Ultraviolet (UV) light room decontamination devices are increasingly used as an adjunct to standard cleaning and disinfection in healthcare facilities. These devices are effective in killing a wide range of pathogens, including Clostridium difficile spores, and they have been shown to reduce the burden of pathogens on surfaces.Reference Nerandzic, Cadnum, Pultz and Donskey 1 – Reference Nerandzic, Thota and Sankar 5 Several quasi-experimental studies have reported reductions in healthcare-associated infections with the use of UV devices in patient rooms.Reference Marra, Schweizer and Edmond 6 Moreover, in a recent cluster-randomized trial, enhanced terminal room disinfection with UV in a subset of high-risk rooms reduced acquisition of targeted pathogens and was associated with reductions in hospital-wide incidence of C. difficile infection (CDI) and vancomycin-resistant enterococci (VRE).Reference Anderson, Chen and Weber 7 – Reference Rutala, Kanamori and Gergen 9
There is increasing interest in expanding the use of UV devices to areas outside of patient rooms. Ultraviolet devices designed specifically to decontaminate items such as keyboards, touchscreens, and cell phones have been shown to be effective.Reference Mathew, Cadnum, Sankar, Jencson, Kundrapu and Donskey 10 – Reference Alhmidi, Cadnum, Piedrahita, John and Donskey 12 Room decontamination devices have also been used to decrease contamination in operating rooms.Reference Simmons, Dale, Holt, Passey and Stibich 13 , Reference El Haddad, Ghantoji, Stibich, Fleming, Segal, Ware and Chemaly 14 Radiology departments are another area where room decontamination devices could potentially be used. Numerous patients, including many of those colonized or infected with multidrug-resistant pathogens, pass through radiology procedure rooms each day.Reference Jencson, Cadnum, Wilson and Donskey 15 In a recent study involving spatial and temporal mapping of patient movement, passing through a computed tomography scanner in the emergency department after a patient with CDI was associated with increased risk of developing CDI.Reference Murray, Yim, Croci, Rajkomar, Schmajuk, Khanna and Cucina 16 Given that patients typically contact only a central procedure table, it is plausible that providing a short cycle focusing primarily on contacted areas might be beneficial. Because our radiology department was planning to purchase UV devices as an adjunct to standard cleaning, we evaluated the efficacy of multiple UV room decontamination devices in a radiology procedure room.
Methods
Point-prevalence survey of environmental contamination in a radiology department
We conducted a point-prevalence culture survey of frequently touched surfaces in the radiology department at the Cleveland VA Medical Center, a 215-bed acute-care facility. The radiology department contains 20 patient rooms, including 5 computed tomography rooms, 2 angiography suites, 5 digital radiography rooms, 6 ultrasound rooms, and 2 magnetic resonance imaging rooms. Environmental services personnel clean all radiology rooms at the end of each work day and provide additional cleaning as needed, including after procedures for patients on contact precautions. Bleach disinfectant wipes are used after CDI patients complete procedures, and quaternary ammonium disinfectant wipes are used for all other cleaning. Radiology staff have access to disinfectant wipes and are responsible for assisting in keeping their imaging equipment clean.
BBL CultureSwabs (Becton Dickinson, Cockeysville, MD) premoistened with sterile normal saline were used to collect cultures from 5×10 cm areas of 5–6 sites on central procedure tables in 10 radiology department rooms during the work day. The sites included areas commonly touched by patients (eg, head rest, body of table, foot of table) and the table control buttons that are commonly touched by personnel. The cultures were processed for MRSA, VRE, fluoroquinolone-resistant gram-negative bacilli, Clostridium difficile, and Candida spp using previously reported methods.Reference Nerandzic, Cadnum, Pultz and Donskey 1 , Reference Cadnum, Shaikh and Piedrahita 17
Test strains used for comparison of UV devices
We studied 1 strain each of C. difficile, methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant Enterococcus faecium (VRE). The C. difficile strain was American Type Culture Collection (ATCC) strain 43598; the MRSA strain was a clinical isolate of pulse-field gel electrophoresis (PFGE) type USA800; and the VRE strain was a clinical VanB-type VRE strain.
