Introduction
The human zona pellucida (ZP) is the protective coat of the oocyte. It is an extracellular glycoprotein matrix that surrounds the oocyte with a paracrystalline three-dimensional network structure. It serves several functions, such as to mediate sperm binding, induce the acrosomal reaction, prevent polyspermy and protect the embryo until implantation. It is composed by four sulphated highly glycosylated proteins arranged in filaments as repeating units of ZP2–ZP3–ZP4 heterodimers, cross-linked by ZP1 dimers. ZP3 is responsible for primary sperm adhesion and binding, followed by acrosomal reaction induction with the assistance of ZP4. ZP2 acts as secondary binding and activates proacrosin into acrosin, a protease needed to penetrate the ZP (Lefièvre et al., Reference Lefièvre, Conner, Salpekar, Olufowobi, Ashton, Pavlovic, Lenton, Afnan, Brewis, Monk, Hughes and Barratt2004; Furlong et al., Reference Furlong, Harris and Vazquez-Levin2005; Caballero-Campo et al., Reference Caballero-Campo, Chirinos, Fan, Gonzalez-Gonzalez, Galicia-Chavarria, Larrea and Gerton2006; Chiu et al., Reference Chiu, Wong, Chung, Lam, Pang, Lee, Sumitro, Gupta and Yeung2008; Reid et al., Reference Reid, Redgrove, Aitken and Nixon2011). Another physical function of the ZP in early cleavage embryos is to promote normal blastomere interaction through the formation of intercellular junctions, thus enabling maximum contact between blastomeres prior to compaction (Bloor et al., Reference Bloor, Metcalf, Rutherford, Brison and Kimber2002). These proteins are synthesised in the endoplasmic reticulum, transferred to the Golgi and delivered to the oocyte membrane by exocytosis (Green, Reference Green1997).
The thickness of the ZP was estimated to be about 19.5 ± 2.2 μm using polarized light microscopy (Pelletier et al., Reference Pelletier, Keef and Trimarchi2004) or 16.18 ± 2 μm by normal microscopical inspection (Balakier et al., Reference Balakier, Sojecki, Motamedi, Bashar, Mandel and Librach2012). ZP fibres are highly ordered and thus have a birefringent structure (retards the refractive index of polarized light). Using polarized light microscopy, it was possible to reveal three layers in the ZP, outer (6.1 ± 1.7 μm), middle (3.7 ± 0.9 μm) and inner (9.8 ± 2.1 μm) (Pelletier et al., Reference Pelletier, Keef and Trimarchi2004).
Balakier et al. (Reference Balakier, Sojecki, Motamedi, Bashar, Mandel and Librach2012) have shown that the ZP thickness was not influenced by demographic, clinical and embryological parameters. In contrast, higher ZP thickness variation has been related to better embryological and clinical outcomes (Palmstierna et al., Reference Palmstierna, Murkes, Csemiczky, Andersson and Wramsby1998) and the same observations were observed regarding higher light retardance of the ZP (Shen et al., Reference Shen, Stalf, Mehnert, Eichenlaub-Ritter and Tinneberg2005; Rama Raju et al., Reference Rama Raju, Prakash, Krishna and Madan2007; Montag et al., Reference Montag, Schimming, Koster, Zhou, Dorn, Rosing, van der Ven and van der Ven2008, Reference Montag, Koster, van der Ven and van der Ven2011; Madaschi et al., Reference Madaschi, Aoki, Braga, Figueira, Francisco, Iaconelli and Borges2009; Braga et al., Reference Braga, Figueira, Queiroz, Madaschi, Iaconelli and Borges2010; Ebner et al., Reference Ebner, Balaban, Moser, Shebl, Urman, Ata and Tews2010).
Human oocyte dysmorphisms attain a large proportion of retrieved oocytes from assisted reproductive technology (ART) treatment cycles. Dysmorphisms are divided in extracytoplasmic and intracytoplasmic defects. There are several intracytoplasmic morphological abnormalities, a few with proven impact on implantation (reviewed in Sá et al., Reference Sá, Cunha, Silva, Luís, Oliveira, Teixeira da Silva, Barros and Sousa2011). Extracytoplasmic defects involve an abnormal morphology of the ZP, perivitelline space and polar body (Rienzi et al., Reference Rienzi, Vajta and Ubaldi2011). Regarding reports on ZP abnormalities, oocytes with dark ZP were associated with normal fertilization and embryo cleavage rates (Alikani et al., Reference Alikani, Palermo, Adler, Bertoli, Blake and Cohen1995; De Sutter et al., Reference De Sutter, Dozortsev, Qian and Dhont1996; Balaban et al., Reference Balaban, Urman, Sertac, Alatas, Aksoy and Mercan1998; Ebner et al., Reference Ebner, Yaman, Moser, Sommergruber, Feichtinger and Tews2000; Meriano et al., Reference Meriano, Alexis, Visram-Zaver, Cruz and Casper2001; Plachot et al., Reference Plachot, Selva, Wolf, Bastit and de Mouzon2002; Ten et al., Reference Ten, Mendiola, Vioque, de Juan and Bernabeu2007; Rienzi et al., Reference Rienzi, Ubaldi, Iacobelli, Minasi, Romano, Ferrero, Sapienza and Baroni2008) whereas oocytes showing a thin ZP had no fertilization. In oocytes with absent ZP a viable pregnancy was only attained when surrounded by granulosa cells (Stanger et al., Reference Stanger, Stevenson, Lakmaker and Woolcott2001). Oocytes with oval ZP showed a normal fertilization rate, an abnormal embryo cleavage pattern and no relation to female age or stimulation data (Ebner et al., Reference Ebner, Shebl, Moser, Sommergruber and Tews2008), with birth of a healthy boy (Esfandiari et al., Reference Esfandiari, Ryan, Gotlieb and Casper2005).
