Introduction
Porcine reproductive and respiratory syndrome (PRRS) is present worldwide and is the most economically important infectious disease of swine production (Neumann et al., Reference Neumann, Kliebenstein, Johnson, Mabry, Bush, Seitzinger, Green and Zimmerman2005). PRRS disease was first described in the United States in 1987 (Keffaber, Reference Keffaber1989; Loula, Reference Loula1991) and a few years later in the Netherlands (Wensvoort et al., Reference Wensvoort, Terpstra, Pol, ter Laak, Bloemraad, de Kluyver, Kragten, van Buiten, den Besten, Wagenaar, Broekhuijsen, Moonen, Zetstra, de Boer, Tibben, de Jong, van't Veld, Groenland, van Genep, Voets, Verheijden and Braamskamp1991). The disease has many clinical manifestations but the two most prevalent are severe reproductive failure in sows and gilts (characterized by late-term abortions, an increased number of stillborns, mummified and weak-born pigs) (Keffaber, Reference Keffaber1989; Bilodeau et al., Reference Bilodeau, Dea, Sauvageau and Martineau1991; Loula, Reference Loula1991; Pol et al., Reference Pol, van Dijk, Wensvoort and Terpstra1991; Christianson, Reference Christianson1992; Albina, Reference Albina1997) and respiratory problems in pigs of all ages associated with a non-specific lymphomononuclear interstitial pneumonitis (Keffaber, Reference Keffaber1989; Bilodeau et al., Reference Bilodeau, Dea, Sauvageau and Martineau1991; Loula, Reference Loula1991; Collins et al., Reference Collins, Benfield, Christianson, Harris, Hennings, Shaw, Goyal, McCullough, Morrison, Joo, Gorcyca and Chladek1992; Rossow et al., Reference Rossow, Morrison, Goyal, Singh and Collins1994; Halbur et al., Reference Halbur, Paul, Meng, Lum, Andrews and Rathje1996b; Albina, Reference Albina1997).
The etiological agent, PRRS virus (PRRSV) was identified in 1991 by investigators in the Netherlands and shortly after in the United States (Wensvoort et al., Reference Wensvoort, Terpstra, Pol, ter Laak, Bloemraad, de Kluyver, Kragten, van Buiten, den Besten, Wagenaar, Broekhuijsen, Moonen, Zetstra, de Boer, Tibben, de Jong, van't Veld, Groenland, van Genep, Voets, Verheijden and Braamskamp1991; Benfield et al., Reference Benfield, Nelson, Collins, Harris, Goyal, Robison, Christianson, Morrison, Gorcyca and Chladek1992; Collins et al., Reference Collins, Benfield, Christianson, Harris, Hennings, Shaw, Goyal, McCullough, Morrison, Joo, Gorcyca and Chladek1992). The PRRSV is an enveloped, single-stranded positive sense RNA virus, approximately 50–65 nm in diameter that is classified in the order Nidovirales, family Arteriviridae, genus Arterivirus along with equine arteritis virus (EAV), lactate dehydrogenase-elevating virus (LDV) of mice and simian hemorrhagic fever virus (SHFV) (Benfield et al., Reference Benfield, Nelson, Collins, Harris, Goyal, Robison, Christianson, Morrison, Gorcyca and Chladek1992; Cavanagh, Reference Cavanagh1997). PRRSV genome is approximately 15 kb in length. The RNA viral genome is capped at the 5′ end and polyadenylated at the 3′ terminus and encodes at least nine open reading frames (ORFs) (Dea et al., Reference Dea, Gagnon, Mardassi, Pirzadeh and Rogan2000a), each of which is expressed via the generation of a 3′-coterminal nested set of subgenomic (sg) mRNAs (Allende et al., Reference Allende, Lewis, Lu, Rock, Kutish, Ali, Doster and Osorio1999; Gorbalenya et al., Reference Gorbalenya, Enjuanes, Ziebuhr and Snijder2006). ORFs1a and 1b, which constitute almost two-third of the genome, encode non-structural proteins (nsps), such as RNA polymerase, that are required for virus replication (Allende et al., Reference Allende, Lewis, Lu, Rock, Kutish, Ali, Doster and Osorio1999). At least 12 nsps are generated as a result of serial cleavages of two polyproteins expressed from ORF1a and ORF1ab, whose RNA is transcribed by a ribosomal frame shift between ORF1a and 1b (Snijder, Reference Snijder1998; Snijder and Meulenberg, Reference Snijder and Meulenberg1998). Three N-glycosylated minor envelope proteins (GP2a, GP3 and GP4) are translated from ORF2a, 3 and 4 and form heterotrimers by disulphide linkage (Wissink et al., Reference Wissink, Kroese, van Wijk, Rijsewijk, Meulenberg and Rottier2005). ORF2b, which is completely embedded in ORF2a, encodes another non-glycosylated minor protein named E (Wu et al., Reference Wu, Fang, Farwell, Steffen-Bien, Rowland, Christopher-Hennings and Nelson2001). ORF5 encodes the major envelope glycoprotein (GP5) that forms a heterodimer with the membrane non-glycosylated protein (M) encoded by ORF6 (Mardassi et al., Reference Mardassi, Massie and Dea1996). The viral capsid is composed of only one nucleocapsid protein (N), which is encoded by ORF7 and is highly immunogenic in infected animals (Dea et al., Reference Dea, Gagnon, Mardassi, Pirzadeh and Rogan2000a).