Preparation of C. difficile spores
Spores were prepared as described previously.Reference Nerandzic and Donskey 18 The spores were stored in phosphate-buffered saline containing 0.1% (v/v) Tween 80 at −80°C. Prior to testing, spore preps were confirmed to be at least 99% dormant, bright-phase spores.Reference Nerandzic and Donskey 18
Comparison of irradiance measurements for the devices
Absolute spectral irradiance measurements were taken using an Ocean Optics JAZ spectrometer equipped with a cosine corrector and a UV+VIS grating (200–850 nm). All measured spectra were interpolated to 1 nm spacing over the range of 250–800 nm. The ambient fluorescent lighting in the test space remained on during the test, and the absolute spectral irradiance of the ambient light was also measured and was subtracted from the measured spectra of the disinfection devices. Measurements were taken at a height of 86.4 cm from the floor and 91.4 cm from the light-emitting portion of the devices. The average absolute irradiances in µW/cm2 for UV-C (250–279 nm), UV-B (280–319 nm), UV-A (320–399), and visible light (400–800 nm) were calculated based on readings taken over several seconds. For the nonstandard device with 3 vertical towers, we measured the irradiance for 1 of the towers with the bulbs in a fixed position directed at the detector.
Comparison of efficacy of multiple UV devices in a radiology procedure room
The efficacy evaluation protocol was approved by the institutional review board and the Biosafety Committee of the Cleveland VA Medical Center. We tested devices available at the Cleveland VA Medical Center or other local hospitals and invited manufacturers of other UV room decontamination devices to provide a device for testing. Table 1 shows characteristics of the 8 devices tested. The time required to move the devices from the corner of the room and set up for operation varied from 18 to 26 seconds for the standard vertical towers and from 26 to 59 seconds for the nonstandard devices. The manufacturers did not provide input on study design and were not provided with data from the study. The evaluations were conducted in a single procedure room used for computed tomography. Initial experiments were conducted with a single device to determine optimal placement of the device for reduction in pathogens placed at multiple locations on the patient exam table.
Table 1 Characteristics of the Ultraviolet Light Devices
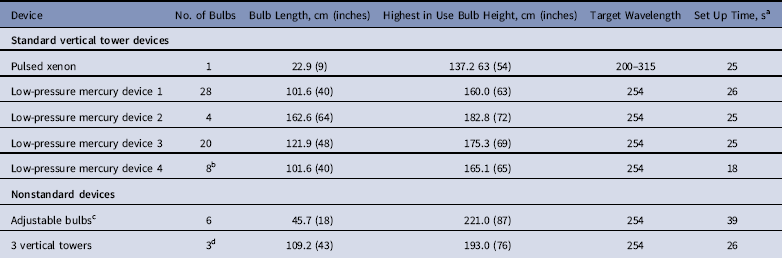
a Time required to move the devices from the corner of the room and set up to begin the ultraviolet light cycle.
b Low-pressure mercury device 4 can be used with 2 towers (16 bulbs total) but was tested as a single vertical tower.
c The device has 3 adjustable bulbs that can be oriented to provide closer proximity to the surface of interest; this device is the same device used for the robotic mobile unit that moves along the side of the table during the cycle.
d Each of the 3 vertical towers has 3 bulbs (ie, 9 total when operated with all 3 towers).
For each pathogen, 10-μL aliquots containing 1×106 colony-forming units (CFU) in sterile water containing 5% tryptone, 0.4% mucin, and 5% bovine serum albumin were spread to cover 20-mm diameter circular stainless-steel carriers and allowed to air dry. The carriers were adhered to petri dish lids. The computed tomography table was set to a height of 40 inches from the floor; an additional test with the table lowered to 20 inches was conducted for a pulsed-xenon device because the bulbs were located lower to the ground than the other devices. Carriers were placed in 5 locations on the table (Fig. 1). The carriers placed at the foot of the table and the mid-point of the table were oriented horizontal to the floor. Given the complex configuration of a headpiece used to hold the patient’s head during computed tomography of the head, 3 carriers were placed at this location including 2 vertically oriented carriers (ie, perpendicular to the floor) inside the left and right wings of the headpiece and 1 horizontally oriented carrier in the center.
