INTRODUCTION
Echinococcus granulosus has a world-wide distribution and is an important zoonotic pathogen. A number of distinct genotypes of the parasite exist, possibly representing distinct species (Thompson and McManus, Reference Thompson and McManus2002). Parasites of the G1 genotype are the most widespread in their distribution and are responsible for the majority of human infections (McManus and Thompson, Reference McManus and Thompson2003). The G1 genotype of E. granulosus is promiscuous in its range of suitable intermediate host species. Domestic livestock are the most important hosts; however, numerous herbivorous wildlife species may also act as hosts (Eckert et al. Reference Eckert, Deplazes, Craig, Gemmell, Heath, Jenkins, Kamiya, Lightowlers, Eckert, Gemmell, Meslin and Pawlowski2001; Jenkins and Macpherson, Reference Jenkins and Macpherson2003). In Australia, a number of species of macropodid marsupials have become infected with the G1 genotype of E. granulosus, maintaining sylvatic transmission of the parasite via wild dogs and dingoes (Jenkins and Morris, Reference Jenkins and Morris1991; Bowles et al. Reference Bowles, Blair and McManus1992; Jenkins and Macpherson, Reference Jenkins and Macpherson2003) The transmission dynamics of this cycle differ markedly from the domestic cycle in that wild dogs (dingoes and dingo/domestic dog hybrids) typically have much heavier burdens of the adult parasite compared to domestic dogs (Jenkins and Morris, Reference Jenkins and Morris1991; Lahmar et al. Reference Lahmar, Kilani and Torgerson2001) and the minimum time required for development in the macropodid intermediate host can be as little as 9 months compared to several years in sheep (Gemmell et al. Reference Gemmell, Lawson and Roberts1986; Barnes et al. Reference Barnes, Hinds, Jenkins and Coleman2007a). This potentially very prolific cycle provides a source for the introduction of the parasite into domestic livestock (Grainger and Jenkins, Reference Grainger and Jenkins1996), is a potential direct source of infection for humans, and causes mortality, primarily due to pulmonary impairment, in a number of species of macropodid marsupial including several species listed on the IUCN's Red List of Threatened Species (Johnson et al. Reference Johnson, Speare and Beveridge1998; IUCN, 2006; Barnes et al. Reference Barnes, Hinds, Jenkins and Coleman2007a, Reference Barnes, Goldizen, Morton and Coleman2008).
Control of transmission of E. granulosus is possible, particularly through measures to prevent infections in the definitive hosts, although eradication of the parasite has only been achieved in island settings (Craig et al. Reference Craig, McManus, Lightowlers, Chabalgoity, Garcia, Gavidia, Gilman, Gonzalez, Lorca, Naguira, Nieto and Schantz2007). A vaccine has been developed for use in intermediate hosts of E. granulosus which greatly reduces susceptibility to infection (Lightowlers et al. Reference Lightowlers, Lawrence, Gauci, Young, Ralston, Maas and Heath1996, Reference Lightowlers, Jensen, Fernandez, Iriarte, Woollard, Gauci, Jenkins and Heath1999). Although vaccination of wildlife species presents many obstacles, this has been achieved in Europe and North America to protect wildlife from rabies using an oral recombinant vaccinia virus (Blancou et al. Reference Blancou, Kieny, Lathe, Lecocq, Pastoret, Soulebot and Desmettre1986; Rupprecht et al. Reference Rupprecht, Hanlon, Slate, Schudel and Lombard2004). In addition, Haydon et al. (Reference Haydon, Randall, Matthews, Knobel, Tallents, Gravenor, Williams, Pollinger, Cleaveland, Woolhouse, Sillero-Zubiri, Marino, Macdonald and Laurenson2006) have demonstrated that low-coverage vaccination strategies can be successful in reducing the reproductive number of an infectious agent below 1 thereby reducing the extent of rabies in the endangered Ethiopian wolf. Wildlife vaccination is usually considered for 3 reasons: when the disease is a public health risk, when there is an economic impact on livestock production or when there is a threat to an endangered species (Cross et al. Reference Cross, Buddle and Aldwell2007). All 3 are relevant to E. granulosus transmission and impacts in Australia. Vaccination may be particularly applicable to the control of hydatid infection in closely managed populations of endangered species and could be used pre-release in captive breeding and re-introduction programmes that form part of recovery plans for such species (Nolan and Johnson, Reference Nolan and Johnson2001; Lundie-Jenkins and Lowry, Reference Lundie-Jenkins and Lowry2005; Menkhorst and Jarman, Reference Menkhorst and Jarman2005, 2007 a). In order to assess the potential for the use of vaccination as a control measure for hydatid infection in macropodid marsupials, we undertook an experimental study of the efficacy of vaccination in reducing or preventing E. granulosus infection in the tammar wallaby, Macropus eugenii. This species is maintained in captive breeding colonies and has been shown to be highly susceptible to infection with the parasite (Barnes et al. Reference Barnes, Hinds, Jenkins and Coleman2007a).
