Introduction
Whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) is one of the most important pests of tomato (Solanum lycopersicum L.) due to the direct and indirect damage it causes to this crop worldwide. Integrated pest management combining different tactics, including the use of tolerant or resistant varieties, was proposed for whitefly control (Ellsworth & Martinez-Carrillo, Reference Ellsworth and Martinez-Carrillo2001). Among the genes responsible for plant resistance (R-genes) to pests or pathogens, Mi-1 is a special case as it mediates tomato resistance to both Middle East-Asia Minor 1 (MEAM1) and Mediterranean (MED) species (previously B and Q biotypes) of B. tabaci (Nombela et al., Reference Nombela, Williamson and Muñiz2003), as well as three species of root-knot nematodes Meloidogyne spp. (Roberts & Thomason, Reference Roberts and Thomason1986), the potato aphid Macrosiphum euphorbiae (Thomas) (Hemiptera: Aphididae) (Rossi et al., Reference Rossi, Goggin, Milligan, Kaloshian, Ullman and Williamson1998) and the potato psyllid Bactericerca cockerelli (Casteel et al., Reference Casteel, Walling and Paine2006).
Plants reacting to pests or pathogens usually show more resistance as they advance in their natural development (Panter & Jones, Reference Panter and Jones2002; Ebrahim et al., Reference Ebrahim, Usha, Singh and Mendez-Vilas2011). As an example, Bas et al. (Reference Bas, Mollema and Lindhout1992) demonstrated that resistance to the greenhouse whitefly (Trialeurodes vaporariorum) in wild tomato genotypes (Lycopersicon hirsutum f. glabratum) increases with plant age.
The effect of plant development (considering both plant age and size) on the resistance to pathogens or insects has also been shown on plants bearing R-genes. In some cases, plant development has been correlated to an increase on gene expression as described for the rice gene Xa3 that confers resistance against Xanthomonas oryzae pv. oryzae (Cao et al., Reference Cao, Ding, Cai, Zhao, Lin, Li, Xu and Wang2007), while other R-genes show a similar expression all along plant development (Century et al., Reference Century, Lagman, Adkisson, Morlan, Tobias, Schwartz, Smith, Love, Ronald and Whalen1999; Martínez de Ilarduya & Kaloshian, Reference Martínez de Ilarduya and Kaloshian2001). Among these genes, the Mi-1 gene not only shows a similar expression during the different phases of tomato development but also after the attack of nematodes or aphids (Martínez de Ilarduya & Kaloshian, Reference Martínez de Ilarduya and Kaloshian2001).
Tomato seedlings bearing the Mi-1 gene become resistant to nematodes soon after germination (Dropkin, Reference Dropkin1969). On the contrary, Mi-1-mediated resistance of tomato to aphids is developmentally regulated as it is not expressed until plants reach 6 weeks of age (Kaloshian et al., Reference Kaloshian, Lange and Williamson1995). Mi-1-mediated resistance to B. tabaci was initially demonstrated in 8-week-old tomato plants (Nombela et al., Reference Nombela, Beitia and Muñiz2000, Reference Nombela, Beitia and Muñiz2001, Reference Nombela, Williamson and Muñiz2003). In another study where only younger plants were tested, no differences in relation to the presence/absence of the Mi-1 gene were found (Pascual et al., Reference Pascual, Avilés, Nombela, Muñiz and Beitia2000). However, none of these studies was specifically designed to compare plants of different ages or sizes in order to reliably conclude whether Mi-mediated resistance to B. tabaci is (or not) developmentally regulated.
In the present work, tomato plants of different ages, bearing and lacking the Mi-1 gene, were simultaneously tested in free-choice (antixenosis) and no-choice (antibiosis) assays, to assess the real influence of plant age on the Mi-1-mediated resistance to B. tabaci. In addition, plants of the same age but with different sizes were compared in other assays to verify whether the degree of plant development can also affect the Mi-1-mediated resistance to this insect pest.
Materials and methods
Insect material
Adult males and females from the MED species of B. tabaci (formerly Q-biotype, in accordance with De Barro et al., Reference De Barro, Liu, Boykin and Dinsdale2011) were used in the free-choice assays, meanwhile only adult females were used in the no-choice assays. A population of this whitefly species was reared for several generations in our laboratory on susceptible tomato cv. Marmande.
