).
Systematic paleontology
Class Chondrichthyes Huxley Reference Huxley1880
Subclass Elasmobranchii Bonaparte Reference Bonaparte1838
Elasmobranchii incertae sedis
Genus Platylithophycus Johnson and Howell, Reference Johnson and Howell1948
Platylithophycus cretaceus (Johnson and Howell, Reference Johnson and Howell1948)
Figures 1–3, 4.1, 5, 6, 7.1, 7.3
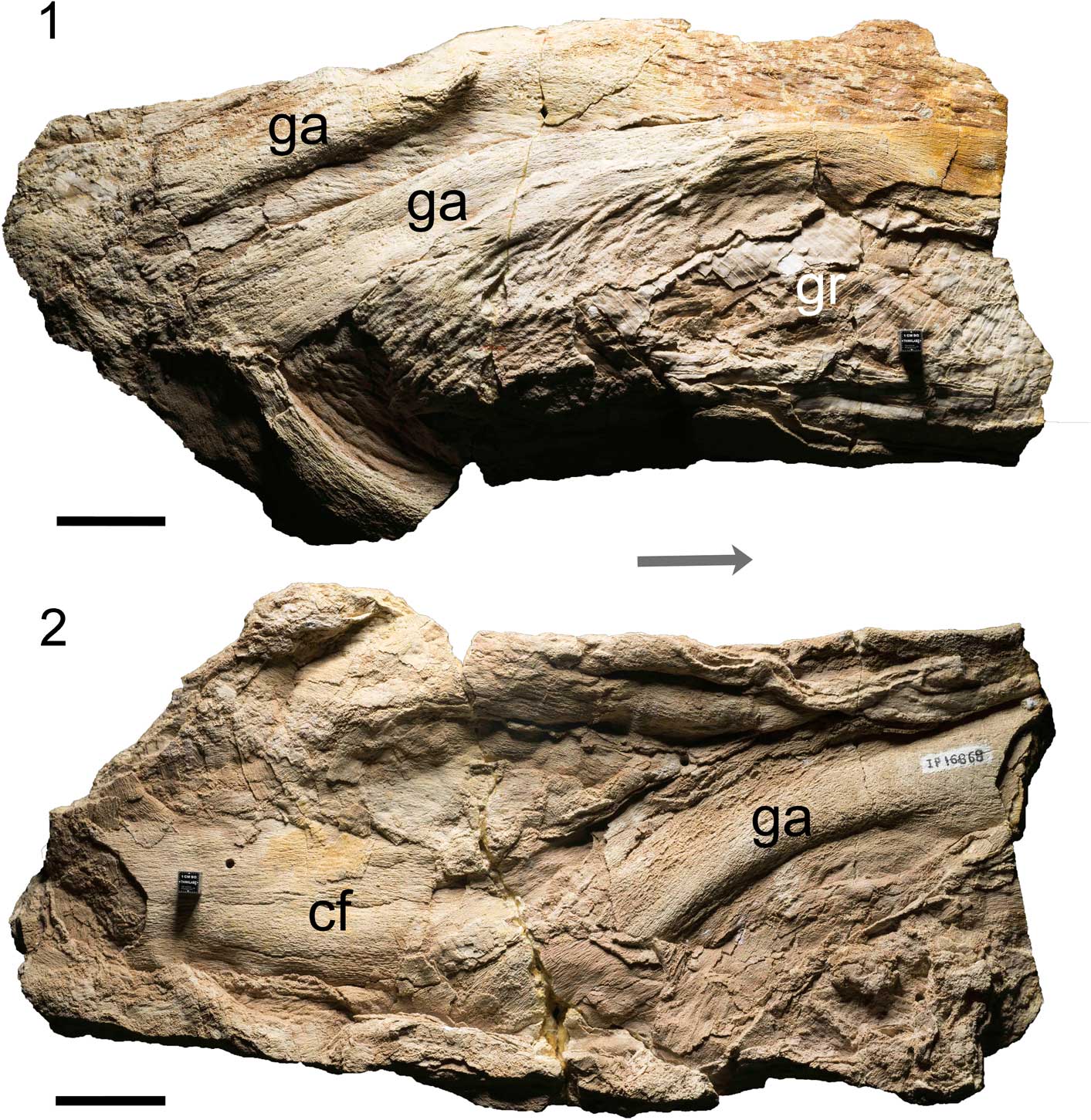
Figure 1 Holotype of Platylithophycus cretaceus (UNSM IP 16868), showing arc of the features interpreted here as gill arches. (1) Lateral view; (2) medial view. Abbreviations: ga=gill arches, gr=gill rakers, cf=cartilage fragments. Arrow indicates anterior, scale=5 cm. Photo courtesy of M. Eklund.