MATERIALS AND METHODS
Animals
Tammar wallabies were obtained from a breeding colony maintained at CSIRO Sustainable Ecosystems, Canberra. All tammars were born in captivity and had no previous exposure to E. granulosus. They were kept in open grassy yards (30×50 m) at CSIRO Sustainable Ecosystems, and supplemented with a diet of ewe and lamb pellets (15% protein; Young Stockfeeds, Young, NSW); water was available ad libitum.
All experimental procedures were approved by the CSIRO Sustainable Ecosystems Animal Ethics Committee (Application Number 05/06-07).
Study design
In a pilot study, 4 juvenile tammar wallabies (7–8 months of age, not yet fully independent of their mother) were vaccinated with 2×1 ml doses of EG95 (50 μg plus either 0·1 mg or 1·0 mg Quil A (Brentag Biosector, Denmark)) 5 weeks apart. Six weeks after the booster vaccination, they were challenged with 8000 E. granulosus eggs (see Barnes et al. Reference Barnes, Hinds, Jenkins and Coleman2007a). Six non-vaccinated tammars of the similar age (i.e. now all 10–11 months of age) were challenged concurrently with the same dose of eggs. One vaccinated tammar in this group died during the trial from causes unrelated to the trial. These animals were monitored until necropsy at 9–16 months post-infection (p.i.).
For the main trial, 40 wallabies (10–11 months of age) were allocated to 4 experimental groups of 10 animals. Twenty tammars were vaccinated with EG95 (50 μg plus 1·0 mg Quil A in a volume of 1 ml) at time 0, and then received an identical booster injection 4 weeks later. Ten of these tammars (4 females and 6 males) and 10 control, non-vaccinated tammars (all females) were challenged 1 month later with 8000 E. granulosus eggs (immediate challenge group). One vaccinated tammar in this group died during the trial from causes unrelated to the trial. These animals were necropsied at 12 months post-immediate challenge.
The remaining 2 groups of animals (10 vaccinated – 6 females and 4 males; and 10 non-vaccinated – 8 females and 2 males) were challenged with the 8000 E. granulosus eggs 9 months after the second vaccination (delayed challenge group). These animals were necropsied 8–12 months post-delayed challenge.
Vaccine preparation
The EG95 vaccine was prepared as a glutathione S-transferase fusion protein and purified. Each dose was prepared as a sterile solution containing 50 μg protein plus Quil A adjuvant as described by Lightowlers et al. (Reference Lightowlers, Lawrence, Gauci, Young, Ralston, Maas and Heath1996).
Collection of eggs for infection of experimental tammars
For each experimental infection, fresh mature, gravid E. granulosus were collected from the intestine of a naturally-infected wild dog shot by an Officer of the Rural Lands Protection Board, Yass, New South Wales, during the normal course of his duties. The eggs were recovered as described by Barnes et al. (Reference Barnes, Hinds, Jenkins and Coleman2007a). When required, the eggs were re-suspended and counted using a modified Fuch's Rosenthal chamber and diluted in distilled water to 4000 eggs/ml.
