INTRODUCTION
Photoacclimation is an important process for photosynthetic organisms on coral reefs because they experience extremes of irradiance over a narrow depth range in this tropical habitat (Mass et al., Reference Mass, Kline, Roopin, Veal, Cohen, Iluz and Levy2010; Dubinsky & Falkowski, Reference Dubinsky, Falkowski, Dubinsky and Stambler2011). Photosynthetically active radiation (PAR) can reach high and even damaging levels near the sea surface, where rates of photosynthesis level off due to light intensities that either inhibit or saturate photon receptors (Mass et al., Reference Mass, Kline, Roopin, Veal, Cohen, Iluz and Levy2010). Many coral reef cnidarians use photoprotective substances such as mycosporine-like amino acids (MAAs) as sunscreen against the damaging effects of intense ultraviolet radiation (UVR) in shallow tropical waters (Shick et al., Reference Shick, Ferrier-Pages and Allemand2003). On coral reefs, high PAR and UVR act synergistically with elevated water temperatures to cause bleaching in some stony corals (Lesser, Reference Lesser1996; Anderson et al., Reference Anderson, Zepp, Machula, Santavy, Hansen and Mueller2001). Giant sea anemones also bleach more in shallow areas exposed to high irradiance than in deeper reef areas (Hobbs et al., Reference Hobbs, Frisch, Ford, Thums, Saenz-Agudelo, Furby and Berumen2013). Shallow reefs that occur in waters with high concentrations of dark brown leachate from rotting sea grasses, such as those in some reef lagoons, may exhibit reduced bleaching due to shading effects of the dissolved pigments (Iluz et al., Reference Iluz, Vago, Chadwick, Hoffman and Dubinsky2008). At the other extreme, diminishing levels of irradiance with depth set the lower limits of coral reef growth at about 40–70 m depth, depending on water clarity (Fricke & Schuhmacher, Reference Fricke and Schuhmacher1983; Jarrett et al., Reference Jarrett, Hine, Halley, Naar, Locker, Neumann, Twichell, Hu, Donahue, Jaap, Palandro and Ciembronowicz2005; Grigg, Reference Grigg2006). Many reef corals are limited to shallow areas on reefs due to their inability to survive at low irradiance on the deep reef slope (Fricke & Schuhmacher, Reference Fricke and Schuhmacher1983). Cnidarians that survive in low light employ a variety of mechanisms to capture the sparse photons that reach the deep reef slope. Some alter the body shape and skeletal growth patterns of the host animal to become more flattened (Fricke & Schuhmacher, Reference Fricke and Schuhmacher1983). Others alter their microhabitat use with depth, occupying shaded reef areas such as crevices and vertical walls when shallow, but occurring more on open horizontal areas when deep (Chadwick-Furman & Loya, Reference Chadwick-Furman and Loya1992; Kuguru et al., Reference Kuguru, Achituv, Gruber and Tchernov2010). In addition, many stony corals increase the concentration of chlorophyll-a per endosymbiotic microalgal cell (Symbiodinium zooxanthellae; Blank & Trench, Reference Blank and Trench1986) with increasing depth and decreasing irradiance (Falkowski & Dubinsky, Reference Falkowski and Dubinsky1981; Dubinsky et al., Reference Dubinsky, Stambler, Ben-Zion, Mccloskey, Muscatine and Falkowski1990; Stambler et al., Reference Stambler, Levy and Vaki2008; Mass et al., Reference Mass, Kline, Roopin, Veal, Cohen, Iluz and Levy2010). This photoacclimation mechanism results in higher densities of photon receptors in coral tissues, and allows them to maintain a steady rate of photosynthesis regardless of irradiance intensity (Stambler, Reference Stambler and Por2008). Reef cnidarians express these mechanisms most extremely near their shallow and deep range limits, where irradiance reaches extreme levels.
Many reef-building corals have been examined for photoacclimation mechanisms, but little is known about this process in soft-bodied cnidarians on tropical reefs, including the photosynthetic sea anemones (Actiniaria), soft corals and sea fans. A few species that form clonal aggregations of small polyps have been examined; the sea anemone Aiptasia pulchella and the corallimorpharians Rhodactis rhodostoma and Discosoma unguja all vary microalgal cell abundances in their tissues and chlorophyll content per microalgal cell with irradiance levels on reefs (Muller-Parker, Reference Muller-Parker1987; Kuguru et al., Reference Kuguru, Winters, Beer, Santos and Chadwick2007, Reference Kuguru, Achituv, Gruber and Tchernov2010). Some temperate sea anemones also adjust their concentrations of microalgal cells with irradiance level (Muller-Parker & Davy, Reference Muller-Parker and Davy2001). Giant sea anemones are conspicuous organisms on many tropical reefs and play major ecological roles as symbiotic hosts to obligate cleaner shrimps in the Caribbean Sea (Huebner et al., Reference Huebner, Dailey, Titus, Khalaf and Chadwick2012), and to both fish and shrimps in the Indo-Pacific region (Fautin & Allen, Reference Fautin and Allen1997; Chadwick et al., Reference Chadwick, Duris, Horka and Por2008; Huebner & Chadwick, Reference Huebner and Chadwick2012). Information concerning their photoacclimation mechanisms is important for understanding how they are able to occupy diverse microhabitats on reefs.
