Introduction
Physical and mental health interact across the lifespan. Psychiatry has moved away from a Cartesian perspective that separates mind from body and toward an integrated biopsychosocial model of ‘empirically based pluralism’ (Kendler, Reference Kendler2012). Many risk factors affect both physical and mental wellness (Cohen, Edmondson, & Kronish, Reference Cohen, Edmondson and Kronish2015; Freitas, Deschênes, Au, Smith, & Schmitz, Reference Freitas, Deschênes, Au, Smith and Schmitz2016; Ho et al., Reference Ho, Feng, Fam, Mahendran, Kua and Ng2014; Keyes, Reference Keyes2004; Richard et al., Reference Richard, Rohrmann, Vandeleur, Schmid, Barth and Eichholzer2017). Although an extensive literature exists on the comorbidity of mental and physical health problems, and having a diagnosed mental disorder is associated with increased risk of a subsequent medical condition (Momen et al., Reference Momen, Plana-Ripoll, Agerbo, Benros, Børglum, Christensen and McGrath2020), the role of psychopathology in the development of physical illness has not yet been as clearly elucidated. Understanding whether psychopathology symptoms confer the risk for the onset of physical illness could have important implications for reducing global disease burden.
Depression has been associated with a range of cardiovascular, metabolic, and related conditions, including acute coronary syndrome and coronary artery disease (CAD) (Carney & Freedland, Reference Carney and Freedland2017; Lichtman et al., Reference Lichtman, Froelicher, Blumenthal, Carney, Doering, Frasure-Smith and Wulsin2014), cardiovascular disease (Cohen et al., Reference Cohen, Edmondson and Kronish2015; Rajan et al., Reference Rajan, McKee, Rangarajan, Bangdiwala, Rosengren, Gupta and Yusuf2020), diabetes (Kan et al., Reference Kan, Pedersen, Christensen, Bornstein, Licinio, MacCabe and Rijsdijk2016; Semenkovich, Brown, Svrakic, & Lustman, Reference Semenkovich, Brown, Svrakic and Lustman2015), erectile dysfunction (Liu et al., Reference Liu, Zhang, Wang, Li, Cheng, Guo and Zhu2018), hypertension (Meng, Chen, Yang, Zheng, & Hui, Reference Meng, Chen, Yang, Zheng and Hui2012), ischemic heart disease (Xian et al., Reference Xian, Scherrer, Franz, McCaffery, Stein, Lyons and Kremen2010), sleep apnea (BaHammam et al., Reference BaHammam, Kendzerska, Gupta, Ramasubramanian, Neubauer, Narasimhan and Moscovitch2016; Harris, Glozier, Ratnavadivel, & Grunstein, Reference Harris, Glozier, Ratnavadivel and Grunstein2009), and stroke (Pan, Sun, Okereke, Rexrode, & Hu, Reference Pan, Sun, Okereke, Rexrode and Hu2011). Depressed mood has been identified as an independent risk factor for cardiovascular problems, including myocardial infarction, CAD, cerebrovascular diseases, and cardiovascular disease (Van der Kooy et al., Reference Van der Kooy, van Hout, Marwijk, Marten, Stehouwer and Beekman2007; van Marwijk, van der Kooy, Stehouwer, Beekman, & van Hout, Reference van Marwijk, van der Kooy, Stehouwer, Beekman and van Hout2015). Additionally, current clinical recommendations acknowledge that depression may confer risk for poor prognosis among patients with acute coronary syndrome (Lichtman et al., Reference Lichtman, Froelicher, Blumenthal, Carney, Doering, Frasure-Smith and Wulsin2014). The interaction of depression symptoms with cardiometabolic abnormalities has been found to increase the risk of type 2 diabetes in patients over age 50 (Freitas et al., Reference Freitas, Deschênes, Au, Smith and Schmitz2016). These findings suggest that depression symptoms may play a role in the onset, course, and/or outcome of cardiovascular and metabolic pathophysiology. A recent large-scale study found an association between depression symptoms and incident cardiovascular disease independent of traditional risk factors for non-communicable diseases (Rajan et al., Reference Rajan, McKee, Rangarajan, Bangdiwala, Rosengren, Gupta and Yusuf2020). However, the relationship between depression symptoms and cardiometabolic health may not be causal; instead, depression symptoms, particularly somatic symptoms such as sleep problems, may covary with cardiometabolic health without playing a causal role (Meijer, Zuidersma, & de Jonge, Reference Meijer, Zuidersma and de Jonge2013). It is important to consider the possibility that non-causal mechanisms may underlie associations between depression and cardiometabolic conditions.
