I. Introduction
Public health tort litigation has exploded in recent decades. Private and public plaintiffs increasingly are filing civil lawsuits in an effort to hold parties responsible for public health harms.Reference Gostin and Wiley1 State and local governments, in particular, strapped for resources to address widespread burdens of injury and disease linked to prevalent products, have resorted to litigation to lend additional supports. Increased sophistication in the methods and data used to track epidemiological harms over time and across populations has bolstered the evidence base that can be brought to bear in such cases. Nevertheless, prominent litigation campaigns related to tobacco use, food consumption, lead paint and asbestos exposure, and firearms have met with mixed success.
Opioid litigation represents the latest surge in public health litigation. These lawsuits predominantly seek to hold companies manufacturing, distributing, and selling prescription opioid analgesic medications accountable for the devastating harms of the crisis that in 2017 was responsible for 130 American lives lost per day.Reference Hedegaard, Miniño and Warner2 The litigation has ballooned into well over 2000 cases filed by governments, most of which are consolidated in federal court under a multi-district litigation (MDL) umbrella while the remainder reside in state courts.Reference Gluck, Hall, Curfman and Raymond3 Opioid case settlements originally date back into the early 2000s, but their frequency has recently accelerated in parallel with increases in opioid-related morbidity and mortality.Reference Haffajee and Mello4 For example, Oklahoma settled its suit against Purdue Pharma, the maker of Oxycontin, for $270 million, and Teva Pharmaceuticals for $85 million (while a judgment worth close to $500 million against Johnson and Johnson is under appeal as of this writing); as well, West Virginia settled its suit against McKesson Corporation, a major drug distributor, for $37 million.Reference Diep, Feeley, Becker and Smith5 More recently, three large opioid distributors (McKesson, Cardinal Health, and AmerisourceBergen) and Teva Pharmaceuticals settled with Cuyahoga and Summit counties for $260 million to avert a bellwether trial in the MDL, while payments in the tens of billions from the same four companies are being discussed with various states attorneys generals to globally settle the MDL.Reference Bernstein6 The uses of settlement funds, particularly in the wake of some misuse of tobacco settlement dollars, has been a point of contention in these and ongoing cases.Reference Carr, Davis and Rutkow7 What is to become of the monstrous opioid MDL, riddled with procedural and logistical hurdles given the dozens of plaintiffs and defendants and plethora of claims, remains to be seen.
Nonetheless, opioid litigation has served and can continue to serve a number of important public health and tort litigation objectives. It seeks to fill gaps in prescription opioid and controlled substance regulation at the federal level, legislative capture and inactivity at all levels of government to prevent addiction and opioid harms, and a lack of self-regulation among companies to ensure the safe use of their products. The litigation also has secured some compensation for abating opioid harms, deterred corporate malfeasance by holding many companies accountable for their behavior and requiring them to change it, and enhanced public awareness of the risks of opioid addiction. While far from a panacea, this litigation can complement and spur other regulatory action to provide better oversight over potentially addictive and harmful medications. Moreover, we can learn from past failures in optimizing public health litigation settlements (e.g., from the tobacco Master Settlement Agreement [MSA]) and ideally reduce opioid harms rooted in prescription opioid marketing and distribution.
This article proceeds in three parts. In Part II, I discuss the general goals and role of public health tort litigation. I briefly discuss whether public health tort litigation objectives were achieved in the tobacco, asbestos, and lead paint contexts in Part III. In Part IV, I discuss the demonstrated and potential value of opioid litigation to achieve public health goals. I conclude in Part V. Ultimately, whether public health tort litigation objectives are met by opioid litigation will depend on how the lawsuits proceed, the terms of settlements and/or judgments, and how these terms are carried out. This litigation does hold the potential to help ameliorate some, though certainly not all, opioid-related harms.
This article proceeds in three parts. In Part II, I discuss the general goals and role of public health tort litigation. I briefly discuss whether public health tort litigation objectives were achieved in the tobacco, asbestos, and lead paint contexts in Part III. In Part IV, I discuss the demonstrated and potential value of opioid litigation to achieve public health goals. I conclude in Part V. Ultimately, whether public health tort litigation objectives are met by opioid litigation will depend on how the lawsuits proceed, the terms of settlements and/or judgments, and how these terms are carried out. This litigation does hold the potential to help ameliorate some, though certainly not all, opioid-related harms.