The aim of the present study was to evaluate the clinical, embryological and ultrastructural features of cases with all oocytes presenting an indented ZP, a novel described dysmorphism.
Materials and methods
According to the National Law on Medically Assisted Procreation (Law 32/2006) and the National Council on Medically Assisted Procreation guidelines (CNPMA, 2008), clinical and embryological data bases, eight immature oocytes from a cohort presenting indented ZP and one surplus normal oocyte were used after patient informed and written consent from cases enrolled in ART treatment cycles.
Stimulation protocol
There were six cases and 13 cycles. Women underwent controlled ovarian hyperstimulation (Pinto et al., Reference Pinto, Oliveira, Cardoso, Teixeira da Silva, Silva, Sousa and Barros2009) using a GnRH short antagonist protocol with cetrorelix in three cycles (Merck Serono, Genève, Switzerland) and ganirelix in five cycles (Organon, Oss, The Netherlands). In four cycles a long agonist protocol was used with busereline (Sanofi Aventis, Frankfurt, Germany). In the third cycle of case 5, an ultralong GnRH agonist protocol was used with triptoreline (Ipsen, Paris, France) combined with cetrorelix at final stimulation. For stimulation, recombinant follicle stimulating hormone (rFSH) was used (Puregon; Organon; Gonal-F; Merck Serono) except in cases 5 and 6 in which rFSH was combined with human menopausal gonadotrophin (HMG; Ferring, Kiel, Germany). For triggering final oocyte maturation, human chorionic gonadotrophin (HCG) was administered (10,000 IU; 15,000 IU in the third cycle of case 1) (Pregnyl; Organon). There were two exceptions to HCG, in the fourth cycle of case 5 in which the agonist busereline was used and in case 6 where recombinant HCG (Merck Serono) was administered. Estradiol serum levels (Elecsys 2010, Roche, France: up to 2009; VIDAS Estradiol II, Biomerieux, Marcy-L'Etolie, France: since 2009) were assayed by electrochemiluminescence (ECL) up to 2009 and enzyme-linked fluorescence assay (ELFA) since 2009 at the day of HCG administration. Basal FSH was assayed by microparticle enzyme immunoassay (MEIA; Abbott Lab, Abbott Park, IL, USA) (Table 1).
Table 1 Stimulation protocol of embryo transfer cycles from the six patients with indented zona pellucida

aAssociated with HMG.
Ab, first trimester abortion; Age-F, female age (years); Age-M, male age (years); Agonist, GnRH agonist; Antag, GnRH antagonist; bFSH, basal FSH (mIU/ml); CP, clinical pregnancy; Date, year of oocyte retrieval; Days, days of stimulation; E2, estradiol (pg/ml); End, endometrium thickness (mm); ET, embryo transfer; ETC, embryo transfer cycles; FSH, total dose of FSH administered (IU); HCG, dose administered (IU); NB, newborn; no 2PN, absent normal fertilization (2PN, 2PB); no ET, no embryo transfer; no MII, absence of mature metaphase II oocytes; P, patients; PCOS, polycystic ovarian syndrome; TI, time of infertility (years).
Oocyte denudation and intracytoplasmic sperm injection (ICSI)
Cumulus–oocyte complexes were denuded under paraffin oil (light mineral oil: liquid paraffin, Medicult Origio, Jyllinge, Denmark; Ovoil-100, VitroLife, Kungsbacka, Sweden) with hyaluronidase (80 UI; ICSI Cumulase, Medicult; Hyase-10X, VitroLife) and incubated in 4-well tissue culture dishes (embryo tested, NUNC, Roskilde, Denmark; Falcon, Becton Dickinson, Franklin Lakes, New Jersey, USA) in in vitro fertilization medium (Universal IVF Medium, Medicult; G-IVF-PLUS, VitroLife) until microinjection. Microinjection was performed as described (Tesarik & Sousa, Reference Tesarik and Sousa1995; Sousa et al., Reference Sousa, Cremades, Silva, Oliveira, Teixeira da Silva, Viana and Barros2002), under paraffin oil, in SPM (Medicult) or G-MOPS-Plus (VitroLife), using polyvinylpyrrolidone (PVP; Clinical Grade; Medicult) and holding and microinjection micropipettes (Swemed, Goteborg, Sweden) in inverted microscopes (Nikon DIAPHOT 200; Nikon ECLIPSE 300; Nikon, Tokyo, Japan) equipped with thermal stages (37 °C), Hoffman optics (Nikon) and Narishige micromanipulators (MO-188; Narishige, Tokyo, Japan).