Plant material and growth
A tomato cultivar carrying the Mi-1 gene (Motelle) and another cultivar lacking this gene (Moneymaker) were used in all assays. These cultivars are near-isogenic lines (Laterrot, Reference Laterrot1987) differing only in the presence of a 650 kb introgressed region from Lycopersicon peruvianum (currently Solanum peruvianum) containing the Mi-1 gene, in chromosome 6 of Motelle (Ho et al., Reference Ho, Weide, Ma, van Wordragen, Lambert, Koornneef, Zabel and Williamson1992). Tomato seeds were germinated and grown in a climatic chamber at a temperature regime of 24:20°C (Light:Dark), L16:D8h photoperiod and 70% r.h. Plants were grown in 1-liter plastic pots filled with autoclaved vermiculite (number 3, Projar, Spain), irrigated every 15 days with a nutritive complex 20-20-20 (Nutrichem 60; Miller Chemical, Hanover, PA, USA) at a concentration of 3 g l–1, and with water when needed in the meantime.
In the experiments to estimate the effects of plant age on the Mi-mediated resistance to B. tabaci, 3-, 5- and 8-week-old tomato plants from each genotype were simultaneously infested and compared. All these plants were grown under the previously detailed climatic conditions.
In the assays to study the influence of plant size, only 8-week-old tomato plants were used. Half of the plants of each genotype were grown as previously described, but the other half were grown from germination at a constant temperature of 19°C to get a smaller size than plants grown at 24°C. At the time of infestation, big plants had 12–13 leaves and they were 70–72 cm high, while small plants had six–seven leaves and they were 20–24 cm high (fig. 1).

Fig. 1. Tomato plants of cultivars Motelle (Mi-1/Mi-1) and Moneymaker (mi-1/mi-1) compared in the free-choice and no-choice assays to study the influence of plant size. Only 8-week-old tomato plants were used. From left to right: small Motelle, big Motelle, small Monemaker and big Moneymaker.
Free-choice assays
The effects of plant age and plant size were evaluated in independent free-choice assays, and each assay was repeated twice. To study the influence of plant age, 20 plants from each genotype and age (120 plants total) were used in each assay. To evaluate the effect of plant size, 18 plants from each genotype and size (72 plants total) were used.
In each assay, plants were transferred from the climatic chamber to an insect-free greenhouse and randomized in a complete block design at a temperature regimen of 25:18°C, L16:D8h photoperiod and 70–88% r.h. Each plant was equidistant from the adjacent pots and the distance among plants was enough to prevent plants from touching each other. Plants were infested with more than 5000 adult whiteflies by shaking an infested plant placed in the centre of the greenhouse. After 6 days, the numbers of adult whiteflies were counted daily in situ on all leaves of every plant until the emergence of new adults (19 and 21 days in the studies of size and age, respectively). Counts were made early in the morning before the adults became active. Three days after the beginning of emergence of new adults, the total numbers of pupae and empty pupal cases on all leaves of every plant were recorded.
No-choice assays
Separated no-choice assays were carried out to evaluate the effects of plant age and plant size, repeating each assay twice. To check the influence of plant age, 15 plants from each genotype and age (90 plants total) were tested in each no-choice assay. To evaluate the effect of plant size, 15 plants from each genotype and size (60 plants total) were used. For each assay, plants were placed in a growth chamber at constant temperature of 24°C, L16:D8h photoperiod, and 65–75% r.h. Five adult females were confined into a plastic clip-cage (Nombela et al., Reference Nombela, Beitia and Muñiz2001) attached to the abaxial surface of one leaflet located in the upper-most fully expanded leaf of each plant. After 6 days, females and clip-cages were removed and eggs laid on each leaflet were recorded. Twenty-one days later, the numbers of fourth-stage (N4) nymphs on each plant were recorded. Moreover, the number of new adults was deduced from the observed number of empty pupal cases.
Statistical analysis
When data from every assay were adjusted to a normal distribution, they were analyzed by a one-way ANOVA and means compared by the Tukey's HSD test. When data were not adjusted to a normal distribution, they were log10 transformed (or arcsen√p for percentages) before analysis. When data, after transformation, were still not adjusted to a normal distribution, means were compared by the Mann–Whitney U test (Statgraphics, 1997). This was the case for the mean number of adults per day in the study of age effect, and the mean number of pupae + new adults per plant in the study of size effect, both during free-choice assays.
The intrinsic growth rate (r m) of the whitefly population on each tomato cultivar and age or size was calculated using the following expression r m = [ln(Nt/No)]/t (Birch, Reference Birch1948), where Nt corresponded to the total number of individuals of the new generation (N4 + new adults) found at the end of the assay, at t days after infestation (t = 19 for size-study and t = 21 in the case of age-study) with a known number (No) of females (Statsoft, 1994). Mean r m values were compared by the Tukey's HSD test.