II. Role of Public Health Tort Litigation
Whereas tort litigation traditionally was conceived as a response to individual wrongs and harms, the “new public health litigation” embraces the collectivist view of tort law as a means for protecting population health and well-being.Reference Parmet and Daynard8 The goals of public health litigation fall along a continuum, from obtaining compensation, to changing the defendant's future behavior, to ultimately destroying the defendant. Common causes of action (or theories of liability) asserted by governments and groups of consumers in public health tort suits — such as public nuisance, unjust enrichment, and fraudulent misrepresentation — stem from this collectivist approach.9 This form of indirect regulation is generally reactive to injury and disease, because a plaintiff must have suffered actual injury to substantiate a claim.10
Tort litigation can be an effective means of public health policy making when other branches of government more typically responsible for policy making (i.e., the legislative and executive branches) and the market have failed to effectively regulate behavior.Reference Jacobson and Warner11 In a “dynamic view,” courts can be viewed as independent institutions, capable of effectively producing social change under such circumstances. Moreover, the courts can serve as a catalyst for action, as happened with civil rights litigation, by educating the public and other governmental branches about harms, rights infringements, and injustices.Reference Rosenberg12 The courts can act more independently than other branches, and thus be less subject to industry capture or political pressures. They also can serve to vindicate rights, particularly of more marginalized, less powerful populations.
But the courts are also inherently constrained in ways that can inhibit social change.13 They are bound by the Constitution and precedent in their decisions and ability to vindicate rights. They also are inherently undemocratic, albeit they have some inter-dependence with other branches of government, for instance because federal judges are appointed by the sitting President. Courts also lack substantive expertise in areas and are not equipped to process sophisticated scientific evidence, and so tend to rely on litigants for this information. Importantly, courts are ill-suited to comprehensively establish, implement and enforce policies — tasks typically left to sister branches of government. Litigation presents procedural drawbacks to making effective policy as well. It is expensive and lengthy, reactive (rather than proactive) to harms, and may not provide an appropriate remedy to all injuries sustained.
Although courts are imperfect policy making entities, civil tort litigation can achieve three important objectives that relate to policy: compensation, deterrence, and accountability.14 First, litigation seeks to obtain compensation on behalf of injured parties from the wrongdoers who inflicted harm, under the theory that money can help repair economic and even noneconomic damages suffered. Second, tort litigation aims to deter injury-causing behavior committed specifically by the defendant(s) to the litigation and/or generally among this class or type of defendant. This deterrent effect can be achieved through defendants' fear of financial liability that induces them to engage in safer behavior, price increases necessitated by major damages awards, or even requirements that defendants change certain behavior or engage in industry-funded educational activities (e.g., educational advertising campaigns to raise awareness about tobacco harms).15 Third, tort litigation seeks to hold wrongdoers accountable for their actions, for instance by assessing punitive damages or by publicly finding them liable for wrongdoing. Equity jurisdiction — or the ability of courts to issue injunctions that compel a defendant to refrain from or carry out certain action — can serve an additional civil litigation goal that often overlaps with deterrence and accountability objectives.16
III. Past Public Health Litigation
Previous public health litigation has achieved many civil litigation goals, as summarized in Table 1, although it has been far from a perfect solution to the morbidity and mortality related to tobacco, asbestos, lead paint, or other harmful product exposures. Although it is beyond the scope of this article to review these litigation landscapes, objectives, and consequences in detail, brief summaries are instructive in considering the value of opioid litigation. The most frequently drawn analogy to the opioid litigation is to the tobacco litigation, given that both involve potentially addictive substances and dozens of government plaintiffs suing product manufacturers. Decades of tobacco litigation culminated in the 1998 MSA between the four major tobacco manufacturers and 46 states attorneys general plus six other jurisdictions worth $206 billion over 25 years plus $9 billion per year in perpetuity thereafter.17 Arguably, the final “wave” of tobacco litigation, in which state governments leveraged epidemiological evidence to demonstrate public health harms, was reactive to regulatory failures and changed public health policy.18 Regulatory capture of the Food and Drug Administration (FDA) and members of Congress likely contributed to a paucity of tobacco regulation at the federal and state levels in the administrative and executive branches prior to the MSA, although it should be noted that the FDA did try to promulgate comprehensive tobacco regulations in the 1990s that were ultimately struck down by the Supreme Court in FDA v. Brown & Williamson Tobacco Corp.19 As demonstrated by the Tobacco Papers divulged in this final litigation wave, the tobacco industry was not self-regulating for the public's benefit. Instead, evidence established that major tobacco companies knew of their products' addictive properties and nevertheless conspired to suppress this information and mislead consumers.Reference Pringle20 After the MSA and arguably related byproducts thereof, other branches of government closed certain regulatory gaps in tobacco regulation. For instance, the Congress passed the Family Smoking Prevention Tobacco Control Act in 2009, which gave the FDA greater authority to regulate tobacco marketing to youth and required warning labels on tobacco products.21