Embryo culture and score
After microinjection, oocytes were washed and cultured in ISM1 (Medicult) or G1-Plus (VitroLife), under oil, in 4-well dishes or multi-small-well EGPS dishes (Sun IVF; Guilfor, USA) inside a multigas incubator (6% CO2, 5% O2, 89% N2, filtrated humidified air; Sanyo, Tokyo, Japan). Embryos were cultured in sequential media. For Medicult, in ISM1/BlastAssist system medium-1 up to day 3 and then in ISM2/BlastAssist system medium-2 up to day 5; for embryo transfer, embryos were placed in 0.5–1 ml of Universal Transport Medium (UTM) for 10 min to 1 h. For VitroLife, in G-1 Plus until day 3 and then in G-2 Plus up day 5; for embryo transfer, embryos were placed in EmbryoGlue. Normal fertilization was assessed at 16–19 h after microinjection. Embryo quality evaluation was performed using established score guidelines. High quality embryos (A/B) were those with the correct number of blastomeres, of similar size and regularity, and with less than 25% of fragments (Vandervorst et al., Reference Vandervorst, Liebaers, Sermon, Staessen, De Vos, Van de Velde, Assche, Joris, Van Steirteghem and Devroey1998). High quality blastocysts were BL1 and BL2 with good morphology and BL3–BL5 if the inner cell mass and trophectoderm were A/B (Gardner et al., Reference Gardner, Phil, Lane, Stevens, Schlenker and Schoolcraft2000).
Embryo transfer, luteal support and pregnancy confirmation
Ultrasound-guided (Aloka, Tokyo, Japan) embryo transfer was performed with a Sure-View-Wallace Embryo Replacement Catheter (23 cm, CE123; Smiths Medical Int., Kent, UK). For luteal support it was used two endovaginal tablets of 100 mg natural-micronized progesterone, 8/8 h (600 mg day) (Jaba, Besins Int, Montrouge, France) from the day of oocyte aspiration up to 10–12 weeks of gestation or when βHCG was negative. In the fourth cycle of case 5, where an agonist was used for triggering final oocyte maturation, it was associated one oral tablet of 2 mg estradiol hemihydrate, 12/12 h for the same time of progesterone (Isdin, Novo Nordisk, Bagsvaerd, Denmark), and HCG (1500 IU) at days 3 and 6 after oocyte pick-up. Clinical pregnancy was established by ultrasound visualization at 7 weeks of gestation of a gestational sac.
Cell imaging and transmission electron microscopy
Live cell images were taken in the inverted microscope (×10 and ×20 objectives) with the use of Cronus-3 imaging system (Research-Instruments Ltd, Cornwall, UK). Eight immature oocytes (4 GV; 1 MI spontaneously matured from a GV; 1 MII spontaneously matured from a GV; 2 MI), and one normal surplus oocyte from a cycle without dysmorphic oocytes (there was no normal mature surplus oocytes from the dysmorphic cases) were processed for transmission electron microscopy. Oocytes were fixed with Karnovsky (2.5% glutaraldehyde, 4% paraformaldehyde, 0.15 M sodium cacodylate buffer) (Sigma-Aldrish, St. Louis, USA; Merck, Darmstadt, Germany) for 30 min at room temperature and then for 2 h at 4 °C, washed in 0.15 M sodium cacodylate buffer, pH 7.3 (Merck), post-fixed with 2% osmium tetraoxide (Merck) in buffer containing 0.8% potassium ferricyanide (Merck) for 2 h at 4 °C, washed in buffer (10 min), serially dehydrated in ethanol (Panreac, Barcelona, Spain), equilibrated with propylene oxide (Merck) and embedded in Epon (Sigma). Semithin and ultrathin sections were prepared with a diamond knife (Diatome, Hatfield, Switzerland) in a LKB ultramicrotome (Leika Microsystems, Wetzlar, Germany). Ultrathin sections collected on 200 mesh formvar carbon-coated copper grids (Taab, Berks, England), stained with 3% aqueous uranyl acetate (20 min) (BDH, Poole, England) and Reynolds lead citrate (10 min) (Merck). Ultrathin sections were observed in a JEOL 100XII transmission electron microscope (JEOL, Tokyo, Japan) operated at 60 Kv (Sousa & Tesarik Reference Sousa and Tesarik1994; El Shafie et al., Reference El Shafie, Sousa, Windt and Kruger2000).
Statistical analysis
Two types of controls were used. All consecutive ICSI cycles during 2005–2011 and those ICSI cycles from the same pool that had embryo transfer at day 2 and day 3, once embryo transfer was mainly performed at these days (four cycles at day 3 and day 2), with only one cycle at day 5. Statistical analysis was carried out through the IBM SPSS Statistics 20 program for Windows. We used the chi-squared test (Fisher Exact Test, two-sided) and the Independent Samples T-test for equality of means (two-sided). A value of P < 0.05 was considered to be statistically significant.
Results
From January 2005 to September 2011 there were 3996 ICSI cycles, of which 13 (six patients, 0.33%) had all oocytes presenting an indented ZP. In addition, all of these oocytes showed total or partial absence of the perivitelline space, absence of resistance to ZP and oolemma penetration during microinjection, and low ooplasm viscosity during microinjection aspiration.
These six infertile patients presented a female factor of infertility: polycystic ovarian syndrome, oocyte factor, uterine factor, ovarian factor (small ovaries) and bilateral ovarian endometriosis. All female patients had normal karyotypes and normal basal FSH serum levels. The mean female age was 33 years old (32–36) and the mean time of infertility was 3 years (1–5), both at the time of the first treatment. Male patients had normal semen analysis and karyotypes. The mean male age was 36 years old (30–43). In all cases ejaculated sperm was used for ART treatments (Table 1).
In three patients with more than one ART cycle (case 1 and 5: four cycles; case 2: two cycles), this dysmorphism was recurrent and affected again all oocytes. Of the 13 ART cycles performed, four had no embryo transfer, three of them (case 1: one cycle; case 5: two cycles) due to absence of mature oocytes and one (case 1) due to failed fertilization (Table 2). In all cases, at embryo transfer, the endometrium had a trimalinar aspect, with embryo transfer being easy with a linear flow, except in case 3, which was punctual.