The virus is genetically, antigenically and pathogenically heterogenous (Dea et al., Reference Dea, Gagnon, Mardassi, Pirzadeh and Rogan2000a; Meng, Reference Meng2000). Currently, PRRSV isolates are divided into two distinct genotypes, represented by Lelystad virus (LV) in Europe (Wensvoort et al., Reference Wensvoort, Terpstra, Pol, ter Laak, Bloemraad, de Kluyver, Kragten, van Buiten, den Besten, Wagenaar, Broekhuijsen, Moonen, Zetstra, de Boer, Tibben, de Jong, van't Veld, Groenland, van Genep, Voets, Verheijden and Braamskamp1991) and ATCC VR-2332 in North America (Benfield et al., Reference Benfield, Nelson, Collins, Harris, Goyal, Robison, Christianson, Morrison, Gorcyca and Chladek1992; Dea et al., Reference Dea, Bilodeau, Athanasious, Sauvageau and Martineau1992). Even though European Union (EU) and North American (NA) isolates possess several biological and immunological similarities, including a nearly identical genome organization, both strains are genetically and antigenically distinct (Mardassi et al., Reference Mardassi, Mounir and Dea1994a; Magar et al., Reference Magar, Robinson, Dubuc and Larochelle1995; Nelsen et al., Reference Nelsen, Murtaugh and Faaberg1999). Sequence analyses have shown approximately a 60% nucleotide identity between the Type I (EU) and Type II (NA) genotypes (Allende et al., Reference Allende, Lewis, Lu, Rock, Kutish, Ali, Doster and Osorio1999; Nelsenet al. 1999; Plagemann, Reference Plagemann2003; Forsberg, Reference Forsberg2005; Hanada et al., Reference Hanada, Suzuki, Nakane, Hirose and Gojobori2005). Several studies have shown that a high degree of genetic variability exists within the NA-type of PRRSV (Meng et al., Reference Meng, Paul, Halbur and Lum1995a; Morozov et al., Reference Morozov, Meng and Paul1995; Kapur et al., Reference Kapur, Elam, Pawlovich and Murtaugh1996; Andreyev et al., Reference Andreyev, Wesley, Mengeling, Vorwald and Lager1997; Gagnon and Dea, Reference Gagnon and Dea1998; Pirzadeh et al., Reference Pirzadeh, Gagnon and Dea1998). While early studies suggested that a lower degree of variability might exist in EU PRRSV type (Suarez et al., Reference Suarez, Zardoya, Martin, Prieto, Dopazo, Solana and Castro1996b; Drew et al., Reference Drew, Lowings and Yapp1997), later studies in some countries, such as Denmark and Italy, reported a high divergence within EU-type isolates (Madsen et al., Reference Madsen, Hansen, Madsen, Strandbygaard, Botner and Sorensen1998; Nielsen et al., Reference Nielsen, Storgaard and Oleksiewicz2000; Oleksiewicz et al., Reference Oleksiewicz, Botner, Toft, Grubbe, Nielsen, Kamstrup and Storgaard2000; Forsberg et al., Reference Forsberg, Oleksiewicz, Petersen, Hein, Botner and Storgaard2001, Reference Forsberg, Storgaard, Nielsen, Oleksiewicz, Cordioli, Sala, Hein and Botner2002). In 2006, the emergence of a potential highly pathogenic variant of PRRSV was reported as the possible cause of large-scale outbreaks with high mortality in China (Li et al., Reference Li, Wang, Bo, Tang, Yang, Jiang and Jiang2007; Tian et al., Reference Tian, Yu, Zhao, Feng, Cao, Wang, Hu, Chen, Hu, Tian, Liu, Zhang, Deng, Ding, Yang, Zhang, Xiao, Qiao, Wang, Hou, Wang, Yang, Kang, Sun, Jin, Wang, Kitamura, Yan and Gao2007). It is now obvious that PRRSV isolates show considerable genetic variation and could be classified in several phylogenetic clusters within each genotype (Yoshii et al., Reference Yoshii, Kaku, Murakami, Shimizu, Kato and Ikeda2005; Kiss et al., Reference Kiss, Sami, Kecskemeti and Hanada2006; Mateu et al., Reference Mateu, Diaz, Darwich, Casal, Martin and Pujols2006; Fang et al., Reference Fang, Schneider, Zhang, Faaberg, Nelson and Rowland2007).