Table 1. Public Health Tort Litigation Role and Objectives Achieved

The tobacco litigation also achieved many civil tort litigation objectives. The MSA was the largest-ever settlement implemented in the U.S. that provided substantial compensation to states.Reference Healton22 Admittedly, the money was not optimally used by many states towards preventing tobacco use harms, and some estimates suggest that only 2-3.5% of MSA revenues were used for smoking control and prevention programs.Reference Haile, Krueger-Andes, Jones and Silvestri23 Because MSA funds were not earmarked for specific activities, states were free to divert the money for purposes unrelated to tobacco, such as servicing debt and bridging budget gaps, although these diverted amounts varied by state.24 Despite some failures, the MSA did have a short-term specific deterrent effect. Namely, it necessitated that tobacco manufacturers significantly increase the prices of cigarettes, which corresponded to a decrease in consumer demand and achieved (at least to a degree) the goal of smoking prevention and cessation.25 Other deterrent effects and accountability goals were achieved by the MSA's behavior change requirements, most notably the enjoinders on marketing to youth (including the end of the “Joe Camel” advertising campaign) and mandatory funding for a large counter-marketing campaign spearheaded by the American Legacy Foundation.Reference Schroeder26 The litigation achieved accountability objectives by publicly shaming the tobacco industry, largely through evidentiary disclosures of documents that demonstrated the tobacco industry's deliberate manipulation of consumers and were shared on a public website.27 These disclosures and the widely publicized nature of the litigation itself enhanced the public's understanding of tobacco's addictiveness and dangers and diminished the industry's credibility.28 There is limited evidence that the MSA had a lasting deterrent effect on the tobacco industry in the U.S. and abroad, however, given the growth of marketing to youth and international populations and sustained contributions to population health disease burden attributable to new exposures after the settlement.29
Large public health litigation campaigns related to other products also present interesting comparisons to opioids. Asbestos, a natural product with remarkable fire-retardant properties, was used widely in homes, public facilities, and workplaces from the 1930-1970s in the U.S. The product's carcinogenic nature largely evaded public scrutiny for decades, even though these risks were known to its manufacturers as early as the 1930s, because symptoms of mesothelioma and other cancers manifest 10-50 years after asbestos exposure.Reference Hensler30 Moreover, the industry worked to suppress the science on asbestos risks and opposed efforts to minimize asbestos in the workplace throughout the 1950s and 1960s, demonstrating ineffective market self-regulation when it came to the public's health.31 Evidence of regulatory capture and failures prior to 1970 abounded in the asbestos domain.Reference White32 However, groundbreaking research in the 1960s and 1970s led by Dr. Irving Selikoff that linked asbestos exposure in the workplace to malignant diseases helped to justify regulation and litigation. Subsequent regulation at the federal and state levels has sought to regulate exposure and handling of the substance to a degree, as demonstrated by the federal Occupational Safety and Health Act passed in 1970 to regulate workplace exposures and the Comprehensive Environmental Response, Compensation and Liability Act of 2000 that designates asbestos-containing material as a hazardous substance.Reference Leonardi33
Aiming to fill various asbestos regulatory gaps enjoyed some success in more recent years. Notwithstanding one significant victory in the case Borel v. Fibreboard — wherein the Fifth Circuit upheld strict liability against asbestos manufacturers — small plaintiff firms representing private parties predominantly floundered in asbestos litigation efforts through the 1970s thanks to causation challenges and statute of limitation defenses.34 But by the 1980s, armed with better epidemiological evidence, plaintiffs firms started more consistently to win asbestos cases. Some wins even secured punitive damages awards. This had the perverse effect of triggering bankruptcy filings by many asbestos manufacturers, thereby limiting recovery amounts in future years; moreover, plaintiffs' firms retained large amounts of the compensation in fees, leaving less for the injured parties.35 By the 1990s, asbestos litigation showed signs of consolidation, in the form of an MDL and various class actions at the federal level. However, these cases suffered from aggregation problems and rejections of class action settlements on procedural grounds of collusion between plaintiff and defense bars.36 Despite the defeats in federal court, private plaintiffs succeeded in many state court efforts to obtain some compensation and public accountability, as well as to send a strong general deterrence message to asbestos manufacturers for fear of large liability exposure.37 But the litigation did not spur equity judgments (in the form of injunctions on behavior), nor did it prompt certain key regulatory changes to promote the public's health — such as an outright ban on the use of asbestos, requirements that the product be removed, or a national injury compensation fund.38
Another product that has long posed public health risks and spurred related litigation is lead paint. The federal government actually implemented a ban on lead-based paint in residential housing in 1978, prior to the influx in public health litigation in this domain. To this day, the Centers for Disease Control and Prevention (CDC) estimates that approximately 4 million households include children that are exposed to high levels of lead, largely due to older lead-based paints that children may consume.39 Lead is known to have very harmful effects on all systems of the body and has particularly deleterious effects on child development.