Table 2 Embryological outcomes in the six patients with indented zona pellucida

aOf the total COC, 1 was degenerative at each cycle (Deg).
bOf the 12 COC, 1 was degenerative and one fractured (Deg).
2PN, number of normal fertilized oocytes; Ab, clinical abortion; AB, total number of embryos of quality AB obtained at each day of culture; AH, assisted hatching; CP, clinical pregnancy; COC, number of cumulus–oocyte complexes; Day, total number of embryos obtained at each day of culture; Deg-ICSI, number of oocytes degenerated after ICSI; ET, number and score of transferred embryos; ETC, embryo transfer cycles; MII, number of mature metaphase II oocytes; NB, newborn; no 2PN, absence of normal fertilization (2PN, 2PB); no ET, no embryo transfer;
Case 1 had four consecutive cycles, one IVF (2005) and three ICSI (2005–2006). In the first cycle no spermatozoa were found bound to the ZP. In the second cycle there were no mature oocytes. There was no pregnancy in the other two cycles after transfer of three embryos at day 3 and of one embryo at day 2 (Table 2).
Case 2 had two ICSI cycles (2006). In the first cycle two embryos (day 3) and in the second cycle one embryo (day 3) were transferred but there was no pregnancy (Table 2).
Case 3 had one ICSI treatment cycle (2006), with transfer of a single blastocyst (day 5), but there was no pregnancy (Table 2).
Case 4 had one ICSI treatment cycle (2008) with transfer of a single embryo (day 3), without pregnancy (Table 2).
Case 5 had four consecutive ICSI cycles (2009–2010). In the first two cycles there were no mature oocytes. In the third cycle there was transfer of one embryo (day 2), but no pregnancy was achieved. In the fourth cycle, there was transfer of two embryos (day 2), with a single clinical pregnancy that ended in the delivery by Cesarean section, after 38 weeks of gestation, of a healthy girl (2385 g), with no complications (Table 2). In this successful cycle, the basal FSH was 8.6 mIU/ml (increased in relation to the other cycles), the antagonist ganirelix was used (as in two other cycles), the endometrium thickness was 10 mm (as in another cycle), the total dose of gonadotrophins was 3200 IU (the highest level of cases using ganirelix), the time of stimulation was 8 days (the lowest of all cycles), the level of serum estradiol was 528.48 pg/ml (the lowest of all cycles) and the agonist busereline was used instead of HCG to create a wave of endogenous FSH in order to increase the possibility of retrieved mature oocytes.
Case 6 had one ICSI treatment cycle (2011), with transfer of two embryos (day 2), with a single clinical pregnancy that ended in a first trimester abortion (Table 2).
Overall, from 3996 ICSI cycles, there were 13 treatments ART cycles (0.33%) that had all oocytes with indented ZP. From these, there were nine embryo transfers (69.2%), with two clinical pregnancies (one ended in a first trimester abortion) (15.4%) and one newborn (7.7%). In three cases that had more than one treatment cycle this dysmorphism was recurrent and attained all oocytes (cases 1, 2 and 5). Comparisons between cases with and without pregnancy revealed no significant differences in relation to female age, time of infertility, days of stimulation, total doses of gonadotrophins, serum estradiol levels, endometrium thickness or type of protocol used. Significant differences were observed regarding basal FSH (P = 0.005) which was higher in the pregnant group. Comparisons in relation to HCG could not be performed as the pregnant group did not use HCG.
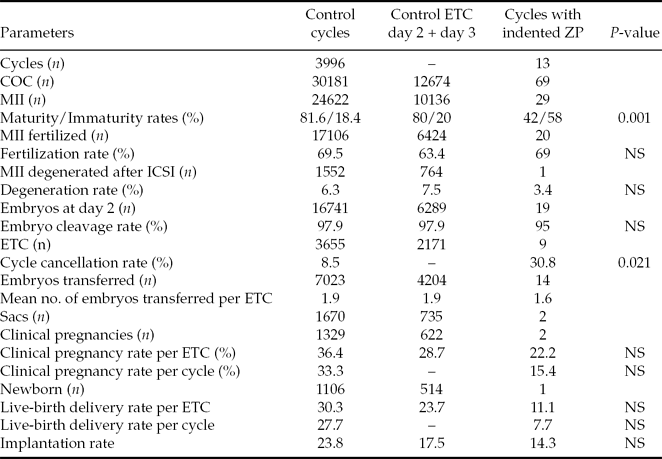
When compared with controls, data showed significant low oocyte maturity and high cycle cancellation rates, no significant differences regarding fertilization, degeneration and embryo cleavage rates, and low, although not significant, pregnancy and live-birth delivery rates (Table 3). No differences were found regarding female age, time of infertility, basal FSH, days of stimulation, total doses of gonadotrophins or serum estradiol levels. Significant differences were observed in cases with dysmorphic oocytes, which exhibited lower HCG doses (P < 0.001) and a higher endometrium thickness (P = 0.018).
Table 3 Embryological outcomes in controls and in the six patients with indented zona pellucida

COC, cumulus–oocyte complexes; ETC, embryo transfer cycles; MII, mature metaphase II oocytes; NS, not significant.