Results
Influence of plant age
Free-choice assays
The daily mean number of B. tabaci adults per plant (n = 20 plants) counted on the 8-week-old plants was always higher on Moneymaker than on Motelle (fig. 2a). In the same assay, the difference between cultivars on the 5-week-old plants was much less pronounced or non-existent (fig. 2a). Whitefly preference for Moneymaker was not observed on 3-week-old plants (fig. 2a) and even, more insects were occasionally recorded on Motelle than on Moneymaker plants. The mean value (n = 21 days) for the 8-week-old plants was statistically higher (P = 0.000) on Moneymaker than on Motelle (table 1). Difference between cultivars in this parameter for 5-week-old plants was also significant although less marked (P = 0.047). On the contrary, no significant differences (P = 0.926) between Moneymaker and Motelle were found on 3-week-old plants (table 1).
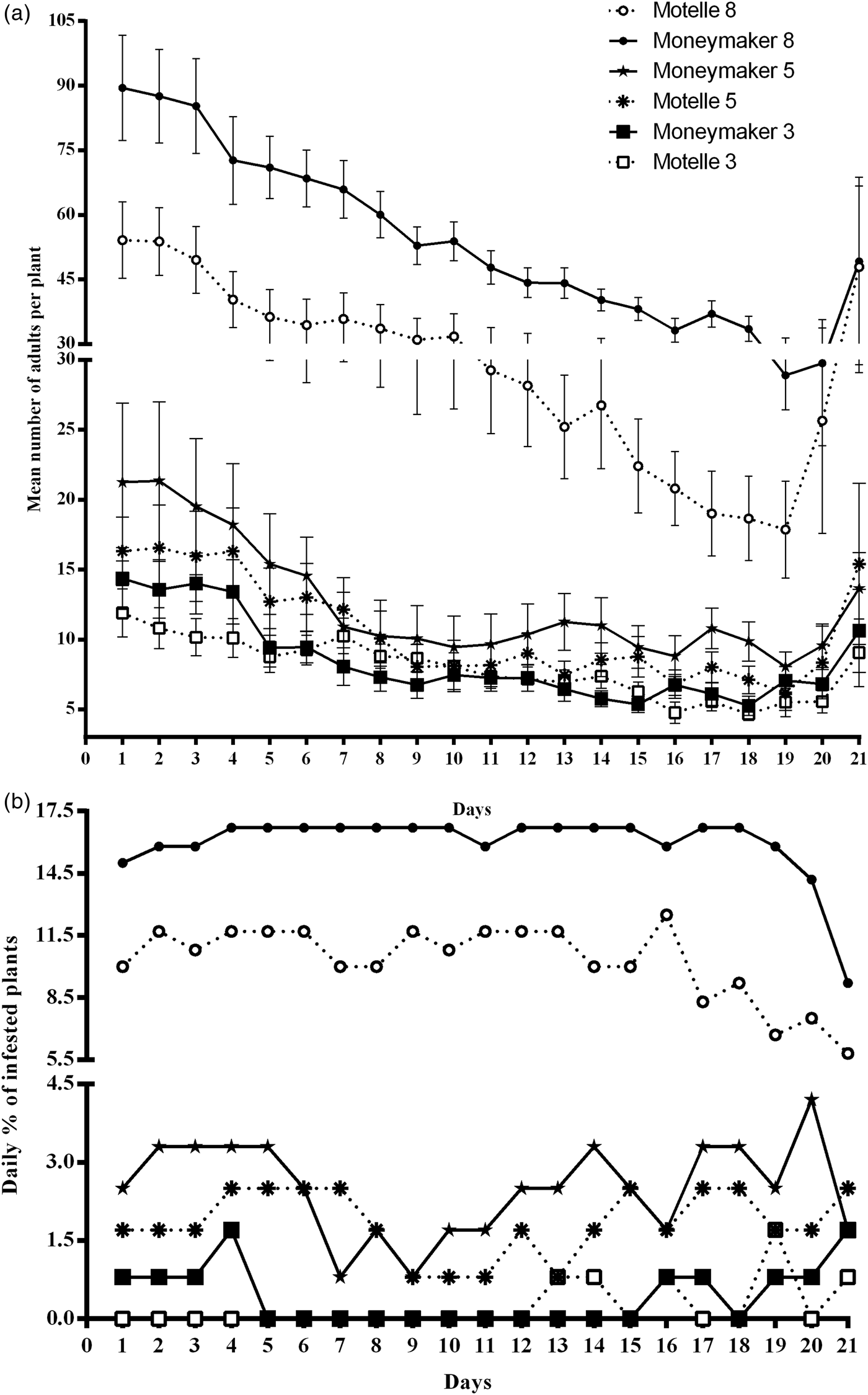
Fig. 2. Daily infestation of tomato cultivars Motelle (Mi-1/Mi-1) and Moneymaker (mi-1/mi-1), during the free-choice assays to test the influence of plant age under greenhouse conditions. The daily counting started at 6 days post-infestation. (a) Infestation rates of adult whiteflies daily recorded on 8-, 5- and 3 week-old plants of Motelle and Moneymaker. Values represent the mean of 20 plants of each age and genotype. (b) Percentage of infested plants calculated every day from the number of plants of each cultivar and age infested with a number of adult whiteflies at least equal or higher than the mean number of adults averaged that day from all 120 tested plants of the free-choice assay.