Like tobacco and asbestos, lead paint spurred several waves of litigation. Beginning in 1987 and concentrated in the 1990s, private plaintiffs sued lead paint and pigment manufacturers under theories of negligence, consumer protection, and conspiracy to suppress information about risks.Reference Smith40 In 1999, the theories claimed and plaintiffs making the claims in the litigation shifted — namely to public nuisance causes of action brought by governments. These new consolidation and collectivist strategies, as were pursued in the tobacco and asbestos litigation contexts, enjoyed some limited successes in the lead paint context.Reference Tipps41 But ultimately, this avenue faced causation hurdles, whereby plaintiffs were challenged to show that the defendant paint manufacturers “controlled the instrumentality that caused the nuisance.”42 One recent plaintiff success in a case in California that took 17 years to wind its way through the courts affirmed a jury award under the public nuisance theory of liability, however.Reference Hiltzik and Hiltik43 Its impact is only just beginning to unfold. But as a general matter, lead paint litigation perhaps addressed some market failures, but had less potential to fill regulatory gaps or specifically deter an industry that was already banned in the U.S. Plaintiff wins have been sparse and led to only limited compensation, or achievement of other public health goals. Moreover, the long length of time required for the asbestos and other public health litigation enterprises to achieve substantial “wins” has complicated their ability to abate public health harms in a direct and timely way.
IV. The Value of Opioid Litigation to Address a Public Health Crisis
a) An Overview of the Opioid Litigation Landscape
What value has opioid litigation brought to addressing the public health crisis, and what is its future potential? As alluded to and much like the tobacco and other litigation domains discussed, opioid litigation has proceeded in several distinct but overlapping waves, culminating in a third wave characterized by mass tort suits alleging population harms. Table 2 provides an overview of these waves of litigation and includes for each: predominant suits, public perception of opioid analgesics, common claims, and usual winner. Opioid cases involve a diverse set of claimants: individuals harmed by opioid analgesics brought cases in the first wave; classes of injured individuals brought suit in the second wave; and governments (state, county, tribal, and federal) sued in the third wave that continues to present.44 Fewer cases have been brought by hospital and health care organizations, suing for the costs they have borne from opioid prescribing-related harms. The most common defendant in early cases and to this day is Purdue Pharma, although other opioid analgesic manufacturers frequently named include Johnson and Johnson (and its subsidiaries), Janssen Pharmaceuticals Inc., Insys Therapeutics, Teva Pharmaceuticals, Endo Health Solutions Inc., and Allergan PLC.45 Other common defendants include dominant opioid distributors — McKesson Corporation, Cardinal Health, AmerisourceBergen, Mallinckrodt, and Miami-Luken — as well as major pharmacies like Walgreens, RiteAid, and CVS.46
Table 2. Opioid Litigation Waves

Notes: FDCA, Food Drug and Control Act; CSA, Controlled Substances Act; RICO, The Racketeer Influenced and Corrupt Organizations Act.
Liability theories asserted in opioid litigation and their outcomes have varied depending on the parties to the suit. In the first wave of litigation, individual plaintiffs most typically asserted personal injury claims against manufacturers. Manufacturers were accused of fraudulently misrepresenting in advertising and detailing efforts opioid analgesic effectiveness for treating pain and non-addictive nature, failing to adequately warn consumers about their products' potentially addictive properties, and failing to include tamper resistant formulations for these drugs. These cases typically were dismissed in early stages of litigation, when defendants successfully asserted defenses such as: lack of causation (given the many contributors to addiction and ensuing harms); wrongful conduct on the part of some individual plaintiffs in illegally obtaining prescription opioids; and product misuse on the part of patients.48 The second wave of opioid litigation involved attempts to aggregate individual defendants into classes. However, the classes typically were not certified for lack of commonality among class members because individuals had different trajectories of product use that contributed to their respective harms.49
The third wave of opioid litigation has proven the most viable. Government plaintiffs, armed with greater resources and more robust population-level evidence that establishes patterns of violations and injuries, have asserted aggregate harms under state-based public nuisance, fraud, conspiracy, and unjust enrichment theories and/or federal statutes.50 A significant development in this wave has been the consolidation of approximately 2700 cases into a federal MDL, overseen by Judge Dan Aaron Polster who is keen to achieve a productive, global settlement. As well, a spate of criminal charges against Purdue Pharma executives, which resulted in a $634 million settlement in 2007, and more recently owners (i.e., the Sackler family) have elevated potential liabilities of and spurred a bankruptcy settlement proposal with this closely-held company.Reference Belmonte and Mann51 Recent settlements in a number of state cases are likely to be an indicator of additional settlements or jury awards to come. However, there are significant challenges to achieving an equitable global settlement, including important structural barriers like: contingency fees paid to private firms (working on behalf of governments) who may have ulterior motives to settle quickly; loss of control over the course of the litigation for those plaintiffs not part of the negotiating lead counsel teams; complexity of settling among so many parties with diverse and sometimes competing interests in state and federal courts; and limited engagement of the public health community in the negotiating process.Reference Ausness52
b) Public Health and Civil Tort Objectives of Opioid Litigation