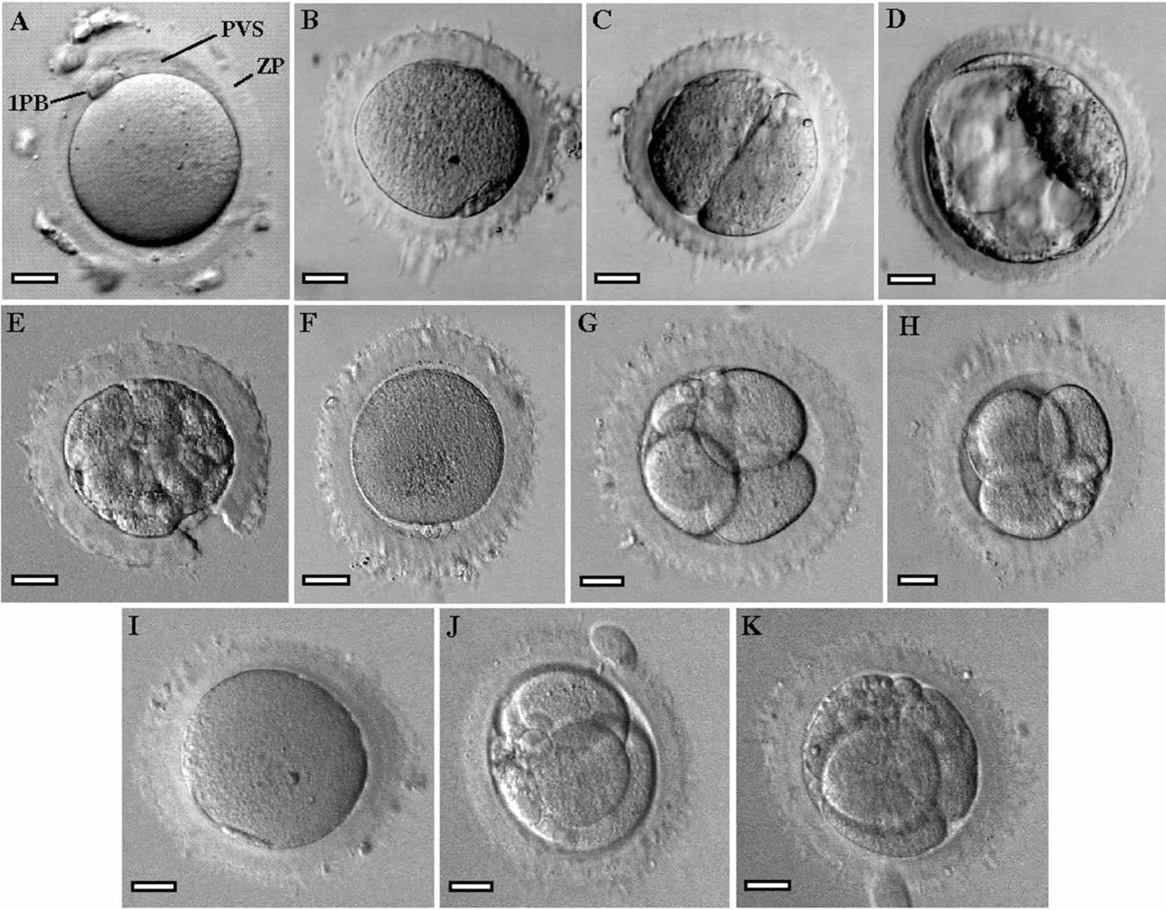
At the inverted microscope, all oocytes displayed the same extracytoplasmic dysmorphism, a dense ZP with an indented outside and absent or decreased perivitelline space. At the region containing the first polar body, the perivitelline space was larger or decreased with a flattened first polar body (Fig. 1).

Figure 1 Images of oocytes and embryos obtained by inverted Hoffman optics microscopy. (A) A mature metaphase II oocyte (MII) with normal morphology. Zona pellucida (ZP), perivitelline space (PVS) and first polar body (1PB). (B–K) MII oocytes with indented ZP, absence or minimal PVS and flattened 1PB, and derived embryos. (B, C) the MII oocyte and the 2-cell embryo transferred from case 1. (D) The blastocyst transferred from case 3. (E) The 10-cell embryo with assisted hatching (hole in ZP) transferred from case 4. (F-H) The MII oocyte and the two 4-cell embryos transferred that gave rise to a clinical pregnancy followed by abortion. (I–K) The MII oocyte and the two embryos (3-cells and 4-cells) transferred that gave rise to a healthy newborn. Bars: 20 μm (A, E, G–K), 25 μm (B–D, F).