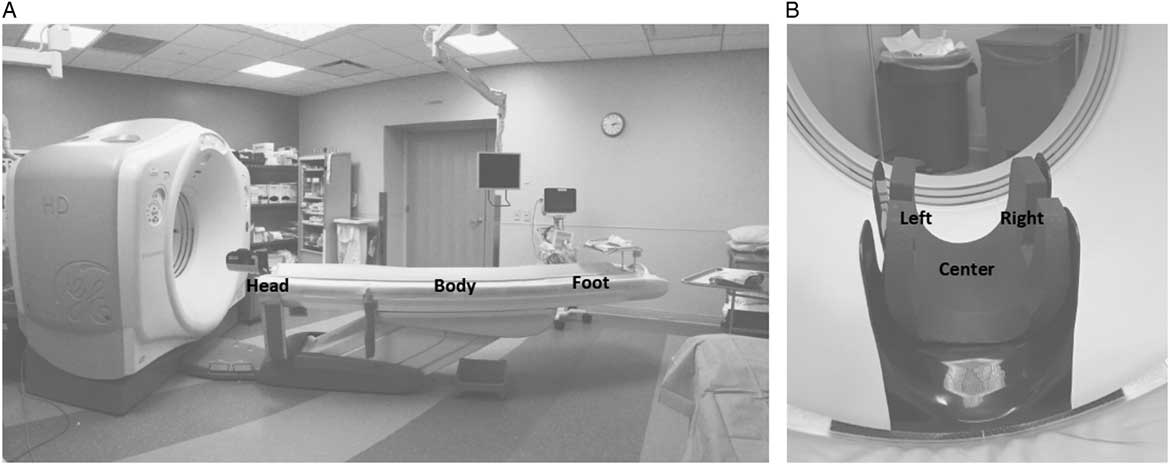
Fig. 1 (A) Radiology computed tomography room used for the comparison of efficacy of the ultraviolet light devices. (B) Removable headpiece used for computed tomography of the head. Steel disk carriers inoculated with 106 colony-forming units (CFU) of the pathogens were placed horizontally on the body (mid-table) and foot of the table and in the center bottom of the headpiece; carriers were placed vertically on the left and right sides of the headpiece. The stationary devices were positioned adjacent to the head of the table; the distances to the carriers placed at the center of the headpiece, body of the table, and foot of the table were 91.5 cm (36 inches), 111.8 cm (44 inches), and 238.8 cm (94 inches), respectively.
For the purposes of the study, the devices were classified as either standard vertical tower devices or nonstandard devices. For the standard vertical tower devices, the device has 1 tower with vertically oriented bulbs and is stationary during operation. Five standard vertical tower devices were tested, including 4 low-pressure mercury devices and 1 pulsed-xenon device. Three nonstandard devices were tested. The first nonstandard device has 3 vertical towers that are intended to run simultaneously to reduce the impact of shadowing when operating in a hospital room. Each tower directs UV light in 1 direction and can be rotated from side to side to provide coverage of surfaces in a patient room or can be in a fixed position to focus on areas of interest. The second nonstandard device has 3 adjustable lamps that can be oriented to provide closer proximity to the surface of interest. The final nonstandard device was a robotic mobile device that moves along the side of the table during the treatment cycle. The robotic device was the same as the device with 3 adjustable lamps but with a robotic base that moved the device in a straight line along the side of the table during the treatment cycle.
The standard vertical tower UV devices were placed near the head of the table (Fig. 1). The device with the adjustable lamps was placed in the same location but the lamps were extended horizontally over the table to provide closer proximity to the table surface and to minimize potential shadowing in the head piece. For the device with 3 vertical towers, 1 tower was placed on each side of the head of the table and 1 was placed at the foot of the table. For the purposes of this study, the device with 3 vertical towers was set such that each tower was in a fixed position facing directly at the table with no rotation. The robotic unit was set to move slowly alongside the table during the 4-minute cycle starting at the foot moving to the head of the table.
Each device was run for a 4-minute treatment cycle; this duration was chosen based on discussions with radiology department staff regarding cycle durations that might have a limited impact on patient flow and because 1 UV device company recommends a 4-minute cycle for radiology departments. After the UV treatment, the carriers were collected and viable organisms were quantified as previously described.Reference Cadnum, Tomas and Sankar 19 All tests were performed in triplicate, and reductions for test carriers were compared with untreated control carriers. For each device, the time to position and set up the device for operation was recorded. For these assessments, the devices were placed in the corner of the room prior to the testing based on the assumption that it would be most efficient to store devices within the treatment room.
Statistical analysis
Analysis of variance was performed to compare the mean log reductions for the standard vertical tower devices and for the nonstandard devices controlling for organism and site. A post-hoc Tukey’s honest significant difference method was used to test pairwise differences between group means. Data were analyzed using SPSS statistical software version 10.0 (SPSS, Chicago, IL).