Radiological and clinical examinations and blood sampling
Each tammar was anaesthetized using 2–3% isoflurane (Attane, Pharmtech) using the methods described by Barnes et al. (Reference Barnes, Hinds, Jenkins and Coleman2007a). Chest radiographs were taken of each tammar at 0, 4, 8, 12 p.i. as described by Barnes et al. (Reference Barnes, Hinds, Jenkins and Coleman2007a). On these occasions each tammar was given a brief clinical examination. The tammars in the pilot study were also radiographed and examined at 9, 14 and 16 months p.i. Tammars were monitored visually every 2–3 days throughout the trial for signs of respiratory distress. All tammars were euthanased and necropsied at the end of each trial or earlier if radiographic changes in the lungs raised concerns that the welfare of the animals was likely to be compromised before the next radiographic examination. Blood samples (3–5 ml) were taken from the lateral coccygeal vein of tammars in the main trial at 0, 1, 2, 6, 8, 10 and 14 months after the initial vaccination.
Infection of experimental tammars with E. granulosus eggs
Infection of tammars was undertaken when the animals were lightly anaesthetized as described by Barnes et al. (Reference Barnes, Hinds, Jenkins and Coleman2007a). Two ml of distilled water containing approximately 8000 E. granulosus eggs were administered using soft plastic tubing inserted into the oesophagus.
Post-mortem examinations
Tammars were euthanased with 140 mg/kg sodium pentobarbitone (Lethobarb, Virbac, Peakhurst, Australia). At necropsy the presence or absence of hydatid cysts was initially assessed by gross visual inspection and palpation. Lung, liver, spleen, kidney, heart and brain were then sectioned at 3–5 mm intervals. The location of each cyst was recorded. The presence or absence of degenerative changes (caseation or calcification) was noted. Cysts were defined as non-viable if they contained only caseous or calcified material and no cyst fluid could be aspirated. When present, fluid was removed from cysts by sectioning or aspiration and examined with a stereomicroscope for the presence or absence of protoscoleces. Any lesions of uncertain aetiology were fixed in 10% buffered formalin. These were later processed using standard histological methods (Lillie, Reference Lillie1954) with sections cut at 4 μm and stained with H & E. Sections were examined to determine the presence or absence of the laminated layer that is diagnostic for cysts of Echinococcus spp (Kilejian et al. Reference Kilejian, Schinazi and Schwabe1961). Sections from cysts of uncertain degenerative status were examined for signs of involution of the laminated layer, degeneration of the germinal membrane and presence of caseous, necrotic or calcified material within the cyst.
ELISA assessment of anti-EG95 antibody titres
Anti-EG95 antibodies in wallaby sera were detected by ELISA. Reagent volumes were 100 μl unless otherwise stated. An optimal concentration of the antigen, EG 95 recombinant protein, (1 μg/ml in 50 mM carbonate buffer, pH 9·6) was incubated in ELISA plates (Microlon, Greiner, Germany) overnight at 4°C. Antigen was discarded and the plate washed 3 times with phosphate-buffered saline and 0·05% Tween20 (Sigma) (PBS-T). Non-specific binding sites were blocked with 200 μl of 5% skim milk powder in PBS (PBS-SM) for 1 h at 37°C. Wallaby sera diluted in PBS-SM was added to the plates and serially diluted from 1/1000. Controls (no antigen present) were included for each serum and a positive control serum was tested on each plate. Plates containing diluted sera were incubated for 1 h at 37°C, and then washed as above. An optimal concentration (1/1000 in PBS-SM) of rabbit anti-kangaroo IgG H&L antiserum (Bethyl Laboratories, USA, A140-104) was added to each well. After incubation at 37°C for 1 h, the antiserum was discarded, the plates washed and goat anti-rabbit HRP conjugate IgG (DAKO, Denmark) was added at 1/1000 in PBS-SM. Following further incubation at 37°C for 1 h, the conjugate was discarded, the plates washed and activated TMB substrate (3,3′,5,5′- tetramethylbenzidene, Sigma) was added to each well. Plates were incubated at room temperature for 15 min; the reactions stopped by addition of 0·5 m H2S04, and the optical density (OD) read at 490 nm. Acceptance of the data from each plate was determined provided the OD of the positive control sera at 2 dilution points (1/32 000 and 1/512 000) fell within 2SD of levels previously calculated from 20 replicate titrations of the positive control. Antibody titres were calculated from the projected titre at which the sera had an OD value of 1·6. Samples having antibody levels which failed to reach this OD at the highest dilution were recorded as being negative.