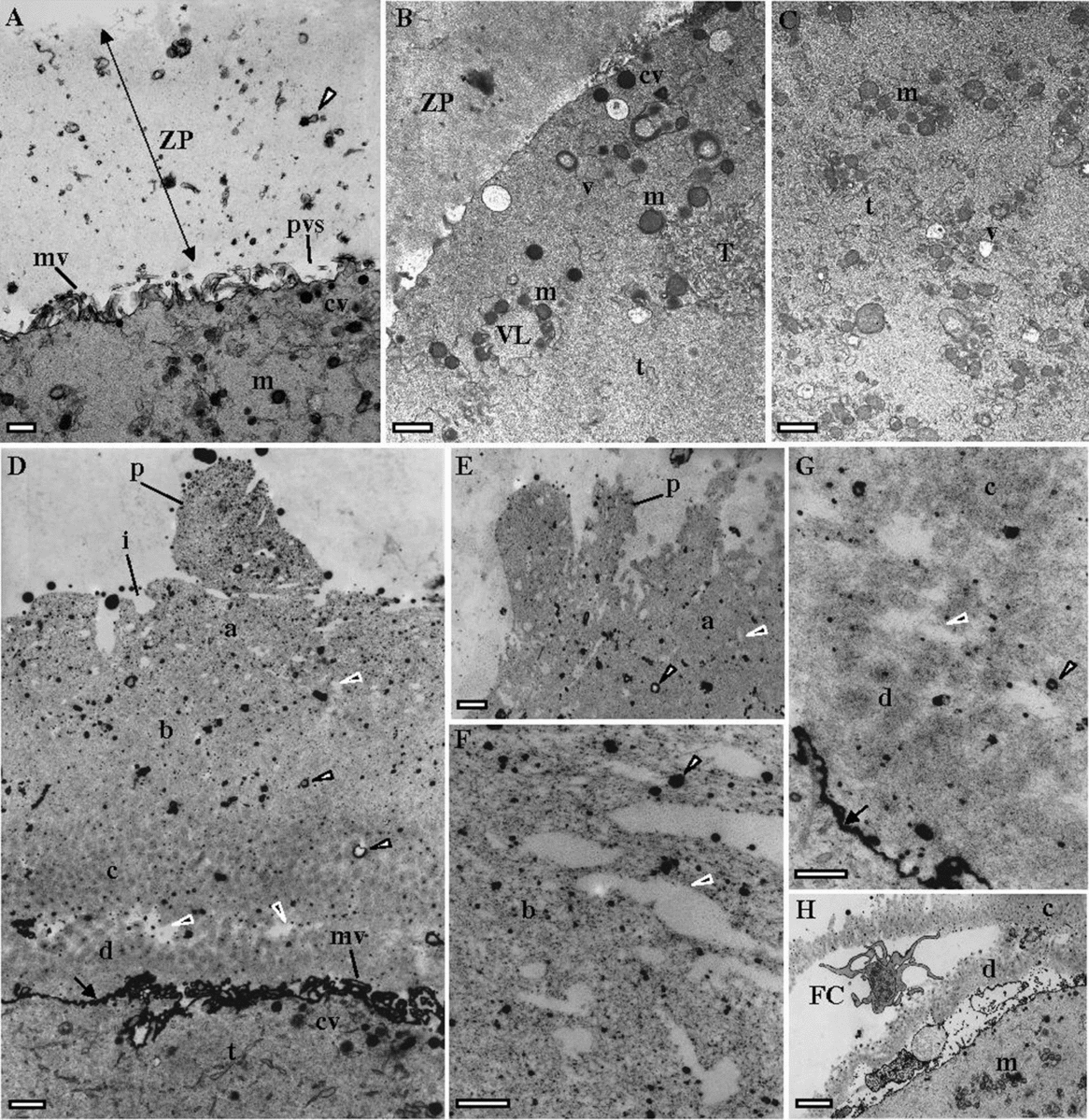
The ultrastructure of dysmorphic oocytes (case 1: 1MI, 1MI matured from 1GV; case 2: 1MI; case 3: 4GV, 1MII matured from 1GV) was compared with a control normal MII oocyte (Fig. 2A–C). The control oocyte was characterized by a homogeneous ZP, with numerous remnants of follicular cell feet (small vesicles) and a perivitelline space filled with small microvilli (Fig. 2A). The oocyte cortex contained cortical vesicles under the oolemma, small and large vesicles of smooth endoplasmic reticulum (SER), surrounded by mitochondria, small SER tubules, and small aggregates of SER tubules surrounded by mitochondria (Fig. 2B). The inner cytoplasm was enriched in mitochondria, SER small vesicles surrounded by mitochondria and small SER tubules (Fig. 2C).

Figure 2 Ultrastructural images taken by transmission electron microscopy. (A-C) a MII oocyte with normal morphology. (A) Oocyte surface with zona pellucida (ZP), remnants of follicular cell feet (white arrowhead), perivitelline space (pvs), microvilli (mv), cortical vesicles (cv) and mitochondria (m). (B) Oocyte cortex with cv, m, smooth endoplasmic reticulum (SER) small tubules (t), SER small (v) and large (VL) vesicles surrounded by m, and small aggregate of SER tubules (T) surrounded by m. (C) Inner cytoplasm with m, t and v. (D–F) a MII oocyte (case 3) with indented ZP and absence or minimal PVS. The ZP presents empty electrolucent regions (black arrowheads) and numerous follicular cell feet (white arrowheads). The surface (a) of the outer ZP layer (b) presents indentations (i) and protuberances (p). (G) The inner ZP layer (c) appears divided by large empty electrolucent regions (black arrowhead) from a inner-most layer (d) that flattens the PVS (arrows). (H) Fractured inner ZP layer with a large remnant of follicular cell cytoplasm (FC). Bars: 1 μm (A–E), 2 μm (F, G), 3 μm (H).