Table 1. Means±standard errors of the parameters evaluated in the free-choice and no-choice assays of the study to analyze the effect of the plant age on the Mi-1-mediated resistance of tomato to B. tabaci. Plants of Motelle (Mi-1/Mi-1) and Moneymaker (mi-1/ mi-1) cultivars were compared, separately for each plant age (8-, 5- and 3-week old).
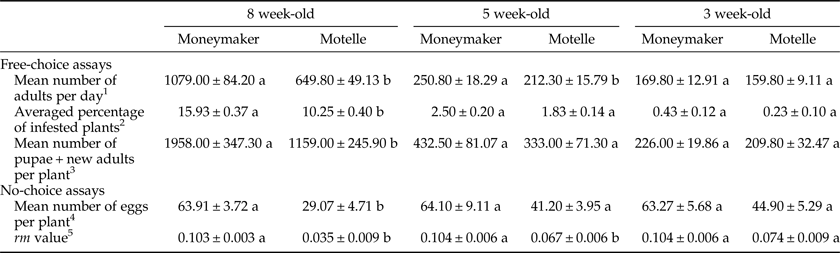
1 Mean number of adult whiteflies recorded daily on 20 tomato plants. Each value represents the mean of 21 days of counting. Different letters for the same plant age indicate significant differences (P < 0.05) between cultivars by the Mann–Whitney test.
2 Averaged percentage of plants infested every day with a number of adults at least equal or higher than the mean number of adults averaged on that day from all 120 tested plants. Each value represents the mean of 21 days of counting. Different letters for the same plant age indicate significant differences (P < 0.05) between cultivars by the Tukey's HSD test.
3 Mean number of new-generation whiteflies ‘[pupae (N4) + new adults (empty pupal cases)] recorded at the end of the assay. Each value represents the mean of 20 plants. Different letters for the same plant age indicate significant differences (P < 0.05) between cultivars by the Tukey's HSD test.
4 Mean number of eggs laid on each plant after five adult female whiteflies were confined to a single leaflet per plant for 6 days. Each value represents the mean of 16 plants. Different letters for the same plant age indicate significant differences (P < 0.05) between cultivars by the Tukey's HSD test.
5 Intrinsic growth rate (r m) of the whitefly population, calculated using the expression r m = [ln(Nt/No)]/t, where Nt corresponded to the total number of individuals of the new generation (N4 + new adults) found at the end of the assay, at t = 21 days after infestation with a known number (No = 5) of females. Each value represents the mean of 16 plants. Different letters for the same plant age indicate significant differences (P < 0.05) between cultivars by the Tukey's HSD test.
As all plants in the free-choice assays were infested, the daily percentages of infested plants were calculated from the number of plants of each cultivar and age infested with a number of adults at least equal or higher than the mean number of adults averaged on that day from all 120 tested plants. Throughout the experiment, the percentages of infested plants of Moneymaker were higher than those of Motelle for 8-week-old plants only (fig. 2b). In contrast, the differences between cultivars were not so clear in the cases of 5- and 3-week-old plants (fig. 2b). When these daily percentages of infested plants were averaged (table 1), the difference between cultivars was highly significant (P = 0.000) for 8-week-old plants only, but not significant for younger plants (P = 0.764 for 3-week-old plants and P = 0.306 for 5-week-old plants).
At the end of the assays, when the new generation of adults started to emerge (21 post-infestation day) and the total number of pupae (N4) and new adults were counted and averaged per plant (n = 20 plants), significant differences (P = 0.015) between Moneymaker and Motelle were found on 8-week-old plants only (table 1), and no significant differences between cultivars were obtained on younger plants (P = 0.940 for 3-week-old plants and P = 0.698 for 5-week-old plants).
No-choice assays
Eggs laid during 6 days on each plant by five confined females were counted and the mean values per plant are shown in table 1. Oviposition values were similar on all Moneymaker plants independently of the plant age. On the contrary, the mean number of eggs on cultivar Motelle decreased as plant age increased. Therefore, statistically significant differences between Moneymaker and Motelle were only found on 8-week-old plants (P = 0.001).
Twenty-one days later, the total number of individuals (N4 + new adults) on each plant was counted and the intrinsic growth rate (r m) of the whitefly population was calculated for each tomato cultivar and age (table 1). The value of this parameter was higher on Moneymaker than on Motelle irrespective of the plant age. However, the difference between cultivars was only significant on 8 and 5 week-old plants (P = 0.000 and P = 0.011, respectively), with the greatest difference recorded on 8-week-old plants.