Statistical analyses
Protection against infection was calculated as the percentage reduction in the mean number of viable hydatid cysts in vaccinated animals in comparison to the mean number of viable cysts in control animals. Other analyses were undertaken using Stata Version 9 (StataCorp, College Station, TX, USA, 2006). To assess associations between the trial (pilot/immediate challenge/delayed challenge), vaccination status (control/vaccinated) and sex on the risk of development of hydatid infection, logistic regression was undertaken with each variable fitted separately and assessed in comparison with the null model using the likelihood ratio test. The odds ratio (OR) and 95% confidence interval (CI) were also calculated for each of these variables, other than the reference group, using the corresponding beta-coefficients and associated standard errors. As the distribution of observed proportions of tammars with various numbers of cysts was closer to that expected with a negative binomial, rather than a Poisson distribution, negative binomial regression was undertaken to assess associations between the trial, vaccination status and sex on the number of cysts that developed. Each variable was fitted separately and assessed in comparison with the null model using the likelihood ratio test. The count ratio (CR, estimated ratio of mean number of cysts for animals in the exposed group relative to the reference group) and 95% CIs were calculated for each variable other than the reference group using the corresponding beta-coefficients and associated standard errors. For both logistic and negative binomial regression, variables with a univariable P<0·25 were progressively included in multivariable models in a forward selection process whereby variables were retained if the associated P-value from the likelihood ratio test was less than 0·05. Interaction terms between 2 significant variables were also tested in the models.
RESULTS
No adverse local or systemic reactions were noted following the administration of EG95. In the pilot study, tammars were euthanased from 9 to 16 months p.i. Hydatid cysts developed in 5/6 control and 0/3 vaccinated tammars.
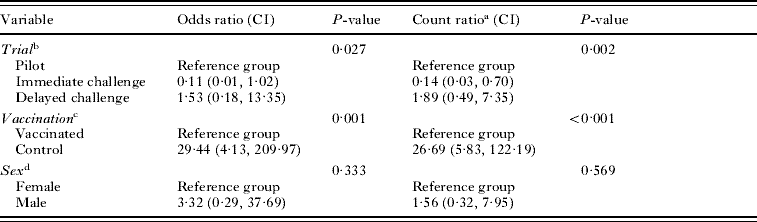
In the main study immediate challenge group, all tammars were euthanased at 12 months p.i. Three of 10 control tammars and 0/9 vaccinated tammars became infected. In the delayed challenge group, tammars were euthanased from 8 to 12 months p.i. Eight of 10 control tammars and 2/10 vaccinated tammars developed hydatid cysts (Table 1). Overall infection rates were 61·5% (16/26) control tammars and 9·1% (2/22) vaccinated tammars. Control tammars were significantly more likely to become infected (OR 29·44; CI 4·13, 209·97; P=0·001, Table 2). There was also a significant association between the trial and likelihood of infection, with tammars in the immediate challenge group less likely (OR 0·11; CI 0·01, 1·02) and those in the delayed challenge group more likely (OR 1·53; CI 0·18, 13·35) to become infected compared to the reference group, the pilot study (P=0·027, Table 2). There was no association between sex of the tammar and the likelihood of becoming infected (P=0·333, Table 2). It was not possible to fit the interaction term between trial and vaccination status to the model.