Irradiance attenuates with water depth, at exponential rates that vary with factors such as inorganic and organic particle concentrations, plankton abundance and other contributors to water turbidity (reviewed in Mass et al., Reference Mass, Kline, Roopin, Veal, Cohen, Iluz and Levy2010). The northern Red Sea receives some of the strongest solar irradiance on earth due to its situation in a desert region that usually lacks cloud cover and thus receives low rainfall, sediment and nutrient inputs (Stambler, Reference Stambler and Por2008). These factors contribute to low light attenuation in coastal waters of the northern Red Sea, where the euphotic zone extends to at least 90 m depth (Stambler, Reference Stambler2005), and three-dimensional coral structure and thus reef development occur to about 60 m depth (Fricke & Schuhmacher, Reference Fricke and Schuhmacher1983). Below this depth, only minute amounts of light penetrate into the twilight zone, defined as 50–150 m deep (Brokovich, Reference Brokovich and Por2008). Depth–irradiance curves exist for some parts of the Red Sea (Fricke & Schuhmacher, Reference Fricke and Schuhmacher1983; Stambler, Reference Stambler and Por2008; Mass et al., Reference Mass, Kline, Roopin, Veal, Cohen, Iluz and Levy2010), but not for Jordanian coral reefs at the northern tip of this enclosed sea.
Individuals of the bulb-tentacle sea anemone Entacmaea quadricolor are the preferred hosts of the Red Sea endemic anemonefish (clownfish) Amphiprion bicinctus (Huebner et al., Reference Huebner, Dailey, Titus, Khalaf and Chadwick2012). These anemones occur throughout the Red Sea region on coral reefs (Chadwick & Arvedlund, Reference Chadwick and Arvedlund2005; Huebner et al., Reference Huebner, Dailey, Titus, Khalaf and Chadwick2012). Photoacclimation mechanisms in E. quadricolor over their wide depth range of at least 0–65 m (Brokovich et al., Reference Brokovich, Einbinder, Shashar, Kiflawi and Kark2008; Bridge et al., Reference Bridge, Scott and Steinberg2012) may be complicated by several factors. Firstly, small clonal individuals of this species may be limited to shallow reef areas, and thus may photoacclimate across a narrower range of irradiance than do large solitary ones in parts of the Indo-Pacific, where both forms are found (Dunn, Reference Dunn1981; Fautin, Reference Fautin1986; Srinivasan et al., Reference Srinivasan, Jones and Caley1999; Scott et al., Reference Scott, Malcolm, Damiano and Richardson2011; Bridge et al., Reference Bridge, Scott and Steinberg2012). However, northern Red Sea individuals of E. quadricolor all belong to the solitary, non-clonal form (Chadwick & Arvedlund, Reference Chadwick and Arvedlund2005; Brokovich et al., Reference Brokovich, Einbinder, Shashar, Kiflawi and Kark2008). Secondly, the tentacles in members of this species may exhibit either a digitiform or bulbous morphology, or sometimes both in one individual (Dunn, Reference Dunn1981; Fautin & Allen, Reference Fautin and Allen1997; Huebner et al., Reference Huebner, Dailey, Titus, Khalaf and Chadwick2012). Tentacle bulbs may form when the anemones are exposed to high irradiance (Delbeek, Reference Delbeek2002), but other factors such as clownfish presence also appear to affect tentacle morphology (Huebner et al., Reference Huebner, Dailey, Titus, Khalaf and Chadwick2012), and this influence varies among geographic regions (Dunn, Reference Dunn1981). Finally, Red Sea individuals of E. quadricolor host microalgal cells belonging to more than one variant of Clade C1 Symbiodinium (Roopin et al., Reference Roopin, Thornhill, Santos and Chadwick2011). Thus, body form, tentacle shape and microalgal type all may interact with photoacclimation processes in this anemone, and these relationships deserve examination in future studies.
Here we describe an initial study on photoacclimation mechanisms by the bulb-tentacle anemone E. quadricolor, in which we present information on variation over a wide depth range (0–43 m) in the abundance, microhabitat use, microalgal cell content and chlorophyll-a concentrations of individuals on coral reefs at Aqaba, Jordan, northern Red Sea.