Influence of plant size
Free-choice assays
The mean numbers per plant (n = 18 plants) of B. tabaci adults were daily calculated and they were always higher on Moneymaker than on Motelle for both big and small plants (fig. 3a), although the difference between both cultivars was more evident in the big plants. From these data, the mean values of daily infestation (n = 19 days) were calculated (table 2) and statistically significant differences between Moneymaker and Motelle were only found on the big plants (P = 0.003).
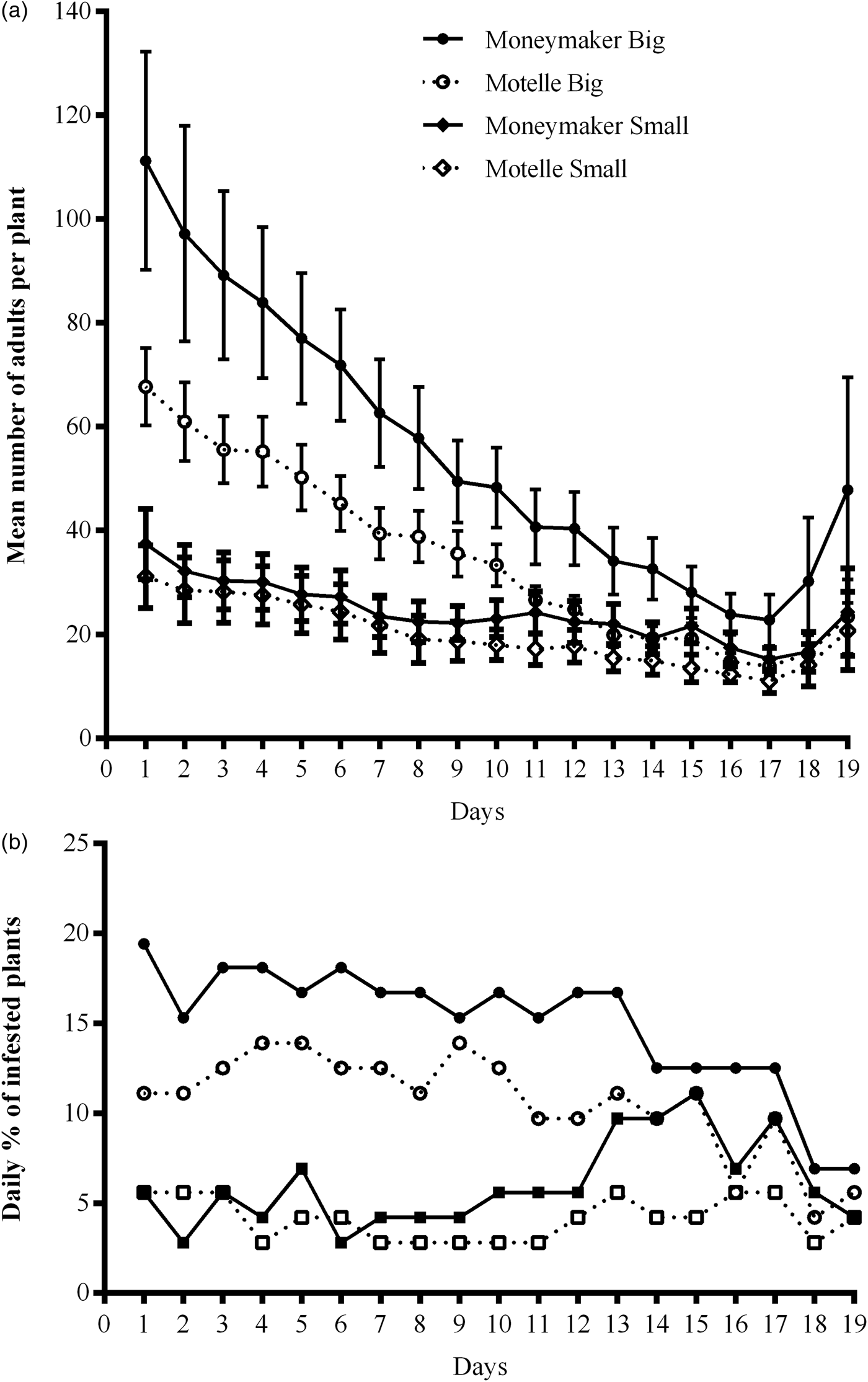
Fig. 3. Daily infestation of tomato cultivars Motelle (Mi-1/Mi-1) and Moneymaker (mi-1/mi-1), during the free-choice assays to test the influence of plant size under greenhouse conditions. The daily counting started at 6 days post-infestation. (a) Infestation rates of adult whiteflies daily recorded on big and small plants of Motelle and Moneymaker. Values represent the mean of 18 plants of each size and genotype. (b) Percentage of infested plants calculated every day from the number of plants of each cultivar and size infested with a number of adult whiteflies at least equal or higher than the mean number of adults averaged that day from all 72 tested plants of the free-choice assay.