Previous research has suggested that somatic (e.g. fatigue, insomnia, and appetite disturbance) and non-somatic depression symptoms may be differentially associated with cardiometabolic outcomes. A 2014 meta-analysis reported that, among patients with heart disease, somatic/affective symptoms, but not cognitive/affective symptoms, were associated with adverse cardiovascular outcomes (de Miranda Azevedo, Roest, Hoen, & De Jonge, Reference de Miranda Azevedo, Roest, Hoen and De Jonge2014). However, both somatic and cognitive dimensions of depression symptoms were independently associated with risk for new cardiac events in a recent study of heart disease patients (Norton et al., Reference Norton, Pastore, Ancelin, Hotopf, Tylee, Mann and Palacios2020). Somatic depression symptoms, both before and after the incidence of cardiovascular disease, have been associated with increased mortality (Freak-Poli, Ikram, Franco, Hofman, & Tiemeier, Reference Freak-Poli, Ikram, Franco, Hofman and Tiemeier2018). Somatic and non-somatic depression symptoms may therefore play differential roles in relation to cardiometabolic health.
Little research has examined genetic influences on the relationship between depression symptoms and cardiometabolic health. However, there is significant genetic overlap between depression and type 2 diabetes (Kan et al., Reference Kan, Pedersen, Christensen, Bornstein, Licinio, MacCabe and Rijsdijk2016), and recent genetic evidence from Mendelian randomization suggests that major depressive disorder (MDD) causally influences CAD but not vice versa (Coleman et al., Reference Coleman, Gaspar, Bryois, Byrne, Forstner, Holmans and Breen2020). It is likely that given pleiotropy (Gale et al., Reference Gale, Hagenaars, Davies, Hill, Liewald, Cullen and Harris2016) and correlated genetic risks (Palmer, Reference Palmern.d.) that may predispose one to psychopathology, cardiovascular and metabolic health problems, and unhealthy lifestyle factors, there are non-causal mechanisms affecting associations between mental and cardiometabolic health across multiple physical and mental health phenotypes.
Studying mental health as a contributor to cardiometabolic conditions could enable a better understanding of biological and psychosocial mechanisms that impact cardiovascular and metabolic health. Most existing longitudinal studies have examined associations between depression symptoms and physical illness over comparatively short follow-up periods (e.g., a 2012 meta-analysis of literature on depression and hypertension found a mean follow-up period of 9.6 years) (Meng et al., Reference Meng, Chen, Yang, Zheng and Hui2012; Semenkovich et al., Reference Semenkovich, Brown, Svrakic and Lustman2015). Additionally, studying the longitudinal relationships between depression and cardiometabolic health enables the identification of targetable risk factors for chronic physical conditions that account for a large proportion of the global disease burden.
In the current analyses, we examined longitudinal associations between total and non-somatic depression symptoms at midlife and the subsequent onset of cardiometabolic and related health outcomes (atrial fibrillation, diabetes, erectile dysfunction, hypercholesterolemia, hypertension, myocardial infarction, sleep apnea, and stroke) in later life, excluding individuals with disease onset before baseline, to establish whether depression may increase the risk of developing cardiometabolic problems. These analyses are novel given the extended period (approximately 27 years) between baseline and follow-up, as well as the inclusion of polygenic risk scores (PRSs) for each of the eight cardiometabolic conditions as covariates to further isolate the effect of depression on long-term health outcomes. Including PRSs as covariates enabled these analyses to partially control for the influence of polygenic risk on the development of each health condition, enabling us to clarify how the experience of depression symptoms may be associated with these health outcomes over and above the influence of common genetic variation. Clarifying the physiological risk that may be conferred by depression symptoms provides insight into the long-term course of health and bolsters the rationale for screening and interventions to address depression earlier in the life course.