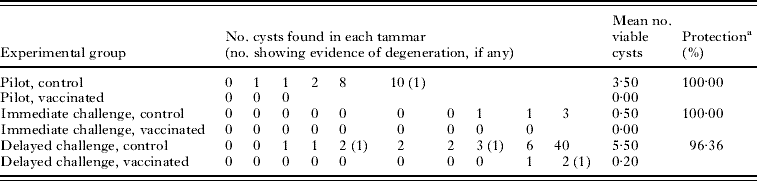
Table 1. Numbers of hydatid cysts and levels of protection achieved in tammar vaccination trials using the EG95 recombinant vaccine
(Immediate and delayed challenge vaccinated tammars were challenged at 1 and 9 months after their last vaccination, respectively.)
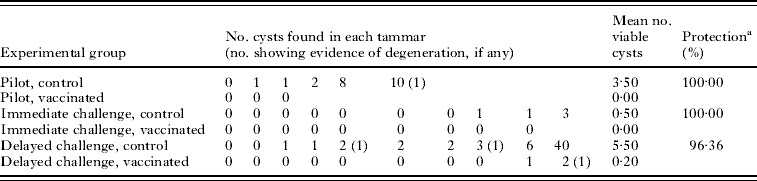
a Calculated as the percentage reduction in the mean number of viable cysts in vaccinated tammars compared with the mean number of viable cysts in control tammars.
Table 2. Associations between trial, vaccination status and sex of the tammar wallaby on the presence (odds ratio) and number of hydatid cysts (count ratio) found at necropsy
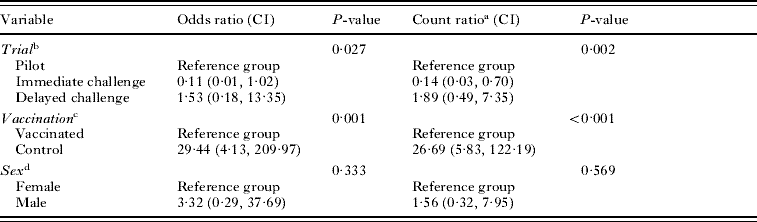
a Estimated ratio of mean number of cysts for animals in the exposed group relative to the reference group.
b Adjusted for vaccination status.
c Adjusted for trial.
d Adjusted for trial and vaccination status.
The numbers of hydatid cysts found in each tammar at post-mortem are shown in Table 1. In control tammars, cyst number ranged from 0 to 40, whereas, among the vaccinated tammars the range was 0–2 cysts with vaccination conferring 96·36–100% protection across the trials. Control tammars were more likely to develop more cysts (CR 26·69, CI 5·83, 122·19, P<0·001, Table 2). There was also a significant association between the trial and the number of cysts developing, with tammars in the immediate challenge group developing fewer cysts (CR 0·14; CI 0·03, 0·70) and those in the delayed challenge group developing more cysts (CR 1·89; CI 0·49, 7·35) compared to the reference group, the pilot study (P=0·002, Table 2). There was no association between the sex of the tammar and the number of cysts developing (P=0·569, Table 2). It was not possible to fit the interaction term between trial and vaccination status to the model.