MATERIALS AND METHODS
Levels of irradiance
This study was conducted from June 2008 to June 2010 on coral reefs adjacent to the Marine Science Station (MSS), Aqaba, Jordan, in the Gulf of Aqaba, northern Red Sea (29.458°N 34.977°E; for site details see Huebner et al., Reference Huebner, Dailey, Titus, Khalaf and Chadwick2012). In 2008, SCUBA divers measured levels of downwelling irradiance (photosynthetically active radiation, PAR) at 1–3 m depth intervals in open water adjacent to the coral reef using an irradiance meter (LI COR LI-185B) encased in an underwater housing. The PAR measurements were taken on 18 June 2008 during the period of maximum daily irradiance (12:00–13:00 hours; reported in absolute units, after Levy et al., Reference Levy, Dubinsky and Achituv2003; Stambler et al., Reference Stambler, Levy and Vaki2008; Mass et al., Reference Mass, Kline, Roopin, Veal, Cohen, Iluz and Levy2010) at 0–42 m depth. The vertical attenuation coefficient (Kd) of PAR was determined from a best-fit curve to these data (after Stambler, Reference Stambler2005). Irradiance also was measured in reef microhabitats occupied by individuals of the bulb-tentacle sea anemone Entacmaea quadricolor that were sampled for microalgal cell and chlorophyll-a concentrations (see below), by placing the light sensor adjacent to the outermost tentacle tips of each anemone in the field (after Muller-Parker, Reference Muller-Parker1987).
Anemone abundance and microhabitat use
In 2008, the abundance and microhabitat use of individuals of E. quadricolor was determined on a coral reef slope that contained a marked study site near the MSS. This site was selected because it was a well-developed coral reef interspersed with sand patches (see maps in Mergner, Reference Mergner and Gomez1981; Maniere & Jaubert, Reference Maniere, Jaubert and Saad1984; Mergner et al., Reference Mergner, Schuhmacher, Kroll and Richmond1992; Khalaf et al., Reference Khalaf, Al-Horani, Al-Rousan and Manasrah2006) and contained abundant sea anemones (Mergner & Schuhmacher, Reference Mergner and Schuhmacher1974; Huebner et al., Reference Huebner, Dailey, Titus, Khalaf and Chadwick2012). All detected individuals of E. quadricolor were counted in three haphazardly-placed belt transects of 2 × 25 m each (50 m2) parallel to shore at each of nine 5 m depth intervals that extended from 0 m to 45 m depth on the reef slope. As part of a separate demographic study on the mid-slope, we quantified microhabitat use by all individuals (N = 72) that occurred at six 16 m depth (after Huebner et al., Reference Huebner, Dailey, Titus, Khalaf and Chadwick2012). For each individual, we recorded the orientation of the oral disc as facing upwards (oriented horizontally) or sideways (oriented vertically), and the type of substrate contacted by the anemone tentacles (rock, sand).
Chlorophyll and microalgal cell concentrations
To assess concentrations of microalgal cells Symbiodinium in the tentacle tips of E. quadricolor, in June 2008, divers haphazardly selected 46 individuals from 3 m to 43 m depth. One tentacle tip (2–3 cm length, after Muller-Parker, Reference Muller-Parker1987; Kuguru et al., Reference Kuguru, Chadwick, Achituv, Zandbank and Tchernov2008; Roopin et al., Reference Roopin, Thornhill, Santos and Chadwick2011) was clipped from each selected anemone and transported to the nearby MSS. Tentacle samples were blotted to remove excess water, and wet mass measured to 1 mg. Microalgal cells were separated from the animal tissue by homogenizing the samples and centrifuging the homogenate to obtain an algal pellet, then the algae were resuspended in filtered seawater, and a haemocytometer (Hausser Scientific) was used to determine microalgal cell abundances at 200× magnification (after Muller-Parker, Reference Muller-Parker1984; Stambler & Dubinsky, Reference Stambler and Dubinsky2005; Kuguru et al., Reference Kuguru, Achituv, Gruber and Tchernov2010). Only three replicates per tentacle sample were examined due to limited time at the field site; previous studies on microalgae in this anemone have shown that this level of replication is sufficient to detect significant variation among treatments (Roopin & Chadwick, Reference Roopin and Chadwick2009; Roopin et al., Reference Roopin, Thornhill, Santos and Chadwick2011).