The goals of the current opioid litigation are widespread and ambitious. This discussion will focus on the third wave of litigation and what governments can and have achieved through litigation, rather than the goals of private party litigation. In terms of public health objectives, Judge Polster said the following in the first of many MDL settlement hearings:
The federal government is probably the least likely branch of government to try and tackle this, but candidly, the other branches of government, federal and state, have punted. So it's here. So I don't think anyone in the country is interested in a whole lot of finger-pointing at this point, and I'm not either …my objective is to do something meaningful to abate this crisis, and to do it in 2018.53
As Judge Polster's comments suggest, other branches of government were slow to respond to the opioid crisis. State policymakers acted before the federal government, but even state responses did not start in earnest until well over a decade after prescribing and deaths began their precipitous climb.Reference Haffajee, Frank, Haffajee and French54
Several high profile examples demonstrate signifi-cant federal regulatory failures and gaps in prescription opioid oversight. For instance, a Drug Enforcement Agency (DEA) whistleblower disclosed that members of Congress close to the pharmaceutical lobby pushed for policies directly requested by the industry. Specifically, Congress passed a 2016 law that stripped the DEA of certain monitoring and enforcement powers, including the ability to freeze suspicious pharmaceutical shipments, at the height of the opioid crisis and following a $102 million lobbying campaign by the industry.Reference Whitaker, Higham and Bernstein55 Federal civil case filings against drug wholesalers fell from 131 in 2011 to 40 in 2014, rebounding somewhat to 64 in 2016 — perhaps providing evidence that the pharmaceutical industry had captured the Department of Justice and affected it's behavior around charges and intervening into DEA enforcement activities.56
The FDA has long been critiqued for its lax response in regulating prescription opioid harms.Reference Bonnie, Zettler and Frydl57 Noted oversights include: passive post-marketing surveillance of these drugs even when they are subject to a Risk Evaluation and Mitigation Strategy (REMS) program;58 approval of potent opioids such as Zohydro (2013) and Dsuvia (2018), despite a panoply of opioid analgesics already on the market and these products' potential for misuse amidst the height of the opioid crisis;Reference Manchikanti, Gottlieb, Sarpatwari, Sinha and Kesselheim59 and lack of incentives to spur innovation in the addiction treatment space (at least until recently). More generally, pharmaceutical companies exert influence over the FDA, calling into question the agency's independence in decision-making. Evidence of this includes: that a large proportion of the FDA's budget has been paid for by the pharmaceutical industry since the 1990s associated with drug application and approval fees;Reference Sekerka and Benishek60 a revolving door between the agency and pharmaceutical industry employees;Reference Silverman61 and “pay-for-play” type deals whereby pharmaceutical executives have paid to meet privately with FDA executives and influence analgesic division recommendations.Reference Whoriskey62
The pharmaceutical industry also has exhibited substantial self-regulation failures when it comes to ensuring the safe use of their opioid products. Drug suppliers should be motivated to protect consumers from harms related to their products: if these products prove dangerous, then consumer demand and company profits will decrease and the image of the company will be tarnished. Nevertheless, firms repeatedly seem to favor short-term profits over long-term product safety, as occurred in the opioid space. Purdue Pharma's sophisticated marketing plan for OxyContin has been documented extensively, and other defendants to the litigation are alleged to have engaged in similar conduct. In its OxyContin detailing efforts, Purdue profiled individual providers, detailing their prescribing patterns, and targeted those who prescribed large quantities of opioids and those with large numbers of patients with chronic pain.Reference Van Zee63 Purdue also created a generous incentive structure for its sales representatives to increase OxyContin sales in their regions and more than doubled the size of its sales force from 1996-2000.64 Sales representatives provided free samples and coupon programs, along with branded gifts, to prescribers to promote the drug.65 Despite a lack of clinical evidence to support its claims, Purdue pushed the message, including through physician front-men, that opioids were non-addictive and could be prescribed liberally to treat non-malignant pain.Reference Haffajee66 The result was a nearly tenfold increase in OxyContin prescriptions for non-malignant pain, from 670,000 in 1997 to 6.2 million in 2002.67 Other companies engaged in similar behaviors. For example, Insys Therapeutics' aggressively targeted non-cancer patient populations (via prescribers) for its powerful fentanyl product approved for the treatment of cancer, Subsys®, to increase market share. Insys did this by conveying misleading marketing messages to its sales force and, by extension, the prescribers they detailed.68
In summary, litigation is bringing to light growing evidence to support allegations that opioid companies failed to self-regulate in the interest of public health, and liability may attach to some of these failures. Certain opioid manufacturers deliberately misrepresented and fraudulently marketed their drugs, despite some known risks and even REMS programs in place for post-marketing surveillance. Certain distributors appear to have supplied opioids in quantities beyond what seemed plausibly medically necessary and colluded with other distributors in neglecting to report suspicious order. Some pharmacies dispensed opioids in alarmingly high dosages. Evidence of these practices and agreements to change behavior have served as the basis for new government regulatory oversight and past settlements with key defendants, as detailed below. Public health litigation thus can and has played a role in addressing serious opioid market failures.