Figure 2 Holotype of Platylithophycus cretaceus (UNSM IP 16868), detail of mineralized cartilage on surface of features interpreted as gill arches, scale=1 cm. Photo courtesy of M. Eklund.

Figure 3 Holotype of Platylithophycus cretaceus (UNSM IP 16868), detail of features interpreted here as gill rakers, scale=1 cm. Photo courtesy of M. Eklund.

Figure 4 Comparison of (1) Platylithophycus (UNSM IP 16868) tesserae with cartilage tesserae of (2) extinct Libanopristis (AMNH FF 3705) and (3) extant Rhizoprionodon (AMNH FF 21652); (2) and (3) are modified from Maisey Reference Maisey2013; scale=1 mm.

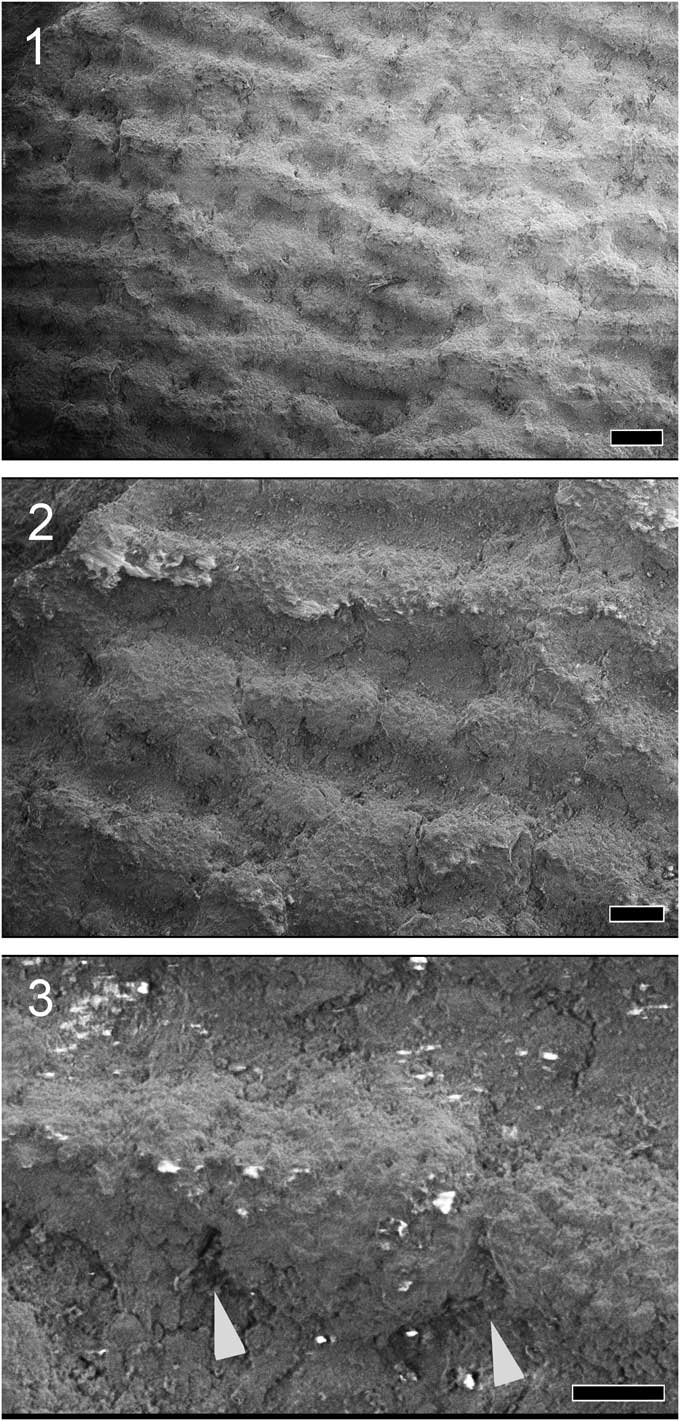
Figure 5 Scanning electron micrographs of the tessellated surface of Platylithophycus (UNSM IP 16868, previously Princeton specimen 26073). (1) Smooth section of undisturbed tesserae, showing intertesseral pores, marked by arrowheads, scale=400 um; (2) tesserae of relatively uniform size, showing intertesseral joints, scale=400 um; (3) detail of (2), magnifying the intertesseral joint area (ij), scale=100 um.