Methods
Description of the sample
This study includes data from the longitudinal Vietnam Era Twin Study of Aging (VETSA). VETSA is a longitudinal study of cognitive and brain aging in men, comprising a subset of twins from the Vietnam Era Twin Registry (VETR) (Kremen et al., Reference Kremen, Thompson-Brenner, Leung, Grant, Franz, Eisen and Lyons2006, Reference Kremen, Franz and Lyons2013). All twin pairs in the VETR served in the US military sometime between 1965 and 1975, although the majority of VETSA participants did not serve in combat or in Vietnam (Tsuang, Bar, Harley, & Lyons, Reference Tsuang, Bar, Harley and Lyons2001). VETSA participants are comparable to the US male population with respect to demographic and health characteristics (Kremen, Franz, & Lyons, Reference Kremen, Franz and Lyons2013). All VETSA participants were randomly selected from the Harvard Twin Study of Substance Abuse (HTS/'baseline') (Tsuang et al., Reference Tsuang, Lyons, Eisen, Goldberg, True, Lin and Eaves1996, Reference Tsuang, Bar, Harley and Lyons2001). Importantly, the HTS did not select on the basis of any diagnostic or other characteristic. The only inclusion criteria for VETSA participants were being between 51 and 59 at the time of initial recruitment and both twins in a pair agreeing to participate at baseline, although individuals were allowed to participate without their co-twin in subsequent waves of the study. For the present study, the sample was restricted to the 787 individual participants with genetically determined white, non-Hispanic European ancestry who participated both during the HTS and the most recent (third) wave of VETSA and who were healthy enough to participate in data collection (see Fig. 1). The sample was limited to subjects with genetically determined European ancestry to enable the inclusion of PRSs as covariates; sample sizes of GWAS studies of non-European cohorts are unfortunately limited for most traits, and PRSs are generally poorer predictors of risk when calculated from a GWAS of one ancestral group and applied to another (Martin et al., Reference Martin, Kanai, Kamatani, Okada, Neale and Daly2019).

Fig. 1. Flowchart of study population and sample size.
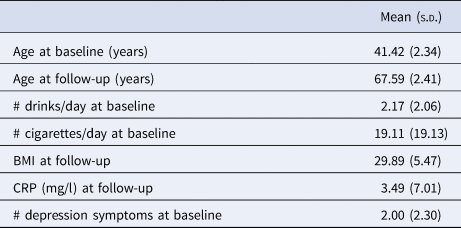
The mean age of participants during the HTS (‘baseline’) was 41.42 (s.d. = 2.34) years, and the mean age at participation in the third wave of VETSA (‘follow-up’) was 67.59 (s.d. = 2.41) years. Table 1 shows the socio-demographic and clinical characteristics of the sample.
Table 1. Sample characteristics (N = 787)

BMI, body mass index; CRP, C-reactive protein.
Measures of mental and physical health
At baseline, participants completed the National Institute of Mental Health Diagnostic Interview Schedule, Version III, Revised (DIS-III-R), a structured psychiatric interview for use in epidemiologic research (Robins, Helzer, Goldring, & Cottler, Reference Robins, Helzer, Goldring and Cottler1989). The DIS-III-R assesses lifetime psychopathology symptoms using criteria from the DSM-III-R (American Psychiatric Association, 1987). The DIS-III-R assesses nine depression symptoms: depressed mood, anhedonia, appetite or weight changes, sleep disturbance, psychomotor disturbance, fatigue, feelings of guilt or worthlessness, difficulty concentrating or making decisions, and thoughts of death, suicidal ideation, or suicide attempt (Robins et al., Reference Robins, Helzer, Goldring and Cottler1989). We used only DIS-III-R symptom counts, not diagnoses, in these analyses. In sensitivity analyses, we excluded symptoms assessing appetite, weight, and sleep disturbance.
At follow-up, participants reported whether a doctor had ever told them that they had any of 65 specific mental or physical health problems. For these analyses, eight cardiovascular and metabolic disorders – atrial fibrillation, diabetes, erectile dysfunction, hypercholesterolemia, hypertension, myocardial infarction, sleep apnea, and stroke – were selected based on their prevalence in the sample as well as on putative relationships with depression based on existing literature (BaHammam et al., Reference BaHammam, Kendzerska, Gupta, Ramasubramanian, Neubauer, Narasimhan and Moscovitch2016; Carney & Freedland, Reference Carney and Freedland2017; Harris et al., Reference Harris, Glozier, Ratnavadivel and Grunstein2009; Kan et al., Reference Kan, Pedersen, Christensen, Bornstein, Licinio, MacCabe and Rijsdijk2016; Liu et al., Reference Liu, Zhang, Wang, Li, Cheng, Guo and Zhu2018; Meng et al., Reference Meng, Chen, Yang, Zheng and Hui2012; Pan et al., Reference Pan, Sun, Okereke, Rexrode and Hu2011; Semenkovich et al., Reference Semenkovich, Brown, Svrakic and Lustman2015). Other cardiometabolic conditions (such as angina and heart murmur) were assessed in VETSA, but were not included in these analyses due to their low incidence in the sample, which could result in the effects of depression on these conditions being undetected due to low power, and/or the unavailability of PRSs for these conditions. Self-reported year of diagnosis was used to determine the onset of each health problem. Cases with the onset of a condition prior to participation in the baseline assessment were excluded from the analyses of that condition. Table 2 shows the incidence of physical health outcomes between baseline and follow-up.