Past opioid litigation settlements also have achieved certain civil tort litigation objectives, a trend poised to continue into the future. Table 3 sets forth key opioid settlement terms among government plaintiffs (states and federal) and various defendants (manufacturers, distributors, and pharmacies) to illustrate satisfied tort litigation objectives. This is not to suggest that all opioid suits have arrived at productive outcomes; many have not settled or have done so on less than ideal terms. For instance, the first settlement included in Table 3 of 2007 between 27 states Attorneys General and Purdue settled for a paltry amount ($19.5 million) given the many plaintiffs involved and failed to attribute much accountability to Purdue Pharma.69 The opioid crisis in states party to this deal only accelerated after the settlement, and Purdue has been accused in subsequent litigation of continuing the very behaviors forbidden in the settlement — namely, making misrepresentations about its opioid product's addictiveness.70 On a whole, however, these settlements largely represent positive developments that are likely to be replicated and built upon in settlements and perhaps judgments to come.
Table 3. Civil Tort Objectives Achieved in Representative Opioid Settlements
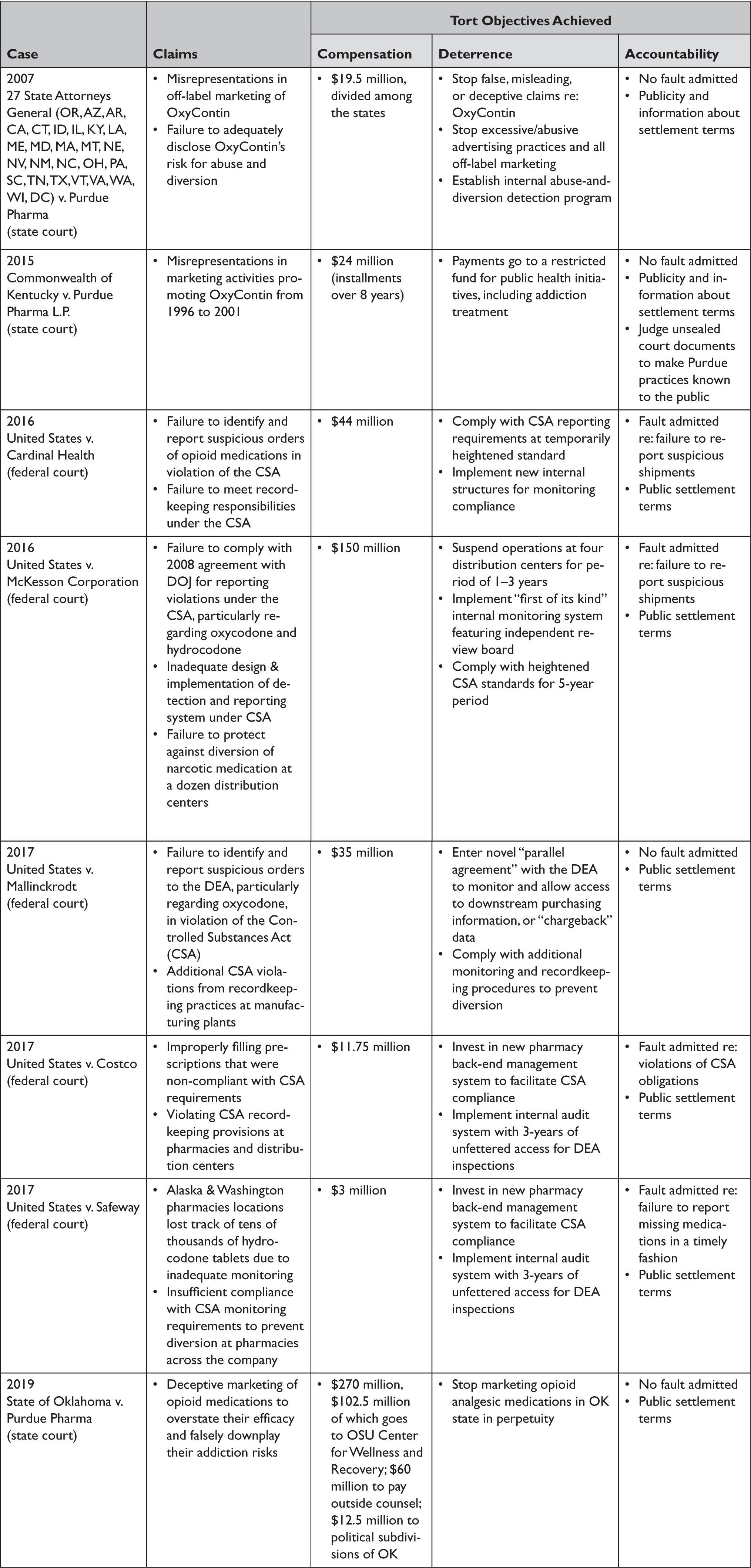
First, in terms of compensation, opioid cases have garnered increasing sums over time, ranging from the $19.5 million in the aforementioned settlement between the states Attorneys General and Purdue Pharma, to $270 million in the 2019 settlement between the State of Oklahoma and Purdue, to almost $500 million in the Oklahoma judgment against Johnson & Johnson.71 More recent compensation amounts reflect a growing understanding of the costs of the crisis, estimated to range nationally from $75 million in 2013 up to $500 billion per year — for a total cost of the epidemic since 2001 of $1 trillion.Reference Rhyan, Manchikanti and Florence72 Governments bear about half of these cumulative costs.Reference Rhyan73 Future opioid litigation could garner much more substantial sums, particularly if parties to the MDL settle as is under discussion with many. Assuming opioid companies do not routinely file for bankruptcy, an MDL global settlement reasonably could be on the order of tens of billions if all parties come to the negotiating table, given the tobacco MSA precedent, extraordinary costs of the crisis, and current settlement talks.74 Although suppliers of opioid analgesics cannot reasonably be expected to bear all opioid-related costs borne by governments or other parties, their pivotal role in fostering addiction by alleged deceitful, misleading, and unlawful means to achieve tremendous profits appears to justify some compensation to ameliorate civil harms.