Table 2. Means±standard errors of the parameters evaluated in the free-choice and no-choice assays of the study to analyze the effect of the plant size on the Mi-1-mediated resistance of tomato to B. tabaci. Plants of Motelle (Mi-1/Mi-1) and Moneymaker (mi-1/mi-1) cultivars were compared, separately for each plant size (big and small plants).
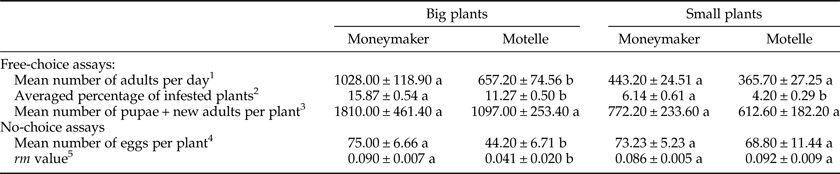
1 Mean number of adult whiteflies recorded daily on 18 tomato plants. Each value represents the mean of 19 days of counting. Different letters for the same plant size indicate significant differences (P < 0.05) between cultivars by the Tukey's HSD test.
2 Averaged percentage of plants infested every day with a number of adults at least equal or higher than the mean number of adults averaged on that day from all 72 tested plants. Each value represents the mean of 19 days of counting. Different letters for the same plant size indicate significant differences (P < 0.05) between cultivars by the Tukey's HSD test.
3 Mean number of new-generation whiteflies ‘[pupae (N4) + new adults (empty pupal cases)]’ recorded at the end of the assay. Each value represents the mean of 18 plants. Different letters for the same plant size indicate significant differences (P < 0.05) between cultivars by the Mann–Whitney test.
4 Mean number of eggs laid on each plant after five adult female whiteflies were confined to a single leaflet per plant for 6 days. Each value represents the mean of 14 plants. Different letters for the same plant size indicate significant differences (P < 0.05) between cultivars by the Tukey's HSD test.
5 Intrinsic growth rate (r m) of the whitefly population, calculated using the expression r m = [ln(Nt/No)]/t, where Nt corresponded to the total number of individuals of the new generation (N4 + new adults) found at the end of the assay, at t = 21 days after infestation with a known number (No = 5) of females. Each value represents the mean of 14 plants. Different letters for the same plant size indicate significant differences (P < 0.05) between cultivars by the Tukey's HSD test.
The percentages of infested plants were calculated every day from the number of plants of each cultivar and size infested with a number of adults at least equal or higher than the mean number of adults averaged that day from all 72 tested plants. The percentages of infested plants of Moneymaker were always higher than those of big Motelle plants (fig. 3b). On small plants, although the percentage of infested plants at the beginning of the assay was similar in both, Moneymaker and Motelle, this value was higher on Moneymaker than on Motelle from the seventh day of counting (fig. 3b). When these daily percentages of infested plants were averaged (table 2), the difference between cultivars was significant for both big (P = 0.000) and small (P = 0.012) plants.
At the end of the assays (19 days post-infestation), the numbers of pupae and new adults per plant (n = 18 plants) were higher on Moneymaker than on Motelle, although with not statistically significant differences, for any plant size (P = 0.304 for big plants and P = 0.557 for small plants) (table 2). Nevertheless, this difference between cultivars was much more evident on the big plants, as pupae counted on Motelle were approximately 60% of those on Moneymaker, while the difference in pupae production on the small plants was only 20%.
No-choice assays
After 6 days, the mean number of eggs per plant was very similar on big and small Moneymaker plants (table 2), but this value on Motelle was significantly reduced on the big plants (P = 0.003), compared with the small ones. However, no significant differences were found between both cultivars on small plants (P = 0.741).
The whitefly intrinsic growth rate (r m) was calculated, as described for the plant-age experiments, for each plant cultivar and size considering the total number of individuals (Nt = N4 + new adults) on each plant. The r m was significantly higher on Moneymaker than on Motelle only for big plants (P = 0.030), but not for the small plants (P = 0.321) (table 2).