Chlorophyll concentrations were not assessed from samples collected in 2008 due to equipment limitations onsite. During a subsequent field season in June 2010, divers haphazardly selected 22 anemones over a wide depth range of 0.1–43 m. From each selected anemone, they collected a tentacle sample that was processed for microalgal abundance analysis as described above. Acetone (90%) was added to a subsample of the microalgal suspension, stored overnight at 4°C for chlorophyll extraction, re-centrifuged, and Chl a concentrations determined using a Spectronic Genensys 5 spectrophotometer (Thermo Electron Corporation, Waltham, MA, USA; after Stambler & Dubinsky Reference Stambler and Dubinsky2005; Kuguru et al., Reference Kuguru, Chadwick, Achituv, Zandbank and Tchernov2008; Mass et al., Reference Mass, Kline, Roopin, Veal, Cohen, Iluz and Levy2010), based on the equations of Jeffrey & Humphrey (Reference Jeffrey and Humphrey1975). All data are presented as means plus one standard deviation unless otherwise indicated.
RESULTS
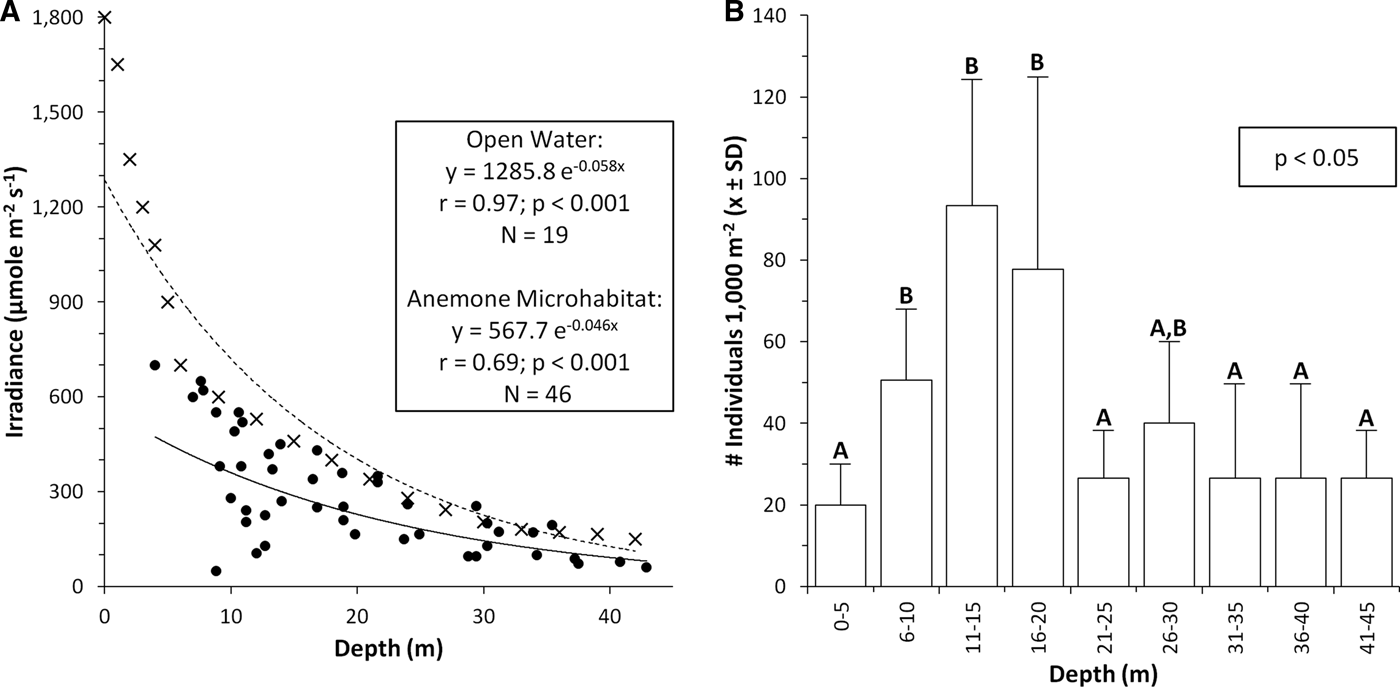
During 2008, the irradiance level in open water near the coral reef was high at the water surface (1800 μm quanta m−2 s−1), and decreased exponentially with depth to only 150 μm quanta m−2 s−1 at 42 m depth, or 8.3% of surface irradiance (Figure 1A). The attenuation coefficient Kd was 0.058 m−1. Levels of PAR in reef microhabitats occupied by individuals of Entacmaea quadricolor varied within each depth and decreased significantly with depth, ranging from 50 to 700 µm quanta m−2 s−1 (Figure 1A). In shallow areas (3–20 m depth), PAR in anemone microhabitats was significantly lower than that in open water (Mann–Whitney U-test, U = 3.90, P < 0.001). Many anemones on the shallow reef occurred in shaded microhabitats such as under overhangs and in crevices. In contrast, on the deep reef slope (21–43 m depth), many anemones occupied unshaded microhabitats where PAR did not differ significantly from that in adjacent open water (U = 101.5, P = 0.07).