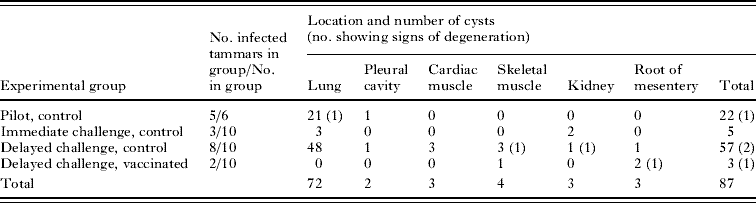
Seventy-two of the 87 (82·8%) hydatid cysts recovered from the 18 infected tammar wallabies were in lung tissue (Table 3). Cysts were also found free in the pleural cavity, in the cardiac and skeletal muscle, in the kidney and at the root of the mesentery (Table 3). Four cysts were classified as non-viable; 3/84 cysts (in lung tissue, skeletal muscle and kidney) in control tammars and 1/3 cysts (in the root of the mesentery) in vaccinated tammars (Tables 1 and 3). Protoscoleces were seen in some cysts from tammars necropsied from 8 months onwards. Due to variation in time of necropsies relative to infection it was not possible to compare the likelihood of cysts becoming fertile between control and vaccinated animals.
Table 3. Distribution of hydatid cysts in various organs for each of the experimental groups in which tammars became infected
(Cysts were not found in tammars from the pilot study vaccinated group or the immediate challenge vaccinated group.)

All vaccinated wallabies sero-converted, with specific IgG titres 1 month after the second vaccination ranging between 15 000 and 340 000, mean 135 000 (Fig. 1). At the time the delayed challenge group were infected, the antibody titres of that group of animals ranged from negative to 7500. Of the 2 wallabies that developed hydatid cysts, 1 had a negative titre and the other a titre of 12 000 at the time of infection. None of the control animals had a detectable level of specific antibody against the EG95 antigen at any time. Only 2 of the vaccinated and delayed challenge animals remained serologically positive 18 months after their initial immunization and the titres in these two animals were <2000. No boost in antibody titre was evident following the challenge infections.

Fig. 1. Antibody titres of tammar wallaby sera after immunization with EG95-GST at 0 and 1 months. Sera from vaccinated animals that were challenged 4 weeks after the booster vaccination (immediate challenge, open arrow) are shown with open symbols and those in which the challenge infection was delayed until 9 months post-vaccination (black arrow) are shown with black symbols. Controls are shown with grey symbols.
DISCUSSION
These findings show that unvaccinated tammars are 29 times more likely to develop hydatid cysts when challenged with E. granulosus eggs compared to vaccinated animals. They are also likely to develop a significantly greater number of cysts. The 96–100% protection afforded by the course of 2 vaccinations is similar to the level of protection noted in the several vaccine trials that have been undertaken in sheep (Lightowlers et al. Reference Lightowlers, Lawrence, Gauci, Young, Ralston, Maas and Heath1996, Reference Lightowlers, Jensen, Fernandez, Iriarte, Woollard, Gauci, Jenkins and Heath1999; Lightowlers, Reference Lightowlers2006).
Both the likelihood of tammars developing cysts and the intensity of infection varied significantly between the trials. The trials varied both in methodology (relative timing of infection and challenge) and in E. granulosus eggs used for the challenge (each batch being from a different wild dog). Differences in infectivity of different batches of parasite eggs may have been a factor. Since the infectivity of the eggs used in the delayed challenge trial appeared to be greater than that used in the others it is not possible to make an objective assessment on whether the reduced protection (96% compared to 100%) afforded by the vaccine in this trial was due to a reduction in efficacy, differences in the susceptibility of the animals at the time of infection or a function of the greater challenge.
Hydatid cysts occur most commonly in the lungs of macropodid hosts; only 0·5% of cysts (1 liver cyst) were found outside of the thoracic cavity in 71 naturally infected eastern grey kangaroos and wallaroos and 5% of cysts (2 kidney cysts) in 11 experimentally infected tammar wallabies (Barnes et al. Reference Barnes, Hinds, Jenkins and Coleman2007a, Reference Barnes, Morton and Colemanb). A remarkable distribution in the location of hydatid cysts was found in the tammars involved in the delayed challenge (Table 2). In vaccinated tammars, the few cysts that were found were present in locations other than the lung, and in the control group, while the majority of the parasites were located in the lungs, cysts were identified also at a variety of other anatomical locations. There was insufficient data to conclude whether this unusual cyst distribution was a feature of the eggs used in the delayed trial or whether the vaccine may have influenced the location of hydatid cysts in vaccinated tammars. There has not been any reference made to vaccination affecting the location of hydatid cysts in sheep (Lightowlers et al. Reference Lightowlers, Lawrence, Gauci, Young, Ralston, Maas and Heath1996, Reference Lightowlers, Jensen, Fernandez, Iriarte, Woollard, Gauci, Jenkins and Heath1999; Heath et al. Reference Heath, Jensen and Lightowlers2003).