Table 2. Incidence of cardiometabolic conditions

a Ns vary due to condition-specific exclusion of early-onset cases or missing data.
b Cases with onset prior to baseline were excluded from analyses.
Covariates
Covariates in all analyses included age at follow-up, alcohol consumption, smoking, body mass index (BMI), C-reactive protein (CRP), and polygenic risk for specific health outcomes to account for known risk factors from the literature on depression and physical health (Capuron et al., Reference Capuron, Su, Miller, Bremner, Goldberg, Vogt and Vaccarino2008; Freitas et al., Reference Freitas, Deschênes, Au, Smith and Schmitz2016; Janszky, Ahlbom, Hallqvist, & Ahnve, Reference Janszky, Ahlbom, Hallqvist and Ahnve2007; Pan et al., Reference Pan, Sun, Okereke, Rexrode and Hu2011). Smoking behavior was measured at baseline as the number of cigarettes participants reported smoking daily during the period of their life when they smoked most, and alcohol consumption was measured at baseline as the average number of drinks per day participants reported consuming over the past year. Analyses used baseline substance use covariates to examine how substance use at baseline was related to the subsequent onset of cardiovascular conditions; BMI and CRP were not available at baseline. BMI was computed using participants' measured height and weight at follow-up, and was consistent with nationally representative data for men over age 60 (Fryar, Kruszon-Moran, Gu, & Ogden, Reference Fryar, Kruszon-Moran, Gu and Ogden2018). High-sensitivity CRP (mg/L) was measured from fasting blood samples at follow-up and used as a marker of inflammation.
Genotyping methods and single nucleotide polymorphism imputation
Each set of health outcome analyses controlled for polygenic risk for the cardiometabolic outcome under study. These scores were included in order to control for underlying biological risk for physical health conditions and partly isolate the potential effects of depression symptoms on physical health outcomes. PRSs were calculated using summary statistics from genome-wide association studies (GWAS) for: atrial fibrillation (Cardiovascular Disease Knowledge Portal, n.d.; Roselli et al., Reference Roselli, Chaffin, Weng, Aeschbacher, Ahlberg, Albert and Ellinor2018); type 2 diabetes (Morris et al., Reference Morris, Voight, Teslovich, Ferreira, Segrè, Steinthorsdottir and McCarthy2012); erectile dysfunction (Bovijn et al., Reference Bovijn, Jackson, Censin, Chen, Laisk, Laber and Holmes2019); total cholesterol (Willer et al., Reference Willer, Schmidt, Sengupta, Peloso, Gustafsson, Kanoni and Abecasis2013); systolic blood pressure (Evangelou et al., Reference Evangelou, Warren, Mosen-Ansorena, Mifsud, Pazoki, Gao and Caulfield2018); CAD (a phenotype including myocardial infarction; PRSs computed from this GWAS were used in myocardial infarction analyses) (Nelson et al., Reference Nelson, Goel, Butterworth, Kanoni, Webb, Marouli and Deloukas2017); sleep apnea (UK Biobank, n.d.); and stroke (Malik et al., Reference Malik, Chauhan, Traylor, Sargurupremraj, Okada, Mishra and Yamaji2018). Individual single nucleotide polymorphism (SNP) effect estimates and p values were extracted from summary statistics. PRSs were computed by PLINK v1.9 using nine different p value thresholds: p < 0.00000001, 0.00001, 0.01, 0.05, 0.10, 0.20, 0.30, 0.40, and 0.50. The PRS used in each of the health condition analyses was chosen based on how highly it correlated with the condition (see online Supplementary eTable S1). Analyses additionally controlled for the first three principal components calculated from genome-wide genotype data of the European-descent subsample in order to account for any cryptic population substructure.