Fig. 1. Levels of photosynthetically active irradiance (PAR) and abundances of bulb-tentacle sea anemones Entacmaea quadricolor on a coral reef adjacent to the Marine Science Station in Aqaba, Jordan, northern Red Sea. Variation in: (A) downwelling PAR with depth, in open water near the reef (circles) and in reef microhabitats occupied by sea anemones (x); (B) anemone abundance with depth. Different letters above columns indicate significant differences in abundance among depths. See text for details.
Individuals of Entacmaea quadricolor occurred throughout the examined depth range, and their abundance varied significantly with depth (Kruskal–Wallis test, H = 19.21, P < 0.05). They were significantly more common at mid-depths on the reef slope (6–20 m) than in either shallower or deeper areas (multiple comparisons tests, P < 0.05; peak abundance = 93.3 ±30.9 individuals per 1000 m2, or roughly 1 individual per 10 m2 at 10–15 m depth, Figure 1B), where they received about 100–500 μm quanta m−2 s−1 PAR (Figure 1A). On the mid-slope, their oral discs mostly faced sideways (oriented vertically, 72.2%, N = 72 anemones examined) with their bases deep in reef holes. Most individuals contacted reef rock (65.3%) or occurred at the rock/sand interface (26.4%), with only a few individuals surrounded by sand (8.3%).
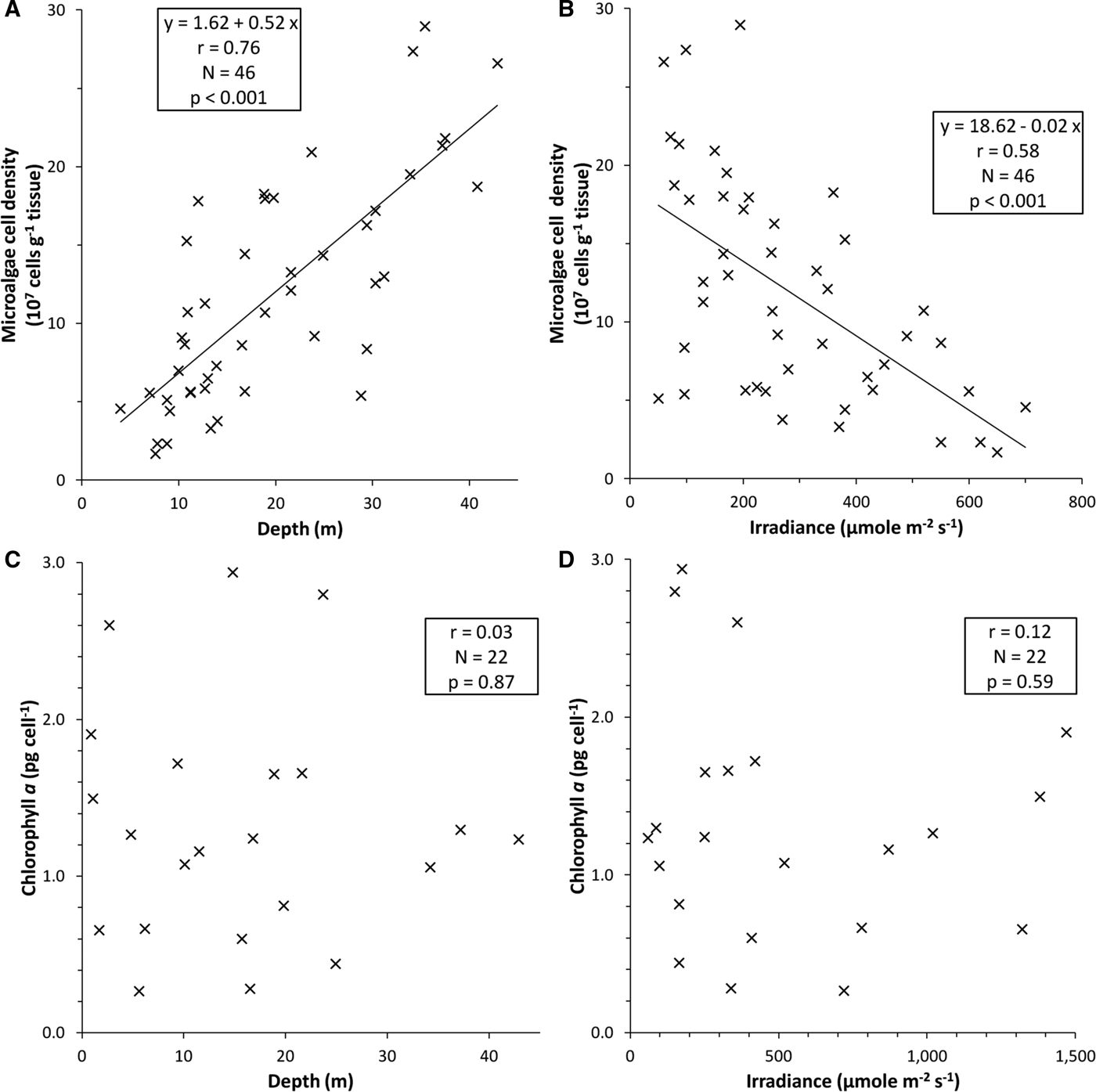
The abundance of microalgal cells within sea anemone tentacles also varied widely among individuals within each depth, but increased significantly with depth (Linear regression, r = 0.76, P < 0.001, Figure 2A). Microalgal concentration was lowest near the water surface (5.07 ±2.59 × 107 cells g−1 tentacle wet mass, N = 10 at 3–10 m depth) and increased about four-fold with depth, reaching 20.31 ±5.57 × 107 cells g−1 (N = 11) at 30–43 m depth (Figure 2A). Microalgal concentration in anemone tentacle tips decreased significantly with the level of PAR in anemone microhabitats (Linear regression, r = 0.58, P < 0.001, Figure 2B), but the relationship was looser than that between microalgal abundance and water depth. Some anemones contained few microalgae even at low irradiance (Figure 2B).