Results
Of 52 sites cultured in 10 radiology rooms, 7 (14%) were positive for 1 or more pathogens. Staphylococcus aureus and Candida spp were each recovered from 2 (4%) sites. VRE, fluoroquinolone-resistant gram-negative bacilli, and C. difficile were each recovered from 1 site.
Figure 2 shows the irradiance readings for the study devices. The UV-C irradiance readings for the 3 standard vertical tower devices tested were similar (range, 106.2–159.9 µW/cm2), whereas the pulsed-xenon device had much lower UV-C irradiance (10.8 µW/cm2). The pulsed-xenon device generated a spectrum with peak irradiance of 309.4 µW/cm2 in the UV-A range of 320–399 nm; 2 pulsed-xenon devices were tested with nearly identical irradiance results. For the nonstandard device with 3 vertical towers, measured UV-C irradiance with 1 tower directed at the detection device was higher than the output of the standard devices; however, during routine operation, the towers of this device rotate to deliver UV to all areas of the room. The nonstandard device with adjustable bulbs had lower measured UV-C output (26.2 µW/cm2) than the standard devices.

Fig. 2 Comparison of irradiance measurements for (A) the standard and (B) nonstandard ultraviolet light devices. Irradiance measurements were taken at a height of 86.4 cm from the floor and 91.4 cm from the light-emitting portion of the devices. The average absolute irradiances in µW/cm2 for the UV-C (250–279 nm), UV-B (280–319 nm), UV-A (320–399), and visible light (400–800 nm) were calculated based on readings taken over several seconds. For the nonstandard device with 3 vertical towers, the irradiance for 1 of the towers was measured with the bulbs in a fixed position directed at the detector.
Figure 3 shows the mean log10CFU reductions of the pathogens for each of the standard vertical tower devices after 4 minutes of UV-C exposure. All low-pressure mercury devices reduced recovery of each of the pathogens significantly more than the pulsed-xenon device (P<.0001 for all comparisons). The performance of the 4 low-pressure mercury devices was similar with ~2 log10CFU or greater reductions in VRE and MRSA and ~1 log10CFU reduction in C. difficile spores. However, device 2 treatment did result in overall reductions that were significantly greater than devices 3 and 4 (P ≤ .02), but not device 1.

Fig. 3 Efficacy of 5 standard vertical tower ultraviolet light decontamination devices in reducing (A) methicillin-resistant Staphylococcus aureus (MRSA), (B) vancomycin-resistant Enterococcus, and (C) Clostridium difficile spores on 20-mm2 steel disk carriers placed on a radiology procedure table. NOTE. LPM, low-pressure mercury. The devices were operated for a 4-minute cycle and reductions in pathogens were measured in comparison to untreated controls. The means of data from triplicate experiments are presented. Error bars indicate standard deviation.
Figure 4 shows the mean log10CFU reductions of the pathogens for the nonstandard devices after 4 minutes of UV-C exposure. By ANOVA, there were no significant differences among the 3 nonstandard devices. The adjustable device with bulbs oriented vertically is shown for comparison but was not included in the analysis. The overall reductions for the nonstandard devices were equivalent to or greater than the reductions achieved by the standard vertical tower devices. Each of the devices achieved ~3 log10CFU reductions in C. difficile spores at the head of the table positions.

Fig. 4 Efficacy of 3 nonstandard ultraviolet light decontamination devices in reducing (A) methicillin-resistant Staphylococcus aureus (MRSA), (B) vancomycin-resistant Enterococcus, and (C) Clostridium difficile spores on 20-mm2 steel disk carriers placed on a radiology procedure table. The nonstandard devices included a device with 3 adjustable lamps that can be oriented to provide closer proximity to the surface of interest, a robotic device that moves along the side of the table during the treatment cycle, and a device that has 3 vertical towers that run simultaneously to reduce the impact of shadowing. The devices were operated for a 4-minute cycle and reductions in pathogens were measured in comparison to untreated controls. The means of data from triplicate experiments are presented. Error bars indicate standard deviation.