Discussion
Effect of plant age on Mi-1-mediated resistance against B. tabaci
These results demonstrated that plant age is a very important factor on Mi-1-mediated resistance against B. tabaci, as variations in the age of tomato plants affect most parameters of whitefly infestation on bearing-Mi-1 tomato plants. So, daily infestation in the free-choice assays of the present work was always greater on Moneymaker than on Motelle for 8-week-old plants, with significant differences in the mean number of daily adults, confirming results from previous studies (Nombela et al., Reference Nombela, Beitia and Muñiz2000, Reference Nombela, Beitia and Muñiz2001, Reference Nombela, Williamson and Muñiz2003). On the contrary, 3-week-old plants did not show differences between both cultivars and the difference among plants with and without the Mi-1 gene was minimal on 5-week-old plants. Moreover, the percentage of plants infested with number of adults equal to or greater than the average obtained daily for each cultivar significantly differed between Moneymaker and Motelle only for 8-week-old plants. Consequently, when adults of the new generation emerged, differences between cultivars were observed in the final infestation levels of 8-week-old plants but not of younger plants.
This plant age-related trend was reinforced by results from the no-choice assays where differences between both cultivars on female oviposition were found only for 8-week-old plants. This is consistent with results from a previous study on young plants, where no differences were found between plants with or without Mi-1 (Pascual et al., Reference Pascual, Avilés, Nombela, Muñiz and Beitia2000). In studies with aphids it was observed that 5- and 7-week-old plants showed differences between Moneymaker and Motelle, although the differences were more evident and constant with 7-week old plants (Kaloshian et al., Reference Kaloshian, Lange and Williamson1995). Differences due to plant age have been also detected on susceptible and resistant tomato varieties during infestation with Tuta absoluta (Leite et al., Reference Leite, Picanço, Guedes and Zanuncio2001). In that case, an increase in the trichomes density with plant age was correlated to a slower larval development on the resistant variety compared with the susceptible one. However, it has been proven that Mi-1-mediated resistance to B. tabaci is independent of the presence of glandular trichomes or their exudates (Nombela et al., Reference Nombela, Beitia and Muñiz2000). This age-related enhanced resistance of plants seems to be a common phenomenon as it has been described for many additional plant–pathogen interactions (reviewed by Ebrahim et al., Reference Ebrahim, Usha, Singh and Mendez-Vilas2011).
One of the parameters used in studies about insect biology is the intrinsic growth rate of a population (r m), which allows to analyze the adaptability of the insect to the host plant, giving an idea about how the insect population changes (González-Zamora & Gallardo, Reference González-Zamora and Gallardo1999; Qiu et al., Reference Qiu, Ren, Mandour and Lin2003; Musa & Ren, Reference Musa and Ren2005; Yang & Chi, Reference Yang and Chi2006; Bonato et al., Reference Bonato, Lurette, Vidal and Fargues2007; Islam & Slunziang, Reference Islam and Slunziang2007; Mansaray & Sundufu, Reference Mansaray and Sundufu2009). Significant differences between Moneymaker and Motelle were found in 8 and 5 week-old plants for the intrinsic growth rate of B. tabaci, although the most dramatic difference was observed on 8-week-old plants. The reason for this was a decrease in the intrinsic growth rate associated with an increase of the plant age on resistant Motelle, but not on susceptible Moneymaker. This indicates that B. tabaci population increased to a lower extent in the next generation on 8-week-old Motelle plants than on 3- and 5-week old plants of the same cultivar.
Effect of plant size on Mi-1-mediated resistance against B. tabaci
We found that, in addition to plant age, plant size is also important for Mi-1-mediated resistance against whiteflies. During the free-choice assays, differences between both cultivars were evident on big plants but not on the small ones. Regarding the final level of infestation, the mean number of pupae and new adults on Moneymaker was almost double than on Motelle, on big plants, while the difference was lower on small plants, although with no significance in either cases. Assuming that pupae survival at this stage is almost 100%, we might suggest a tendency that the difference between both cultivars will be higher for big plants than for small ones. A previous study comparing the survival of two whitefly species (Bemisia tabaci and Trialeurodes vaporariorum) on tomato leaves with different developmental stage indicated that development plays an important role in plant–pests interactions (Zhang & Wan, Reference Zhang and Wan2012). Besides, tomato resistance to aphids depends on leaf development irrespective of its position (Kaloshian et al., Reference Kaloshian, Kinsey, Ullman and Williamson1997). However, the leaf position could be an important factor in the resistance against pathogens (Visker et al., Reference Visker, Keizer, Budding, Van Loon, Colon and Struik2003).
Another study revealed that plant size or growth rate contributed more than other plant traits, such as secondary metabolites, to herbivore resistance (Carmona et al., Reference Carmona, Lajeunesse and Johnson2011). Our results are in agreement with that study as plant size also influenced whitefly reproductive activity during no-choice assays, with significant differences in oviposition between both cultivars only for big plants. Besides, the intrinsic growth rate of B. tabaci on Moneymaker was constant, irrespective of plant size, while this parameter was lower on the big Motelle plants than on the small ones. Only for big plants this parameter was statistically higher in Moneymaker than in Motelle, suggesting that a decrease in B. tabaci population in the next generation would happen only on big plants while it would have a similar increase on both cultivars on smaller plants. A review of Boege & Marquis (Reference Boege and Marquis2005) suggested that the differences occurring during plant growth can influence the allocation of resources to defense against herbivores. This can suggests that other component(s) influenced by plant development can be important for the Mi-1-mediated resistance against whiteflies.