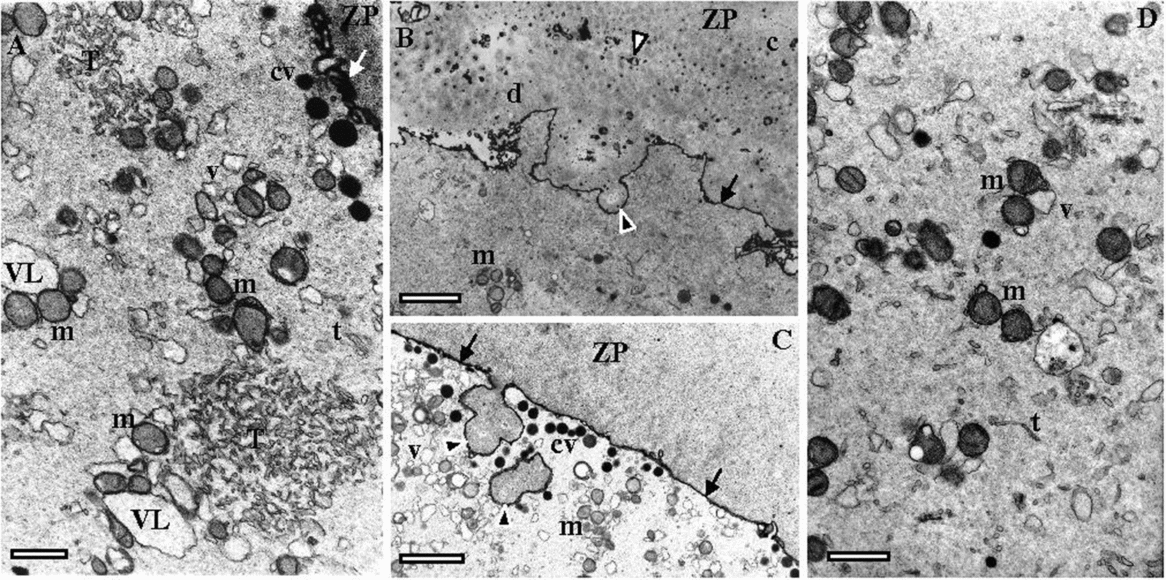
Dysmorphic oocytes presented a slightly larger ZP (about 12.3 μm versus 15.4 μm at the inverted microscope; about 9 μm versus 12 μm at electron microscopy) with an abnormal structure. The ZP was separated into a large outer region and a short inner denser region, with presence of numerous remnants of follicular cell feet (Fig. 2D). The structure of the ZP appeared loose as it was filled with numerous electrolucent spaces, several of them with large dimensions (Fig. 2F). The outer ZP layer presented external indented regions and protrusions (Fig. 2D,E). The inner ZP region was apparently divided into two regions by large electrolucent spaces (Fig. 2D,G). At this region the inner ZP appeared fractured with inclusion of large follicular cell cytoplasm remnants (Fig. 2H). The perivitelline space was absent or decreased in most of the surface, probably due to compression by the presence of a thick ZP inner layer, which also probably compressed microvilli (Fig. 2D,G). The oocyte cortex contained cortical vesicles, small and large SER reticulum vesicles with associated mitochondria, small SER tubules and small aggregates of tubular SER with associated mitochondria (Fig. 3A). At the oocyte surface there were signs of exocytosis of ZP material (Fig. 3B,C). The inner cytoplasm was enriched in mitochondria, SER small vesicles surrounded by mitochondria and small SER tubules (Fig. 3D).

Figure 3 Ultrastructural images taken by transmission electron microscopy. (A–D) MII oocytes (cases 1–3) with indented zona pellucida (ZP) and absence or minimal perivitelline space (arrows). (A) Oocyte cortex with cortical vesicles (cv), mitochondria (m), smooth endoplasmic reticulum (SER) small tubules (t), SER small (v) and large (VL) vesicles surrounded by mitochondria, and small aggregates of SER tubules (T) surrounded by mitochondria. (B, C) Images showing exocytosis (black arrowheads) of ZP material. (D) inner cytoplasm with m, v and t. Follicular cell feet (white arrowhead); ZP inner layer (c); ZP inner-most layer (d). Bars: 1 μm (A), 2 μm (B, C), 1 μm (D).
Discussion
Oocytes with indented ZP showed normal degeneration, fertilization and embryo cleavage rates. The low but not significant clinical pregnancy and live-birth delivery rates may be explained by the small number of patients and cycles, but these are rare cases and only these were observed during 7 consecutive years (0.33%). The main finding of the present report is the association between this ZP dysmorphism (probably due to a defective cross-link between ZP fibrils) and oocyte immaturity (arrest at the MI stage). The capacity to fertilize oocytes by ICSI and embryo development seemed not to be compromised, but clinical outcomes were harmed (probably due to the ovarian factor that enabled a low number of retrieved oocytes with the presence of a very high oocyte immaturity rate).
Studies with knockout mice gave evidence for the function of ZP proteins. ZP3-knockout mice showed that oocytes can develop without ZP but are unable to fertilise (Rankin et al., Reference Rankin, Familari, Lee, Ginsberg, Dwyer, Blanchette-Mackie, Drago, Westphal and Dean1996). Using ZP2-knockout mice, ZP1 and ZP3 were synthesized and originated a thin ZP, being also unable to fertilize. However, when microinjected, they were able to develop up to the blastocyst stage but mice never became pregnant (Rankin et al., Reference Rankin, O'Brien, Lee, Wigglesworth, Eppig and Dean2001). Data from ZP1-knockout mice revealed that oocytes were able to build a loosely organised ZP. These oocytes were able to fertilize and give birth, but the progeny was significantly smaller and had a short lifespan (Rankin et al., Reference Rankin, Talbot, Lee and Dean1999). Taking into account these findings, it is thus possible to suggest that the present observation of a loose structure in oocytes with indented ZP could be due to an abnormal synthesis of ZP1. This situation would affect the normal structure assembly of the ZP fibrils, which could explain the observed low resistance to micropipette penetration of the ZP, as well as the absence of sperm binding in the IVF cycle (case 1) due to an altered correct presentation of ZP sperm receptors. Why oocytes with indented ZP presented exocytosis of ZP material remains unknown.