Fig. 2. Densities of endosymbiotic microalgae Symbiodinium and chlorophyll per microalgal cell within the tentacles of bulb-tentacle sea anemones Entacmaea quadricolor on a coral reef adjacent to the Marine Science Station in Aqaba, Jordan, northern Red Sea. Variation in: (A) abundance of microalgal cells in anemone tentacles with depth; (B) abundance of microalgal cells with levels of irradiance; (C) chlorophyll-a concentrations per microalgal cell with depth; (D) chlorophyll-a concentrations per microalgal cell with irradiance.
Chlorophyll-a concentrations per microalgal cell in anemone tentacles (1.31 ±0.76 pg cell−1, range = 0.27–2.94 pg cell−1, N = 22 anemones) likewise varied widely among individuals within each depth, but did not vary significantly with either depth or PAR level (Linear regressions, r = 0.032 and 0.122, P = 0.87 and 0.59, respectively, Figure 2C, D). Similar to the pattern of microalgal cell abundance, some anemones contained little chlorophyll even at low irradiance or in deep areas (Figure 2C, D).
DISCUSSION
We demonstrate that the giant sea anemone Entacmaea quadricolor inhabits a wide depth range on northern Red Sea coral reefs and appears to photoacclimate to exponentially decreasing PAR with depth, using two major mechanisms. Firstly, shallow individuals occupy shaded reef holes, thus exposing themselves to lower levels of irradiance, including damaging UVR, than in open water. Secondly, individuals increase the abundance of endosymbiotic microalgal cells in their tissues about four-fold on the deep vs shallow reef slope, thereby greatly enhancing their photosynthetic efficiency as irradiance decreases with depth. The microalgae within these anemones do not appear to increase their per-cell chlorophyll concentrations with depth, in contrast to most stony corals (Dubinsky et al., Reference Dubinsky, Stambler, Ben-Zion, Mccloskey, Muscatine and Falkowski1990; Stambler et al., Reference Stambler, Levy and Vaki2008; Mass et al., Reference Mass, Kline, Roopin, Veal, Cohen, Iluz and Levy2010) and other reef cnidarians (Muller-Parker, Reference Muller-Parker1987; Kuguru et al., Reference Kuguru, Winters, Beer, Santos and Chadwick2007, Reference Kuguru, Achituv, Gruber and Tchernov2010).
The depth profile of PAR observed here (attenuation coefficient Kd = 0.058 m−1) is almost identical to those reported from other sites in the northern Red Sea, where Kd = 0.054 ±0.006 m−1 (Stambler, Reference Stambler2005), but one recent study revealed even greater light penetration in open water in this region (Kd = 0.004; Stambler, Reference Iluz, Vago, Chadwick, Hoffman and Dubinsky2008). Thus, values from both Israel and Jordan at the northern tip of the Gulf of Aqaba confirm that high water transparency occurs near coral reefs on both sides of this steep, narrow gulf, and that shallow reef organisms here are exposed to some of the highest levels of irradiance on earth (Stambler, Reference Stambler2005, Reference Stambler and Por2008; Mass et al., Reference Mass, Kline, Roopin, Veal, Cohen, Iluz and Levy2010). Slight deviations from a smooth curve in our light profile may have occurred due to limited accuracy of the diving depth gauge (±0.1 m), refraction of light due to surface waves, variation in absorption across the water column, and changes in surface intensity during the sampling period, which extended over 1 h during a SCUBA dive.