Age-related influence on known R-gene mediated resistances
Taking together all results from both studies, effect of age and size, it can be concluded that Mi-1-mediated resistance against B. tabaci is developmentally regulated, although plant age has more impact than plant size on this resistance. The Mi-1 gene is not the only R gene that confers development-dependant plant resistance. In tomato, the Cf-9B gene, belonging to the gene family Cf-9, confers resistance to Cladosporium fulvum in mature (flowering) plants, while this resistance was not found on seedlings (Panter et al., Reference Panter, Hammond-Kosack, Harrison, Jones and Jones2002). Our results are also in agreement with those from previous studies (Qi & Mew, Reference Qi and Mew1985; Mew, Reference Mew1987; Goel & Gupta, Reference Goel and Gupta1990; Ogawa, Reference Ogawa1993), which demonstrated that rice R genes conferred resistance to Xanthomonas oryzae pv. oryzae (Xoo) only on adult plants. Those studies suggested a correlation between plant resistance and expression level of the corresponding R gene, as described for Xa3 (also known as Xa26), which increases its expression in old rice plants, being correlated with a greater resistance to the pathogen Xoo (Cao et al., Reference Cao, Ding, Cai, Zhao, Lin, Li, Xu and Wang2007). However, this correlation has not always been found: Xa21 resistance gene has the same expression on susceptible and resistant rice plants indicating that this developmentally regulated resistance is not correlated with its expression levels (Century et al., Reference Century, Lagman, Adkisson, Morlan, Tobias, Schwartz, Smith, Love, Ronald and Whalen1999). Mi-1 gene shows a similar expression during the different phases of tomato development, and also after the attack of nematodes or aphids (Martínez de Ilarduya & Kaloshian, Reference Martínez de Ilarduya and Kaloshian2001). During our study of tomato-B. tabaci incompatible interactions (manuscript in preparation), the expression of Mi-1 gene was constant as no differential regulation was detected by microarray analysis. Overexpression of Mi-1.2 gene on transgenic tomato plants did not increase its resistance against aphids nor triggered resistance on smaller plants (Goggin et al., Reference Goggin, Shah, Williamson and Ullman2004).
Regulation of resistance levels by plant development can limit, in some cases, its implementation as a crop protection system. Plants are susceptible to the attack of pathogens and herbivores during a period of plant development (Century et al., Reference Century, Lagman, Adkisson, Morlan, Tobias, Schwartz, Smith, Love, Ronald and Whalen1999), but the mechanisms regulating this phenomenon are still unknown. Two hypotheses that might explain this developmentally regulated resistance on plants bearing the Mi-1 gene has been suggested (Martínez de Ilarduya & Kaloshian, Reference Martínez de Ilarduya and Kaloshian2001): (1) Mi-1.2 is post-transcriptional regulated differently in roots and leaves, or (2) other component, regulated by plant development, is necessary for the resistance against insects but not for nematodes. Since the attempts to generate an antibody specific to MI-1.2 protein were unsuccessful (Goggin et al., Reference Goggin, Shah, Williamson and Ullman2004), no advances in the first hypothesis have been made. Although a Mi polyclonal antibody has been developed (Van Ooijen et al., Reference Van Ooijen, Mayr, Kasiem, Albrecht, Cornelissen and Takken2008), it is not Mi-1.2-specific and does not distinguish among most tomato Mi homologous proteins (all these Mi like proteins are also endogenous). In relation to the second hypothesis, the existence of another component has been demonstrated as the Rme1 gene is necessary for the Mi-1-mediated resistance against nematodes, aphids and whiteflies (Martínez de Ilarduya et al., Reference Martínez de Ilarduya, Moore and Kaloshian2001, Reference Martínez De Ilarduya, Nombela, Hwang, Williamson, Muñiz and Kaloshian2004). Rme1 has not been cloned to date, so it cannot be assured that its function is regulated by plant development similarly to Rcr3 gene in the resistance to C. fulvum mediated by Cf-2 (Dixon et al., Reference Dixon, Golstein, Thomas, van Der Biezen and Jones2000). More studies are necessary to better understand the mechanisms that regulate this Mi-1-mediated resistance to be used as a part of the crop protection system against pests and diseases.
Acknowledgements
This research was funded by a Project (AGL2007-65854/AGR) from the Plan Nacional I + D + I, Spanish Ministry of Education and Science. Clara I. Rodríguez Álvarez was financially supported by a fellowship/contract (AP2006-1035) from the Spanish FPU Program.