A detailed description of VETSA genotyping procedures is available in Logue et al. (Reference Logue, Panizzon, Elman, Gillespie, Hatton, Gustavson and Kremen2019). Whole-genome genetic variation was assessed at deCODE Genetics (Reykjavík, Iceland) using Illumina HumanOmniExpress-24 v1.0A BeadChips (Illumina, San Diego, CA, USA). Before PRS calculations, we cleaned and conducted quality control of genotype data using PLINK v1.9 (Chang et al., Reference Chang, Chow, Tellier, Vattikuti, Purcell and Lee2015). Single nucleotide polymorphism weights (SNPweights) and principal components computed using PLINK v1.9 in conjunction with 1000 Genomes Phase 3 reference data were used to identify a European ancestry subset of the data (1000 Genomes Project Consortium, 2015; Chen et al., Reference Chen, Pollack, Hunter, Hirschhorn, Kraft and Price2013). Principal components were computed based on a linkage-disequilibrium pruned set of 100 000 common (minor allele frequency >0.05) genotyped SNPs. Within the subset of participants with genetically determined European ancestry, principal components were recomputed for use as covariates for population substructure in the analyses. Imputation was performed using MiniMac (Fuchsberger, Abecasis, & Hinds, Reference Fuchsberger, Abecasis and Hinds2015; Howie, Fuchsberger, Stephens, Marchini, & Abecasis, Reference Howie, Fuchsberger, Stephens, Marchini and Abecasis2012) computed at the Michigan Imputation Server (https://imputationserver.sph.umich.edu). The 1000 Genomes Phase 3 EUR data were used as a haplotype reference panel.
Statistical analyses
Analyses were performed in SPSS Statistics Version 26 (2018). Generalized estimating equations were used to account for the non-independence of observations within twin pairs. Separate multivariable models were run using each of the eight health conditions. Analyses were performed using the logit link function to obtain log odds ratios. All models assumed an exchangeable correlation structure, and robust variance estimators were used. All covariates were standardized using z-scores. Analyses were run on complete cases; for a comparison between participants with complete and incomplete data, see online Supplementary eTable S2.
Analyses examined associations between total depression symptoms at baseline and the subsequent onset of health conditions by follow-up, controlling for age, alcohol consumption and smoking at baseline, BMI and CRP at follow-up, and PRS for the health outcome under study. The significance threshold was p < 0.05 after correcting for multiple comparisons across eight sets of analyses, using the false discovery rate proposed by Benjamini and Hochberg (Reference Benjamini and Hochberg1995; Radua & Albajes-Eizagirre, Reference Radua and Albajes-Eizagirren.d.).
Sensitivity analyses were conducted using a measure of depression symptoms that removed somatic items (those assessing weight, appetite, and sleep disturbances) to determine whether the results were sensitive to the exclusion of somatic depression symptoms.
Supplementary analyses were conducted to examine crude associations between total depression symptoms reported at baseline and incident health outcomes 27 years later (see online Supplementary eTable S3). Additional supplementary analyses were conducted without the inclusion of PRSs as covariates, which allowed the inclusion of an additional 408 participants (total N = 1195) for whom genetic data were not available and/or who were not of genetically-determined white, non-Hispanic European ancestry (see online Supplementary eTables S4–5 and Fig. 1).
Results
Table 3 displays standardized results from longitudinal analyses that examined associations between total depression symptoms reported at baseline and incident health outcomes 27 years later, controlling for age, alcohol consumption, smoking, BMI, CRP, and polygenic risk. After correcting for multiple comparisons, total depression symptoms at baseline were significantly longitudinally associated at follow-up with incident diabetes (OR 1.29, CI 1.07–1.57), erectile dysfunction (OR 1.32, CI 1.10–1.59), hypercholesterolemia (OR 1.26, CI 1.04–1.53), and sleep apnea (OR 1.40, CI 1.13–1.74). BMI was significantly associated with atrial fibrillation (OR 1.38, CI 1.03–1.85), diabetes (OR 1.64, CI 1.36–1.99), hypercholesterolemia (OR 1.30, CI 1.06–1.61), hypertension (OR 1.77, CI 1.39–2.25), and sleep apnea (OR 2.23, CI 1.75–2.83). In multivariate models, PRSs for atrial fibrillation (OR 1.90, CI 1.30–2.78), diabetes (OR 1.55, CI 1.26–1.91), CAD (OR 1.58, CI 1.21–2.07, in myocardial infarction analyses), cholesterol (OR 1.75, CI 1.41–2.17), systolic blood pressure (OR 1.53, CI 1.25–1.86, in hypertension analyses), and stroke (OR 1.52, CI 1.15–2.02) all were significantly associated with their respective health variable.
Table 3. Longitudinal associations between depression symptoms at baseline and health conditions at follow-up

CI, confidence interval; BMI, body mass index; CRP, C-reactive protein; PRS, polygenic risk score.
a Association remained significant after correcting for multiple comparisons.
b Polygenic risk scores (PRSs) for health conditions: atrial fibrillation, diabetes, erectile dysfunction, coronary artery disease (for myocardial infarction), cholesterol, systolic blood pressure (for hypertension), sleep apnea, and stroke. All analyses also controlled for first three principal components (not shown).