The vertical oral disc orientation and occupation of shaded reef microhabitats by shallow individuals of E. quadricolor (which also occurs at Eilat; Roopin et al., Reference Roopin, Thornhill, Santos and Chadwick2011) is similar to both the orientation behaviour and microhabitat use of some mobile fungiid corals (Chadwick-Furman & Loya, Reference Chadwick-Furman and Loya1992) and corallimorpharians (Kuguru et al., Reference Kuguru, Achituv, Gruber and Tchernov2010) on nearby reefs. These patterns indicate that in shallow water, some mobile cnidarians orient their oral discs away from downwelling irradiance and occupy shaded microhabitats, potentially as protective mechanisms against the damaging effects of high irradiance (reviewed in Muller-Parker & Davy, Reference Muller-Parker and Davy2001; Mass et al., Reference Mass, Kline, Roopin, Veal, Cohen, Iluz and Levy2010). In contrast, on the deep slope (>20 m depth), similar levels of PAR in anemone microhabitats and adjacent open water indicate that individuals do not appear to prefer shaded areas when ambient irradiance is low. The oral disc orientations of the anemones were not recorded in deep water, so variation in their orientation behaviour with depth remains unknown.
The high abundance of individuals of E. quadricolor measured here (up to 1 individual per 10 m2 at 10–15 m depth) is more than ten times that on reefs nearby in Israel (~6 individuals per 1000 m2) and more than 100 times that on reefs in Egypt (<1 individual per 1000 m2; Chadwick & Arvedlund, Reference Chadwick and Arvedlund2005). Thus, coral reefs at Aqaba support much greater abundances of this anemone than do all other reef areas examined in the Gulf of Aqaba, possibly due in part to the relatively pristine condition of many Jordanian coral reefs (Mergner, Reference Mergner and Gomez1981; Khalaf & Abdallah, Reference Khalaf and Abdallah2003; Khalaf et al., Reference Khalaf, Al-Horani, Al-Rousan and Manasrah2006). Individuals of the obligate anemonefish Amphiprion bicinctus also occur at high abundances of up to 3 individuals per 100 m−2 on some reefs at Aqaba (Khalaf et al., Reference Khalaf, Al-Horani, Al-Rousan and Manasrah2006). At protected sites in the Philippines, giant anemones including E. quadricolor occur at abundances of 2–6 individuals per 1000 m−2 (Shuman et al., Reference Shuman, Hodgson and Ambrose2005), which are lower than those in Jordan, but similar to abundances of giant anemones on Egyptian coral reefs (Chadwick & Arvedlund, Reference Chadwick and Arvedlund2005). Interestingly, recent studies have documented high abundances of E. quadricolor on mesophotic reefs at 50–65 m depth on the Australian Great Barrier Reef (Bridge et al., Reference Bridge, Scott and Steinberg2012), which are similar to the values reported here, and even higher abundances at 10–15 m depth in southern Australia (Scott et al., Reference Scott, Malcolm, Damiano and Richardson2011).
The depth distributional patterns of E. quadricolor are similar throughout the Gulf of Aqaba, in that individuals occur at all depths on the reef slope, but are most abundant at <15 m depth (Chadwick & Arvedlund, Reference Chadwick and Arvedlund2005). The decrease in anemone abundance at very shallow depths (0–5 m) in Aqaba may relate to the high per cent cover of coral competitors (30–50%) near the reef crest on Jordanian reefs (Mergner & Schuhmacher, Reference Mergner and Schuhmacher1974; Mergner, Reference Mergner and Gomez1981; Mergner et al., Reference Mergner, Schuhmacher, Kroll and Richmond1992) in contrast to lower coral abundances on most shallow reefs in Israel (Wielgus et al., Reference Wielgus, Glassom and Fishelson2003; Ben-Tsvi et al., Reference Ben-Tsvi, Al-Zibdah, Bresler, Jamal and Abelson2011) and Egypt (Hawkins & Roberts, Reference Hawkins and Roberts1994; Hassan et al., Reference Hassan, Kotb, Al-Sofyani and Wilkerson2002). Photoinhibition effects on the anemones at high irradiance in very shallow water also may limit their shallow abundances, and cause them to inhabit shaded habitats in shallow areas. Irradiance patterns can change temporally, both daily and seasonally. The angle of the sun to the reef may influence the shading of anemones within hole and crevice habitats, so that anemones shaded at mid-day may be exposed to direct sunlight earlier or later in the day. By measuring irradiance during the summer at mid-day, we revealed patterns of anemone shading during both a season and time of day with high irradiance, when photoinhibition effects may be most intense. In Australia, giant anemones (mostly E. quadricolor) that host anemonefish are most abundant at mid-depths on the reef slope, although in some areas they also are abundant on the deep slope (Bridge et al., Reference Bridge, Scott and Steinberg2012). These anemones may reach their physiological optimum at moderate levels of irradiance (about 400 600 μm quanta m−2 s−1), because very low or high irradiance may cause sub-optimal photosynthetic performance in this and other giant anemones such as Heteractis crispa, which exhibits the same depth distributional pattern (Chadwick & Arvedlund, Reference Chadwick and Arvedlund2005). The anemonefish Amphiprion bicinctus occupies large individuals of E. quadricolor to 65 m depth in the twilight zone, and is one of the few pomacentrids to extend this deep in the Red Sea (Brokovich, Reference Brokovich and Por2008; Brokovich et al., Reference Brokovich, Einbinder, Shashar, Kiflawi and Kark2008). In Australia, the coral reef pomacentrid Chrysiptera rollandi also occurs deep on the reef slope (to 40–50 m), but in contrast to anemonefish, its depth distribution does not relate to the availability of preferred habitat such as a host sea anemone (Hoey et al., Reference Hoey, McCormick and Hoey2007). Variation with depth in other physical factors such as water motion, and in biological processes such as predation and competition, also may in part cause these depth distributional patterns (for example, competition with corals near the water surface at Aqaba, see above). However, photoacclimation responses to irradiance appear to be major contributors to the depth-distributional pattern of E. quadricolor in the Red Sea.