Another positive compensation development is the earmarking of funds, as we have seen in many state cases (e.g., Kentucky and Oklahoma settlements, Table 3). The Oklahoma settlement, negotiated by the Attorney General with Purdue, was particularly encouraging because it specifically allocated funds to the Center for Wellness and Recovery at Oklahoma State University ($102.5 million).75 This center is focused on addiction treatment — both providing care and also developing new research — across the state and will use the money to further these goals.76 Another $12.5 million was allocated to cities and counties to abate their opioid crisis nuisances.77 While this money can be spent in various ways by local governments, the uses must abate opioid harms and cannot be diverted to unrelated purposes, unlike with tobacco MSA funds. (Of note, however, after the Oklahoma settlement was announced the disgruntled legislature passed a law to require that they oversee the allocations of future settlements, as occurred with the MSA.Reference Bernstein78)
Opioid litigation moreover appears to have had a deterrent effect on the companies that historically supplied and continue to supply prescription opioids. This stands in contrast to the lead paint litigation, wherein companies were out of business by the time litigation penalties or behavior change requirements could have any deterrent effect. In the case of opioids, the monetary damages may not have deterred some companies, they do seem to have deterred others. For instance, Purdue Pharma is on the verge of bankruptcy arrangements and Insys Therapeutics, which has paid penalties in both civil and criminal cases totaling at least $230 million, recently filed for bankruptcy.Reference Silverman, Spector, DiNapoli and Raymond79 Bankruptcy filings may prevent these companies from operating in the future, or at a minimum, could make them recalibrate the risks of pursuing profits over public health concerns. On the other hand, bankruptcy filings could limit the financial resources available in future settlements (as happened in the case of asbestos) and thus may act a as a “shield” from public health accountability.80
Beyond monetary penalties, various settlements have included behavior change requirements that specifically deter opioid suppliers from engaging in unlawful acts, as shown in Table 3. For manufacturers, settlements increasingly include terms that forbid prescription opioid marketing or promotion altogether or for a period of time within jurisdictions party to the litigation (e.g., Illinois and Massachusetts for Insys Therapeutics, and Oklahoma and 27 other states for Purdue Pharma).81 For distributors and pharmacies, behavior change requirements often involve suspension of opioid analgesic distribution or sales to jurisdictions where they were excessively supplied, strict reporting of suspicious shipments or sales to the DEA as required under the CSA, and heightened internal monitoring systems that track shipments and flag outliers (Table 3). On the other hand, the litigation could serve to over-deter many drug companies from innovating, manufacturing, and supplying opioid analgesics, which could exacerbate pain suffering for some patients. Evidence of opioid analgesic shortages (let alone relating to the litigation) is so far scant, but this potential unintended consequence is one to carefully monitor.