Table 4 displays results of sensitivity analyses that used a measure of depressive symptoms without somatic items (appetite, weight, and sleep disturbances). After correcting for multiple comparisons, non-somatic depressive symptoms at baseline were significantly longitudinally associated with sleep apnea (OR 1.32, CI 1.09–1.60) at follow-up. Non-somatic symptoms were not significantly associated with incident diabetes, erectile dysfunction, or hypercholesterolemia.
Table 4. Sensitivity analyses: Longitudinal associations between depression symptoms (excluding somatic items) at baseline and health conditions at follow-up

CI, confidence interval; BMI, body mass index; CRP, C-reactive protein; PRS, polygenic risk score.
a Association remained significant after correcting for multiple comparisons.
b Polygenic risk scores (PRSs) for health conditions: atrial fibrillation, diabetes, erectile dysfunction, coronary artery disease (for myocardial infarction), cholesterol, systolic blood pressure (for hypertension), sleep apnea, and stroke. All analyses also controlled for first three principal components (not shown).
Discussion
Total depression symptoms were longitudinally associated, over a 27-year follow-up period, with the incidence of several chronic cardiometabolic health conditions, after controlling for available physiological and behavioral risk factors such as alcohol consumption, smoking, BMI, CRP, and polygenic risk. A lifetime history of depression symptoms, assessed at midlife, was associated with significantly increased odds of subsequently developing diabetes, erectile dysfunction, hypercholesterolemia, and sleep apnea in later life. Thus, having depression symptoms earlier in life may increase risk for the later onset of chronic health conditions above and beyond the contributions of alcohol consumption history, smoking history, BMI, inflammation (CRP), and polygenic risk. The length of follow-up is particularly noteworthy. Lifetime total depression symptoms were assessed at an average age of 41 and they predicted risk for these chronic health conditions over two decades later. Our results suggest that the effects of depression symptoms on cardiometabolic health could be very long-lasting, and that, consistent with prior research, these associations are robust to the inclusion of traditional risk factors for non-communicable diseases such as smoking and alcohol use (Rajan et al., Reference Rajan, McKee, Rangarajan, Bangdiwala, Rosengren, Gupta and Yusuf2020). However, it is also possible that causal factors not assessed in this study could be increasing risk for both depression and later cardiometabolic health, or that non-causal mechanisms explain these relationships. For example, individuals with a history of depressive symptoms may be more likely to access health care generally, therefore enhancing the rate of detection of cardiometabolic conditions compared to individuals without a history of depressive symptoms.
Our finding that total depression symptoms were significantly associated with incident diabetes is consistent with previous research demonstrating that depression increases the risk for type 2 diabetes; this research also indicates that this relationship is bidirectional (Semenkovich et al., Reference Semenkovich, Brown, Svrakic and Lustman2015). We also found that depression was longitudinally related to erectile dysfunction, hypercholesterolemia, and sleep apnea; these findings extend previous work that has identified relationships between depression and cardiometabolic health cross-sectionally or over a shorter timeframe (BaHammam et al., Reference BaHammam, Kendzerska, Gupta, Ramasubramanian, Neubauer, Narasimhan and Moscovitch2016; Harris et al., Reference Harris, Glozier, Ratnavadivel and Grunstein2009; Liu et al., Reference Liu, Zhang, Wang, Li, Cheng, Guo and Zhu2018; Montazer & Wheaton, Reference Montazer and Wheaton2011).
Sensitivity analyses revealed that, when somatic depression symptoms (i.e., weight, appetite, and sleep disturbances) were excluded, the remaining non-somatic symptoms were significantly associated with incident sleep apnea. The associations between non-somatic depression symptoms and erectile dysfunction and hypercholesterolemia were not significant, and the association between these non-somatic symptoms and diabetes did not survive correction for multiple comparisons. This is consistent with previous literature finding that somatic depression symptoms are associated with poor cardiometabolic outcomes (de Miranda Azevedo et al., Reference de Miranda Azevedo, Roest, Hoen and De Jonge2014). Depression-related changes in appetite, weight, and/or sleep may either lead to or indicate physiological changes that impact cardiometabolic health over time. A large-scale study of dimensions of depression symptoms found that somatic symptoms, measured both before and after cardiovascular disease onset, are associated with mortality (Freak-Poli et al., Reference Freak-Poli, Ikram, Franco, Hofman and Tiemeier2018). Future research could investigate whether particular symptom clusters, or even individual symptoms, are uniquely associated with risk for specific cardiometabolic outcomes, particularly in populations with higher overall depression symptom burden than our study.