Figure 6 Scanning electron micrographs showing the thread-like “filaments” of Platylithophycus (UNSM IP 16868, previously Princeton specimen 26072) previously considered part of a sepiid cuttlebone or a calcareous green alga, but here shown to be composed of tessellated cartilage, by a series of increasingly magnified images of a region of the cartilage. (1) Filaments appear uniform at higher magnification, scale=400 um; (2) filaments are revealed to be made up of tesserae, scale=200 um; (3) detail of individual tesserae, intertesseral joints marked by arrowheads, scale=100 um.
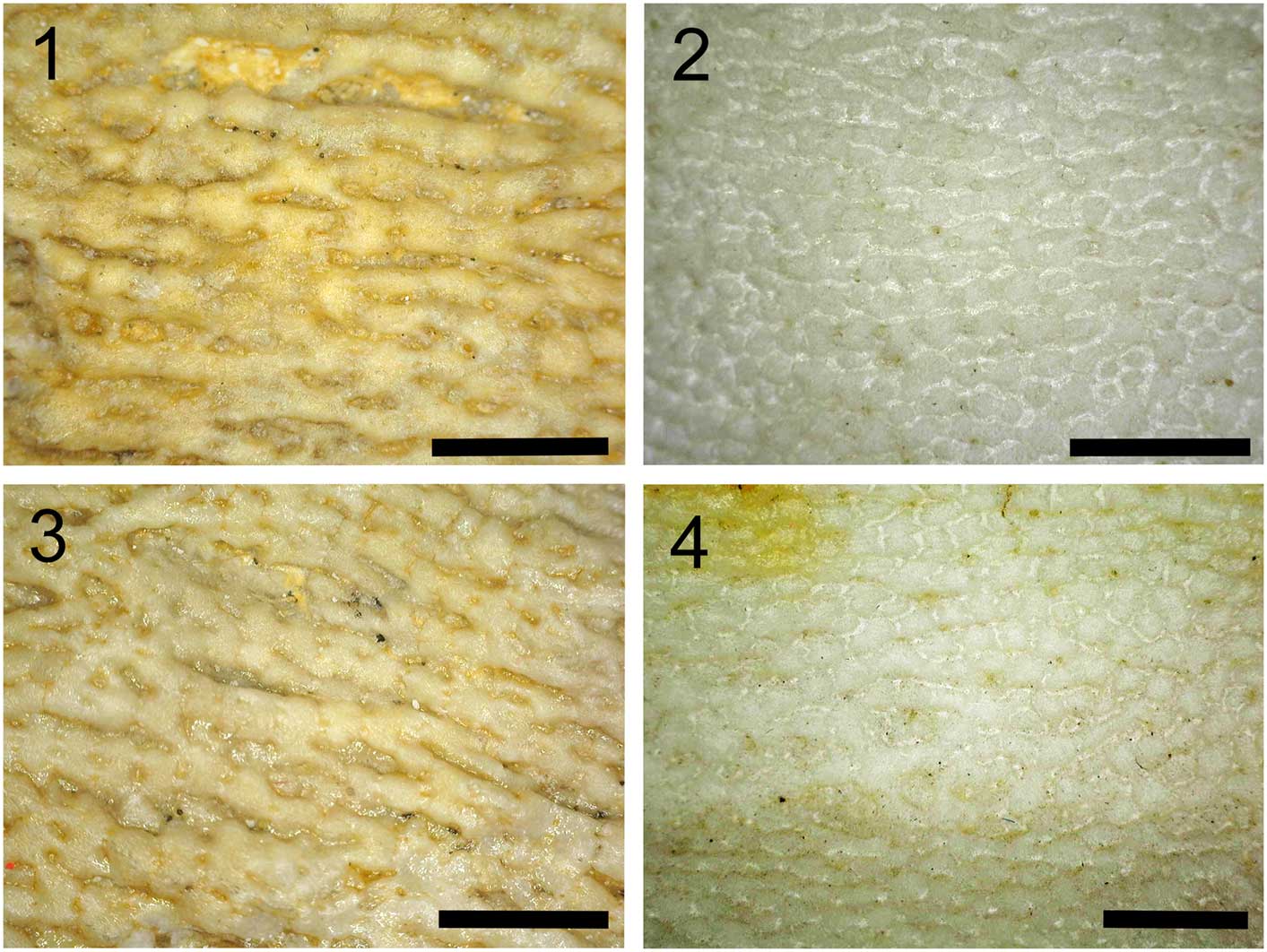
Figure 7 Surface of (1, 3) UNSM IP 16868, showing threadlike aggregations of tesserae on the surface of a gill arch in Platylithophycus, compared with (2, 4) a similar threadlike appearance of cartilage on the surface of the cranium in the extant mackerel shark (Lamna, AMNH FF 20426), scale=2 mm.