The increase with depth in microalgal abundance in the tissues of E. quadricolor is similar to the pattern observed in other sea anemones (reviewed in Muller-Parker & Davy, Reference Muller-Parker and Davy2001) and corallimorpharians (Kuguru et al., Reference Kuguru, Winters, Beer, Santos and Chadwick2007, Reference Kuguru, Achituv, Gruber and Tchernov2010), and in a few species of stony corals (Titlyanov et al., Reference Titlyanov, Titlyanova, Yamazato and van Woesik2001; Stambler et al., Reference Stambler, Levy and Vaki2008). In contrast, most stony corals increase chlorophyll concentration per microalgal cell with decreasing irradiance and increasing depth (Falkowski & Dubinsky, Reference Falkowski and Dubinsky1981; Dubinsky et al., Reference Dubinsky, Stambler, Ben-Zion, Mccloskey, Muscatine and Falkowski1990; Mass et al., Reference Mass, Kline, Roopin, Veal, Cohen, Iluz and Levy2010). The four-fold increase in microalgal abundance with depth observed here greatly enhances the photosynthetic efficiency of E. quadricolor, and appears to allow for the maintenance of large body size in deep individuals. Symbiotic sea anemones such as E. quadricolor may depend heavily on photosynthate from their endosymbiotic microalgae, which can provide up to 100% of anemone metabolic energy needs and be important for the maintenance of body size (Muller-Parker & Davy, Reference Muller-Parker and Davy2001). Individuals of this species on nearby reefs at Eilat do not vary their microalgal abundance with depth between 5 and 20 m, but their microalgal levels decrease significantly with irradiance in the microhabitats they occupy (Roopin et al., Reference Roopin, Thornhill, Santos and Chadwick2011). The latter depth range may be too narrow to detect microalgal trends with depth; however, in shallow water the anemones occur in reef holes, thereby reducing their exposure to high irradiance near the water surface, resulting in the trend with irradiance but not depth. Results obtained here for Aqaba also are similar to those from Eilat, in that shallow individuals harbour concentrations of about 5–20 × 107 microalgal cells g−1 tentacle wet mass at both sites (Roopin et al., Reference Roopin, Thornhill, Santos and Chadwick2011; Figure 2A). The microalgal concentrations reported here also are similar to those in the tissues of other sea anemone species (Stambler & Dubinsky, Reference Stambler and Dubinsky1987) and in starved individuals of E. quadricolor under laboratory conditions, but are lower than for anemones exposed to ammonia supplements or anemonefish in the laboratory, where microalgae can reach >30 × 107 cells g−1 (Roopin & Chadwick, Reference Roopin and Chadwick2009). These patterns demonstrate that exposure to both high nutrients and low irradiance can enhance concentrations of microalgae in the tissues of these sea anemones.
ACKNOWLEDGEMENTS
We thank the faculty and staff of the Marine Science Station at Aqaba for their hospitality and logistical support. We also thank the many research students in this NSF programme who assisted with the laboratory and field work. We dedicate this paper to Yousef Jamal Mahatta, marine physiology technician at the MSS who died shortly after this study was completed, and whose love of the Red Sea and knowledge of sea anemones greatly enhanced this project. This is publication no. 113 of the Auburn University Marine Biology Programme.
FINANCIAL SUPPORT
This research was funded by grant number NSF-OISE 0733604 to N.E.C. from the National Science Foundation, USA.