Of the cardiometabolic outcomes with which total depression symptoms were not significantly associated in full models, myocardial infarction and stroke are likely to be the most reliably reported by participants, given that these health outcomes are discrete events which often require emergency medical care, and thus potentially less likely to be underreported than other health outcomes. One possible explanation for the lack of observed association with myocardial infarction is the relatively low base rate of this outcome in our sample (8.3%). These analyses may have been underpowered, especially as the odds ratio for myocardial infarction (1.25) was of a similar magnitude to other health outcomes that were significantly associated with depression symptoms. Future studies should examine whether myocardial infarction is associated with a history of depressive symptoms in other samples, including samples with a higher base rate of this outcome.
PRSs were significantly associated with their respective cardiometabolic conditions in most models. Although PRSs represent only genetic risk due to common variation, these findings demonstrate the importance of more in-depth screening for both depression and genetic risk factors for cardiometabolic health. These results could also reflect the influence of genes relevant to depression on cardiometabolic outcomes.
Several putative mechanisms responsible for the longitudinal relationships between psychopathology and cardiometabolic health have been suggested. These mechanisms fall into two major categories: behavioral and biological processes. Hypothesized behavioral mechanisms linking depression to cardiovascular and metabolic health problems include lifestyle and compliance factors associated with depression, such as smoking, poor diet, lack of exercise, weight gain, and reduced medication compliance (Chaddha, Robinson, Kline-Rogers, Alexandris-Souphis, & Rubenfire, Reference Chaddha, Robinson, Kline-Rogers, Alexandris-Souphis and Rubenfire2016; Colotto, Rubini, Savoriti, D'Adduogo, & Mercuri, Reference Colotto, Rubini, Savoriti, D'Adduogo and Mercuri2010; Semenkovich et al., Reference Semenkovich, Brown, Svrakic and Lustman2015; Serrano, Tiemi Setani, Sakamoto, Maria Andrei, & Fraguas, Reference Serrano, Tiemi Setani, Sakamoto, Maria Andrei and Fraguas2011). In our analyses, BMI at age 68 was associated with cardiometabolic conditions when controlling for other covariates. Of note, BMI could be considered both a biological and a behavioral process, as it is a complex phenotype associated with a multitude of biological factors (e.g., genetics) as well as non-biological factors (e.g., walkability of one's environment) (Locke et al., Reference Locke, Kahali, Berndt, Justice, Pers, Day and Econs2015; Tarlov et al., Reference Tarlov, Silva, Wing, Slater, Matthews, Jones and Zenk2020). BMI and major psychiatric disorders, including major depression, have extensive polygenic overlap (Bahrami et al., Reference Bahrami, Steen, Shadrin, O'Connell, Frei, Bettella and Andreassen2020), and higher BMI is likely causally associated with the incidence of depression (Tyrrell et al., Reference Tyrrell, Mulugeta, Wood, Zhou, Beaumont, Tuke and Hyppönen2019). Although our study did not examine BMI longitudinally, it is possible that BMI influenced both the development of depression symptoms and of cardiometabolic health outcomes in our sample. It is noteworthy that BMI was significantly associated with five of the eight cardiometabolic conditions examined.
Other hypothesized biological mechanisms linking depression to cardiovascular and metabolic health problems include abnormal cardiac function (e.g., heart rate variability, left ventricular impairment), hyperinflammation, serotonin transport gene polymorphisms, hypothalamic–pituitary–adrenal axis dysregulation, endothelial dysfunction, endocrine changes, and greater platelet activation and aggregation (Chaddha et al., Reference Chaddha, Robinson, Kline-Rogers, Alexandris-Souphis and Rubenfire2016; Monami & Marchionni, Reference Monami and Marchionni2007; Pozuelo et al., Reference Pozuelo, Tesar, Zhang, Penn, Franco and Jiang2009; Schoevers et al., Reference Schoevers, Bremmer, Beekman, Hoogendijk, Deeg and van Tilburg2004; Semenkovich et al., Reference Semenkovich, Brown, Svrakic and Lustman2015; Serrano et al., Reference Serrano, Tiemi Setani, Sakamoto, Maria Andrei and Fraguas2011). In our analyses, CRP was not significantly associated with cardiometabolic conditions when controlling for other covariates. Notably, several antidepressant medications affect these physiological processes, which suggests the possibility that antidepressant treatment could reduce the heightened cardiovascular and metabolic risk burden conferred by depression (Monami & Marchionni, Reference Monami and Marchionni2007). Future work could examine these hypothesized mechanisms directly through mediation analyses. Another possible non-causal mechanism could be indicated by correlated genetic risk for both depression and cardiometabolic health problems; genetic correlations between other complex traits and health outcomes have been found in GWAS data (Bulik-Sullivan et al., Reference Bulik-Sullivan, Finucane, Anttila, Gusev, Day, Loh and Neale2015).