1948 Platylithophycus cretaceum Reference Johnson and HowellJohnson and Howell, p. 632, pl. 93, figs. 1, 2.
1968 Platylithophycus cretaceum; Reference Miller and WalkerMiller and Walker, p. 181, pls. 2, 3.
Holotype
Gill arches and associated cartilage fragments (UNSM IP 16868) from the Niobrara Chalk, Kansas (Johnson and Howell, Reference Johnson and Howell1948, pl. 93, figs. 1, 2).
Diagnosis
Cartilaginous gill arches of a large chondrichthyan fish, embedded in chalk matrix. Calcified cartilage of the gill arches possesses a filamentous appearance (Fig. 2), while the cartilage present on gill rakers (Fig. 3) is tessellated (“canaliculate” according to Miller and Walker, Reference Miller and Walker1968).
Occurrence
Upper Cretaceous Niobrara Chalk, 3 miles northeast of Monument Rocks, Cove County, Kansas (Johnson and Howell, Reference Johnson and Howell1948).
Description
The specimen is 48 cm in length and 24 cm across at its widest point. Gill arch elements are all fairly stout and flattened in shape, which may be a result of taphonomic distortion (Fig. 1). The exposed elements are considered to represent the actual gill-bearing parts of branchial arches (i.e., ceratobranchials and/or epibranchials). More ventral (basi-, hypo-) and dorsal (pharyngo-) elements could not be recognized. Gill rakers are present beneath the elements identified here as ceratobranchials, and are tessellated differently from the skeletal cartilage (Fig. 3).
Materials
University of Nebraska State Museum IP 16868.
Remarks
Platylithophycus cretaceus is founded on material that bears many similarities to the calcified cartilage of a chondrichthyan fish. Its surface structure is remarkably similar to that of tessellated cartilage (Figs. 2, 3), and the overall morphology is that of large gill arches (probably epibranchials and ceratobranchials). Structures interpreted to be gill rakers are present, medial to the gill arches (Figs. 1.1, 3). The size of these elements indicates this was a large chondrichthyan.
Results
The tiled appearance of the surface of Platylithophycus is identical to the tessellated calcified cartilage of both extinct and extant chondrichthyans (Fig. 4). Tesserae are arranged with a sub-hexagonal close-packing arrangement, with individual tesserae defined by an intertesseral joint system (Fig. 5), as well as local presence of intertesseral pores between tesserae (Fig. 5.1).
When previous descriptions of Platylithophycus are reinterpreted in the light of our interpretation of the structure and morphology of the holotype (Fig. 1, UNSM IP16868), several noteworthy features emerge. Johnson and Howell (Reference Johnson and Howell1948) described a “plant consisting of many flat fronds about six inches long and half an inch wide.” The difference in texture between these structures and surrounding filamentous structures baffled these earlier workers, who wrote, “the filaments appear to have grown from both sides of the fronds and they were so numerous that, where the fronds lie flat on the bedding surface, as they do in our specimen, the filaments form an almost matted layer above and beneath them” (Johnson and Howell, Reference Johnson and Howell1948, p. 632). Based on our interpretation of Platylithophycus as having tessellated cartilage, the “filaments” described by Johnson and Howell probably represent differentially calcified cartilaginous gill arches (Fig. 2). The filaments resemble continuous strands at low magnification (Fig. 6.1), but when examined under an electron microscope, they are composed of semi-contiguous individual tesserae, separated from neighboring tesserae by faint traces of intertesseral joints (Fig. 6.2, 6.3). This filamentous structure might initially appear unusual, but similar coalesced strands of tesserae are sometimes present in the cartilage of extant chondrichthyans (e.g., Lamna; Fig. 7).