During oogenesis, several transcription factors act in sequence to govern primordial follicle formation and the expression of the genes encoding ZP proteins (Liang et al., Reference Liang, Soyal and Dean1997; Cui & Kim Reference Cui and Kim2007). Four possible explanations for MI arrest have been suggested: (1) oocytes still did not attain the full competence to mature; (2) absence of normal meiotic recombination; (3) inability to produce the key cell cycle regulating factors; or (4) disruption of microfilament action (Mrazek & Fulka Reference Mrazek and Fulka2003; Brunet & Maro Reference Brunet and Maro2005; Beall et al., Reference Beall, Brenner and Segars2010; Kwon & Lim Reference Kwon and Lim2011). In accordance to these observations, microarrays analysis suggested that gene expression levels can be specific to the degree of oocyte nuclear maturation, such as genes involved in control of DNA repair (some overexpressed in MI oocytes), cell cycle checkpoints (some overexpressed in MI oocytes) and transcription (some not expressed, underexpressed and overexpressed in MI oocytes) (Gasca et al., Reference Gasca, Pellestor, Assou, Loup, Anahory, Dechaud, De Vos and Hamamah2007).
In the present dysmorphism, besides the presence of an abnormal ZP, oocyte immaturity was also evident, mainly due to MI arrest (42% of COC and 80.6% of the immature oocytes). In fact, in relation to controls, we found a significant higher rate of immaturity (58% versus 18.4%), which attained 100% of the oocytes in three cycles (one cycle in case 1 and two cycles in case 5). This high level of immaturity could be associated with any of the above described factors due to the ovarian pathology observed that causes folliculogenesis disruption and an abnormal ovarian hormonal milieu (patients with small ovaries, bilateral ovarian endometriosis and polycystic ovarian syndrome). This situation could also explain why the only term pregnancy obtained was after using an agonist instead of HCG to increase oocyte maturity and reinforced luteal supplementation during the period of implantation with estrogen and low HCG doses.
Under the oolemma, there is a network of fine contractile actin filaments whose structure is dependent on oocyte maturity. This network is needed to keep the oocyte shape and membrane integrity (Dale et al., Reference Dale, Tosti and Iaccarino1995), to build microvilli and assist the cortical contraction (enables the entry of the spermatozoon into the ooplasm). Microfilaments are also involved in chromosome and spindle migration. This network of filaments also supports the normal function of the oolemma. The oolemma contains the sperm receptor and multiple channels that are activated after gamete fusion or artificial activation during ICSI. Oocyte activation induces the exocytosis of the cortical vesicles and activates nutrient absorption. Following gamete fusion, generation of intracellular calcium waves initiates oolemma contraction, activates metabolism, microtubule polymerization, and pronuclear formation and central migration, with most of these events being dependent of F-actin (Sousa et al., Reference Sousa, Barros, Silva and Tesarik1997a,Reference Sousa, Barros, Mendoza and Tesarikb; Dale et al., Reference Dale, Wilding, Coppola and Tosti2010; Hasan et al., Reference Hasan, Matsumoto, Kihira, Yoshida, Sato and Sato2012; Kashir et al., Reference Kashir, Konstantinidis, Jones, Lemmon, Lee, Hamer, Heindryckx, Deane, De Sutter, Fissore, Parrington, Wells and Coward2012). The actin cytoskeleton of the oocyte is also very sensitive to an abnormal ovarian milieu (increased levels of inflammatory cytokines and reactive oxygen species, and unbalanced hormone levels) (Mansour et al., Reference Mansour, Abdelrazik, Sharma, Radwan, Falcone and Agarwal2009), such as that occurring in the present cases with ovarian pathology. In this context, actin-related proteins, such as formins (actin nucleators) have been implicated in premature ovarian syndrome (disruption of DIAPH2) and in polycystic ovarian syndrome (overexpression of FMN2 and DIAPH2) (Kwon & Lim, Reference Kwon and Lim2011). As above described, immaturity at the MI stage may be related with multiple factors that affect not only nuclear maturation but also the cytoskeleton. The observation in oocytes with indented ZP of decreased oolemma resistance and low ooplasm viscosity could thus be explained by disrupted microfilament action.
In conclusion, oocytes with indented ZP appeared associated with low oocyte maturity, high cycle cancellation rates and low live-birth delivery rates. Due to the absence of evidence related to female age and stimulation protocols, and the fact that in five of the six patients there was an ovarian pathology, the presence of this dysmorphism in all oocytes and recurrence in all cycles of three patients clearly suggests a folliculogenesis disturbance due to an ovarian factor. Data also suggest that the use of an agonist instead of HCG with special luteal support might favour a higher cytoplasmic and nuclear maturity in these specific cases.
Acknowledgements
We would like to acknowledge: Jorge Beires, MD, PhD, (Department of Gynecology and Obstetrics, Director of the Unit of Gynecology and Reproductive Medicine, Hospital de S. João, E.P.E, Porto, Portugal) and José Manuel Teixeira da Silva, MD, Gynecologists, for oocyte retrieval; José Correia, MD, Anaesthesist (Department of Anesthesiology, Hospital de S. João, E.P.E, Porto, Portugal); Cláudia Osório, BSc, Biologist, Ana Gonçalves, BSc, Biochemist, and Nuno Barros BSc, Microbiologist, (Centre for Reproductive Genetics Prof. Alberto Barros), for spermatology laboratorial work.
Funding:
R.S. received a PhD grant from Governmental and European Community funds, Foundation for Science and Technology, Ministry of Science, Technology and Superior Education, Portugal (SFRH/BD/23616/2005).
Disclosure statement:
The authors have nothing to declare.