This study has several limitations that should be considered. The sample includes only men, and we limited our main analyses to participants of genetically determined European ancestry to permit the inclusion of PRSs as covariates. These factors limit the generalizability of these findings; however, supplementary analyses in the full sample (N = 1195; see online Supplementary eTables S4–5) follow the same general pattern of results as the main analyses. This may be due to the relatively low proportion of variance that is generally explained by PRSs, which index common genetic variation and may not reflect all genetic influences on the risk of outcomes. Also, cardiometabolic health was assessed using self-report binary (yes/no) measures of doctor diagnosis, which do not account for disease severity; this means that the identification of cases depended on access to healthcare. Although more objective measures exist for some conditions examined (e.g., blood pressure or use of antihypertensive medications), objective measures were not available for all health conditions, and reliance on them could also result in missed cases. As several of the conditions we assessed are often undiagnosed – such as sleep apnea (Finkel et al., Reference Finkel, Searleman, Tymkew, Tanaka, Saager, Safer-Zadeh and Avidan2009) – the true prevalence of these conditions in our sample may well be underestimated. This may mean that the estimates presented in these analyses are conservative: if the true prevalence of these conditions is higher, then the strength or magnitude of their associations with a history of depressive symptoms may be masked or biased toward the null. In total, 103 participants died before the third wave of VETSA; their exclusion from these analyses may indicate that our sample is biased toward healthier participants. This potential selection bias may have resulted in more conservative estimates of the associations between depression and cardiometabolic outcomes. Future work should include medical record information and other objective measures of disease burden when possible. Additionally, the initial requirement in the first wave of VETSA that both twins must participate in the study may have introduced volunteer bias (Neale & Eaves, Reference Neale and Eaves1993). Finally, the odds ratio values in our findings should not be compared directly to each other because the base rates for the health conditions vary.
Although this study was observational and the associations between depression and cardiometabolic health conditions cannot be interpreted causally, the longitudinal design as well as the exclusion of cases of cardiometabolic health problems that were diagnosed before baseline strengthen the conclusions that can be drawn about how total depression symptoms in midlife impact the subsequent development of health problems in later life. These stable, longitudinal patterns of association could also be explained by non-causal correlated genetic risk factors not tested here. Our next step is to fit biometrical genetic twin models to the data to model both cross-temporal causality and correlated genetic risk factors, which may provide alternative, mechanistic explanations. Whether causal or not, depression symptoms were still predictive of cardiometabolic health problems over two decades later. Future research should strive to further elucidate causal relationships and paths between depression and cardiometabolic health over time, and to identify any common causes they may share. Finally, the results suggest that clinicians treating depression should pay careful attention to cardiometabolic risk factors in addition to depression symptoms themselves.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003329172000505X.
Data
Data on CAD/myocardial infarction (for use in constructing PRSs) have been contributed by CARDIoGRAMplusC4D investigators and have been downloaded from www.CARDIOGRAMPLUSC4D.ORG. This research has been conducted using data from UK Biobank, a major biomedical database.
Acknowledgements
This work was, in part, the result of work supported with the resources of the VA San Diego Center of Excellence for Stress and Mental Health. The Cooperative Studies Program of the Office of Research & Development of the United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. All statements, opinions or views are solely those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government, the NIA or the NIH. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution, this research would not have been possible.
Financial support
This work was supported by the National Institute on Aging (H.L.D., M.W.L., R.T., R.E.M., C.E.F., M.S.P., K.N.C., R.V., D.E.G., M.S.-C., M.E.W., N.A.G., M.C.N., X.M.T., N.W., H.X., W.S.K., and M.J.L., R01 AG050595; R.E.M., C.E.F., W.S.K., and M.J.L., R01 AG022381; R.T., R.E.M., W.S.K., and M.J.L., R01 AG022982; C.E.F and D.E.G., R01 AG059329; C.E.F., P01 AG055367; C.E.F., R56 AG037985); the Research Council of Norway (O.A.A., 223273); the European Union's Horizon 2020 Research and Innovation Action Grant (O.A.A., 847776); the National Center for Advancing Translational Science (J.A.E., 1KL2TR001444), and the Virginia Center on Aging (N.A.G.).
Conflict of interest
O.A.A. received speaker's honorarium from Lundbeck and is a consultant for HealthLytix.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.