Discussion
In a point-prevalence culture survey, we found that contamination of radiology tables with healthcare-associated pathogens was not uncommon. Exposure to a 4-minute treatment cycle with 5 standard vertical tower UV-C room decontamination devices was effective in reducing pathogens on carriers in multiple sites on a radiology procedure table. However, the 4 low-pressure mercury devices were significantly more effective than the pulsed-xenon vertical tower device. Nonstandard devices with adjustable bulbs, a robotic base that moves beside the table during the cycle, or with 3 towers, were at least as effective as the standard devices. Each of the devices required <1 minute to move into position and complete the set-up needed to begin a UV cycle. These results suggest that many UV devices that are currently available could provide an effective and efficient adjunct to manual cleaning and disinfection in radiology procedure rooms.
Our findings are consistent with 3 recent reports in demonstrating that measurements of irradiance may be useful in understanding decontamination performance of different devices.Reference Boyce, Farrel, Towle, Fekieta and Aniskiewicz 20 – Reference Tande, Pringle, Rutala, Gergen and Weber 22 If a radiometer is available, measurement of irradiance can be completed quickly and easily. Alternatively, commercial test cards can provide a simple and easy-to-use colorimetric assessment of UV output.Reference Boyce, Farrel, Towle, Fekieta and Aniskiewicz 20 , Reference Masse, Hartley, Edmond and Diekema 21 Such measurements can provide comparative data for different devices, assess delivery of UV to different sites in patient rooms, and confirm that devices are operating correctly.
Although manufacturers may suggest that some devices have features that enhance efficacy, the 4 standard low-pressure mercury devices that were tested had similar irradiance readings and were similarly effective in reducing pathogens on carriers. The pulsed-xenon device provided much lower UV-C output with higher UV-A output and was less effective in reducing pathogens on carriers. A previous study also demonstrated that a pulsed-xenon device was less effective in reducing pathogens on carriers than a low-pressure mercury device.Reference Nerandzic, Thota and Sankar 5 However, pulsed-xenon devices have been shown to reduce bacterial contamination on surfaces in patient rooms, and use of the device has been associated with reductions in VRE and C. difficile infections in some quasi-experimental studies.Reference Nerandzic, Thota and Sankar 5 , Reference Marra, Schweizer and Edmond 6
The nonstandard devices that were tested are intended to allow increased proximity to the sites of contamination and/or to improve exposure in shaded areas. One notable finding was that the device with adjustable bulbs was as effective as the standard low-pressure mercury vertical tower devices despite having substantially lower measured irradiance. The ability to extend the adjustable bulbs of the device horizontally over the table increases proximity to the sites where carriers were placed. The same device was also effective when deployed as a robotic device that moves along the side of the table during the treatment cycle. One potential limitation of the adjustable and robotic devices is that they required more time to set up than the standard devices.
The standard low-pressure mercury vertical towers achieved only a ~1 log reduction of C. difficile spores with a 4-minute cycle. One approach to address this deficiency of these devices might be to provide a longer cycle time after procedures are completed on patients with CDI. Previous studies have demonstrated that exposure times of 10 minutes or longer may provide sufficient UV-C dosing to reduce C. difficile spores by 2 logs or more.Reference Nerandzic and Donskey 18 , Reference Boyce, Farrel, Towle, Fekieta and Aniskiewicz 20 – Reference Masse, Hartley, Edmond and Diekema 21
Our study has some limitations. We assessed contamination of the radiology department with a single point-prevalence culture survey. For the evaluation of reduction on carriers, we evaluated only 3 pathogens and conducted testing in only 1 type of procedure room. Results may differ in other types of rooms or in other outpatient settings. We did not evaluate the efficacy of the devices in reducing real-world contamination on radiology tables. However, we included an organic load to simulate organic material that might be present on surfaces and placed the carriers at multiple sites on the table. Finally, we focused on decontamination of the procedure table based on the presumption that this would be the area most likely to become contaminated and contribute to patient-to-patient transmission. However, we cannot exclude the possibility that other sites in the procedure room might also become contaminated and contribute to transmission.
Acknowledgments
We would like to thank the staff of the Radiology Department at the Cleveland VA Medical Center for assistance with testing and the companies that provided temporary use of ultraviolet light devices for the study.
Financial support
Department of Veterans Affairs (Merit Review grant to C.J.D.)
Conflicts of interest
C.J.D. has received research funding from GOJO, Pfizer, Clorox, Avery Dennison, and Boehringer Laboratories. All other authors report no conflicts of interest relevant to this article.