Johnson and Howell’s “fronds” are reinterpreted here as the cartilage forming serial arrays of chondrichthyan gill rakers (Fig. 3). Comparison of their overall morphology with extant and fossil cartilaginous fishes indicates the fossil represents only the gill arches of the animal. No teeth are visible on the holotype specimen, although some may be hidden inside the matrix and therefore inaccessible without CT scanning or additional preparation.
Discussion
Endoskeletal tessellated calcified cartilage is considered a synapomorphy of conventionally defined chondrichthyans, within the total group Chondrichthyes (including acanthodian fishes that lack this hard tissue; Zhu et al., Reference Zhu, Yu, Ahlberg, Choo, Lu, Qiao, Qu, Zhao, Jia, Blom and Zhu2013). Thus, presence of this unique endoskeletal tissue in Platylithophycus is a hallmark feature of many chondrichthyans. Although the basic structure of tessellated calcified cartilage is highly conserved among these forms, the arrangement and density of individual tesserae, and the depth of calcification within the cartilaginous endoskeleton varies among taxa and even across different parts of the skeleton in a single individual (Dean, Reference Dean2011; Maisey, Reference Maisey2013). Anecdotal evidence exists for a diversity of tesseral configurations, but these have been poorly documented in the literature, and a review of these is beyond the scope of this paper. Our observations nevertheless demonstrate that Platylithophycus is founded on parts of a chondrichthyan whose cartilage exhibits at least two types of tesseral morphology that can be recognized in modern elasmobranchs. Only recently has much attention been devoted to resolving the development of tesserae in extant chondrichthyans (Seidel et al., Reference Seidel, Lyons, Blumer, Zaslansky, Fratzl, Weaver and Dean2016), the regulation of the mineralization process, and the effects of environment on calcification (Dean et al., Reference Dean, Ekstrom, Monsonego-Ornan, Ballantyne, Witten, Riley, Habraken and Omelon2015), but it seems likely that the tessellated calcification in Platylithophycus developed in identical manner to extant elasmobranchs.
While it is possible to diagnose Platylithophycus cretaceus on the basis of its unusual and potentially apomorphic gill raker morphology, the lack of features such as teeth in the holotype specimen mean that this taxon can only be classified as Elasmobranchii incertae sedis, pending the future discovery of more complete remains, which might provide clues as to its identity or possible synonymy with another large Niobrara Chalk elasmobranch.
Several large, predatory lamniform sharks have been described from the Niobrara Chalk, including Cretoxyrhina (Shimada, Reference Shimada1997a, Reference Shimadab), Scapanorhynchus (Hamm and Shimada, Reference Hamm and Shimada2002), and Cretolamna (Shimada, Reference Shimada2007), and it is possible that Platylithophycus belongs to one of these previously described taxa, although modern predatory lamniform sharks do not have densely arranged cartilaginous gill rakers like those of Platylithophycus. Dense arrays of gill rakers are found in modern filter-feeding elasmobranchs, including the whale shark (Rhincodon typus, order Orectolobiformes), basking shark (Cetorhinus maximus, order Lamniformes), megamouth shark (Megachasma pelagios, order Lamniformes), and rays (Manta, Mobula, order Myliobatiformes). However, extant filter feeding sharks do not have the type of gill raker structure observed in Platylithophycus; instead, the rakers consist either of elongate modified denticles as in Megachasma (Paig-Tran and Summers, Reference Paig-Tran and Summers2013, fig. 14), have denticles covering their surface of cartilage as in Cetorhinus (Paig-Tran and Summers, Reference Paig-Tran and Summers2013, fig. 15), or are entirely cartilaginous elements arranged into filtering pads as in Rhincodon (Matthews, Reference Matthews1950; Motta et al., Reference Motta, Maslanka, Hueter, Davis, de la Parra, Mulvany, Habegger, Strother, Mara, Gardiner, Tyminski and Zeigler2010; Paig-Tran and Summers, Reference Paig-Tran and Summers2013, fig. 13). Additionally, the filtering pads of Rhincodon are characterized by a reticulated mesh structure that was not observed in Platylithophycus. Nevertheless, large, filter-feeding rays such as Manta and Mobula have densely packed, cartilaginous gill rakers (Paig-Tran and Summers, Reference Paig-Tran and Summers2013, figs. 5, 6), somewhat like those observed in Platylithophycus. A thorough review of the gill structures in filter-feeding chondrichthyans, including images to which we compared Platylithophycus, was presented by Paig-Tran and Summers (Reference Paig-Tran and Summers2013). Paig-Tran et al. (Reference Paig-Train, Kleintech and Summers2013) provided a detailed comparison of filter pads in devil rays, at both microscopic and macroscopic scales.