Protoscoleces were identified from cysts as soon as 8 months p.i. This is sooner than the 9 months recorded in a previous experimental infection of tammar wallabies (Barnes et al. Reference Barnes, Morton and Coleman2007b) and provides further evidence for the potentially more rapid intermediate phase of the parasite life cycle in the Australian sylvatic cycle compared to the domestic cycle. Overall, the proportion of cysts showing signs of degeneration (4·6%, 4/87) was similar to that recorded in previous experimental infection of tammar wallabies (7·5%, Barnes et al. Reference Barnes, Hinds, Jenkins and Coleman2007a) and is consistently lower than that recorded in sheep (Gemmell, Reference Gemmell1966; Lightowlers et al. Reference Lightowlers, Jensen, Fernandez, Iriarte, Woollard, Gauci, Jenkins and Heath1999). Data are insufficient to analyse differences in cyst degeneration between vaccinated tammars (1/3 cysts) and control tammars (3/84 cysts) but the higher proportion of degenerate cysts in the vaccinated animals suggests that vaccination may have some effect on developing cysts.
Specific antibody raised by the wallabies against the vaccine protein were similar to those seen in EG95 vaccinated sheep (Woollard et al. Reference Woollard, Gauci, Heath and Lightowlers1998). The available evidence suggests that the vaccine has its effects through complement-mediated lysis of the invading oncosphere (Woollard et al. Reference Woollard, Gauci, Heath and Lightowlers2000; Lightowlers and Heath, Reference Lightowlers and Heath2004). The antibody titres seen in the 2 wallabies which did develop hydatid cysts when challenged 9 months after vaccination did not provide a clear association between protection and antibody titre at challenge. Data from the animals which were protected suggested that titres as low as 2000 were protective.
As multiple cysts in lungs and pleural cavity are known to have a rapid detrimental effect on the health of tammars (Barnes et al. Reference Barnes, Hinds, Jenkins and Coleman2007a) and endangered small macropodids (Johnson et al. Reference Johnson, Speare and Beveridge1998; Barnes et al. Reference Barnes, Goldizen, Morton and Coleman2008), the EG95 vaccination in its current injectable form may be beneficial if administered pre-release in captive breeding programmes. The level of protection that has been found here, reducing both likelihood and intensity of infection, following experimental infection with E. granulosus in the tammar wallaby would suggest that the vaccine would also be a valuable tool for widespread control of hydatid transmission through wild animals such as, in Australia, macropodid marsupials. However, for this to be achievable, the challenge remains to develop a practical method for delivery of the vaccine to the numerous species of wild animal that play a role in sylvatic hydatid disease transmission.
Funding for the study from Australian Research Council Linkage grant ARCLP0668354 and National Health and Medical Research grant 350279 is acknowledged. Valuable assistance with the care, maintenance and experimental procedures on the tammars at CSIRO Sustainable Ecosystems was provided by Dave Spratt, John Wright, Katrina Leslie, Kaitlyn Preece, Shawna Foo and Beth Koehn. We are grateful to Calvary Clinic, Canberra and the Canberra Veterinary Hospital, for the use of their automatic X-ray processing unit, Morag Wilson for her advice on radiography and Annie Rose and Anna Galloway for their help with interpretation of radiographs. The authors are indebted to Bill Morris (Vertebrate Pest Control Officer, Yass Rural Lands Protection Board, NSW) for his valuable contribution.