Opioid litigation has changed the behavior of at least some opioid companies that are taking voluntary steps to address the crisis. For instance, several opioid suppliers are donating profits to abate the crisis and seeking to innovate new products that deter abuse, treat pain non-addictively, or manage addiction.82 Purdue Pharma introduced an abuse-deterrent formulation of OxyContin, the first of its kind to be approved by the FDA, in 2010. Purdue also financially supports prescription drug monitoring program development across the states to help prevent diversion, problematic polypharmacy, and “doctor or pharmacy shopping” for opioids and donates to naloxone access and medication for opioid use disorder expansion efforts.83 Purdue Pharma also volunteered to stop marketing prescription opioids to physicians.84 These steps appear to be directly or indirectly tied to the litigation efforts, which changed public perception of opioid analgesics and the companies making them and pressured suppliers to modify any questionable practices that arguably contributed to the crisis.
Finally, the litigation has succeeded in holding opioid suppliers accountable for their alleged wrongdoings, at least to a degree. One of the drawbacks to settlements, as compared to a judge or jury trial verdict in a plaintiff's favor, is the frequent lack of admission of fault on the part of defendants (Table 3). For instance, Purdue Pharma and Johnson & Johnson have yet to admit responsibility in any of the opioid litigation settlements to which either has been a party. Distributor and pharmacy defendants more frequently have admitted fault (Table 3). Admission of responsibility is important as it documents, on the record, that a defendant engaged in legal wrongdoing, and forms the basis of their penalties or behavior change requirements. Even without admission, however, the publicity garnered from the litigation and implied responsibility arising from a settlement agreement or actual liability demonstrated in a judgment serve to hold companies accountable. Evidence divulged in litigation also can induce policymakers to act and start to fill regulatory gaps. For instance, Senator Claire McCaskill's investigations of various opioid distributors and significant opioid legislation have followed on the heals of litigation.85
Future behavior change induced by and initiatives that can be funded by damages arising from the litigation can augment existing abatement efforts and serve to further hold opioid companies accountable for their actions. Settlements or judgments could require opioid suppliers to engage in counter-advertising campaigns, much like in the “Truth” campaign generated from the tobacco MSA, and to fund educational efforts (though not be involved in generating their content) geared towards prescribers, pain specialists, and addiction treatment specialists. Companies could be required to invest in and innovate new (non-addictive) pain and opioid addiction therapies, along with fund research into initiatives with proven effectiveness to triage opioid harms (e.g., robust prescription drug monitoring programs often paired with pain clinic regulation, naloxone distribution, needle exchange programs, provision of medications to treat opioid use disorder and counseling).Reference Mauri, Townsend, Haffajee and Haffajee86 Strict compliance with federal laws (including the CSA's reporting requirements and the FDCA's marketing requirements) and state laws, and limits on marketing and lobbying tactics around prescription opioids (both direct-to-consumer and to professionals) should also be required. Finally, assistance with funding and programming targeted at structural determinants of disease (e.g., housing and employment services for those in recovery) could also be incumbent upon opioid suppliers to hold them accountable for the prescription opioid-related outcomes among vulnerable sectors of the population.
V. Conclusion
Prescription opioid litigation already has addressed many regulatory and market failures and has achieved numerous civil tort litigation objectives. It has arguably contributed more value in these regards than several other public health litigation agendas discussed briefly herein, such as asbestos and lead paint (Table 1), and has further potential to do so. Lessons learned from the tobacco MSA and previous opioid settlements (Table 3) can help to guide best practices going forward to maximize the impact of the current wave of government opioid suits. For example, generous monetary awards that specifically earmark use of funds for addiction treatment and support services, naloxone distribution and administration, pain and addiction therapy innovation, evidence-based diversion prevention efforts, structural determinants of opioid harms, and professional education on germane topics, are warranted. As well, prohibitions on demonstrated illegal and harmful practices — such as failure to report suspicious shipments or diversion under the CSA, and false marketing or representations in violation of the FDCA or state laws — ought to be included in settlements or judgments. Finally, admissions of fault and transparent, public reporting of court records and settlement terms would further serve to hold companies accountable for any wrongdoing. These steps would further tort litigation goals and public health purposes of the enterprise.
Litigation is certainly not a panacea or substitute for other regulatory actions, and instead should serve as a complement. It can highlight deficiencies in other branches of government and help to reform them. Indeed, legislative and executive branches do seem to be acting with greater fervor in recent years, for instance by enacting the SUPPORT for Patients and Communities Act and a panoply of state laws.87 If effectively wielded, litigation can (and already has served to) jumpstart needed funds and behavior changes to seriously address opioid harms and abate the crisis.
Acknowledgments
Dr. Haffajee's work on this article was supported by funding from the National Center for Advancing Translational Sciences of the National Institutes of Health (grant #KL2TR002241) and the Centers for Disease Control and Prevention (grant #U01CE002780).