Rare teeth from a putative manta ray relative, Cretomanta, have been documented from the Upper Cretaceous of northern Africa and North America, as well as from the Niobrara Formation of Saskatchewan, Canada (as Cretomanta canadensis, Case et al., Reference Case, Tokaryk and Baird1990). No skeletal remains of this enigmatic taxon have been found, so it is unknown whether its branchial skeleton is like that of Platylithophycus.
Conclusions
Based on the tessellated structure of Platylithophycus, in combination with the gross morphology of the structures that we interpret as gill arches and gill rakers, P. cretaceus is here interpreted as a large cartilaginous fish, possibly related to extant filter-feeding rays such as Manta and Mobula. This identification potentially expands the range of morphological diversity in the Niobrara elasmobranch fauna (which includes a form that possessed Manta-like teeth; Case et al., Reference Case, Tokaryk and Baird1990). However, we cannot definitively identify P. cretaceus beyond the level of Elasmobranchii incertae sedis because no name-bearing teeth or other identifiable elements such as denticles or fin spines are associated with the holotype specimen.
While we disagree with identifications of Platylithophycus as a plant or invertebrate, we recognize that those earlier comparisons with an alga or squid were based upon reasonable arguments given the interpretations presented. Prior researchers may simply have lacked expertise in vertebrate hard tissue ultrastructure (particularly the unique form of chondrichthyan calcified cartilage). According to paleontological legend, an undergraduate was the first to identify this fossil’s chondrichthyan affinity (a student in a University of Nebraska paleontology class is said to have suggested Platylithophycus looked like fossil cartilage).
Paleontology is home to myriad temporarily misplaced taxa, including medullosan ferns that were once regarded as sponges (Dunn et al., Reference Dunn, Krings, Mapes, Rothwell, Mapes and Kequin2003) and lungfish teeth misattributed to polypore fungi (Brown, Reference Brown1938). Accurate identification and classification of fossils are obviously paramount criteria for estimates of clade age as well as for meaningful reconstructions of paleodiversity and paleoecology. Wilson and Bruner (Reference Wilson and Bruner2004) recommended a thorough review of Niobrara Chalk fish systematics and stratigraphy, citing uncertainties and outstanding issues with taxonomy as possible obstacles for new workers. The Niobrara Chalk is so diverse and its fossils so abundant that there may be other taxonomic puzzles besides Platylithophycus stowed away in teaching collections and museums around the United States, patiently awaiting fresh eyes and renewed interest.
Acknowledgments
We thank M. Gottfried, J. Kriwet, and a third anonymous reviewer for their helpful comments, which improved this manuscript. Thanks also to A. Smith and M. Hill for their management of the Microscopy and Imaging Facility (American Museum of Natural History). A. Summers (University of Washington) and M. Paig-Tran (California State University, Fullerton) provided helpful suggestions regarding the gill structure of mobulid rays. M. Eklund (ThinklabZ) produced exceptional photographs of the holotype. We are particularly grateful to G. Corner and R. Secord (University of Nebraska State Museum) and A. Gishlick (AMNH) for specimen loans, and especially to A. Prybyla (Columbia University), who flew from Nebraska to New York with this large specimen as her carry-on luggage.