The molecular characteristics, biological and immunological functions of the PRRSV structural proteins and nsps and their involvement in the virus pathogenesis are described below.
Virus morphogenesis
Early studies reported that the N protein forms a spheric icosahedral capsid core of 20–30 nm in diameter, which is surrounded by a lipid envelope containing the viral envelope proteins, yielding a relatively smooth spherical virion of about 60 nm in diameter (Benfield et al., Reference Benfield, Nelson, Collins, Harris, Goyal, Robison, Christianson, Morrison, Gorcyca and Chladek1992; Dea et al., Reference Dea, Gagnon, Mardassi, Pirzadeh and Rogan2000a; Doan and Dokland, Reference Doan and Dokland2003a, Reference Doan and Doklandb). Recently, Spilman et al. (Reference Spilman, Welbon, Nelson and Dokland2009) described the structure of PRRSV virions based on cryo-electron microscopy (EM) analysis and tomographic reconstruction of virions grown in MARC-145 (monkey kidney) cells. They reported that the virus has a pleomorphic morphology, a spherical to oval shape with a size ranging from about 50 to 65 nm, a hollow, layered core of around 40 nm diameter and a smooth outer surface studded with a few envelope protein complexes. The structural analysis indicates that the PRRSV core consists of a helical nucleocapsid (Fig. 1) wrapped into a hollow ball (Spilman et al., Reference Spilman, Welbon, Nelson and Dokland2009), unlike the earlier studies (Benfield et al., Reference Benfield, Nelson, Collins, Harris, Goyal, Robison, Christianson, Morrison, Gorcyca and Chladek1992; Dea et al., Reference Dea, Gagnon, Mardassi, Pirzadeh and Rogan2000a; Doan and Dokland, Reference Doan and Dokland2003a, Reference Doan and Doklandb). The latest results are not surprising since other members of the Nidovirales, such as coronavirus, possess a helicoidal capsid.

Fig. 1. Schematic representation of the PRRSV particle. The locations of the structural proteins: GP2a, E, GP3, GP4, GP5, M and N (encoded by ORFs 2–7) are shown. The virion possesses a non-segmented single-stranded RNA genome, enclosed in a nucleocapsid protein (N), yielding a helicoidal capsid structure. The N protein is the sole component of the viral capsid and interacts with itself through covalent and non-covalent interactions (homodimer). The major envelope viral protein (GP5) forms a heterodimer structure with the membrane non-glycosylated protein (M) which dominates the virion surface. The minor structural proteins (GP2a, E, GP3 and GP4) are incorporated into virions as a multimeric complex. The minor structural viral proteins multimeric complex also interacts with the GP5-M heterodimer (not illustrated). Although GP3 is a structural viral protein, there is one report it is a non-structural and secreted viral protein (which suggests that this could be a strain-dependent phenomenon).
Being an enveloped virus, PRRSV survivability outside the host is affected by temperature, pH and exposure to detergents. It is known that PRRSV can survive for extended intervals at temperatures ranging from −70 to −20°C (Benfield et al., Reference Benfield, Nelson, Collins, Harris, Goyal, Robison, Christianson, Morrison, Gorcyca and Chladek1992); nonetheless, viability decreases with increasing temperature. Specifically, recovery of PRRSV has been reported after exposure for up to 20 min at 56°C, 24 h at 37°C and 6 days at 21°C (Benfield et al., Reference Benfield, Nelson, Collins, Harris, Goyal, Robison, Christianson, Morrison, Gorcyca and Chladek1992). The PRRSV remains stable at pH ranging from 6.5 to 7.5, but infectivity is reduced at pH<6.0 or >7.65 (Bloemraad et al., Reference Bloemraad, de Kluijver, Petersen, Burkhardt and Wensvoort1994). Detergents reduce infectivity of the virus and lipid solvents such as chloroform and ether are particularly efficient at disrupting the viral envelope and eliminating infectious virions (Benfield et al., Reference Benfield, Nelson, Collins, Harris, Goyal, Robison, Christianson, Morrison, Gorcyca and Chladek1992). Even if PRRSV is relatively fragile in the environment, appropriate weather (wind, temperature, humidity, etc.) may favor the transmission of the virus through aerosols up to 4.7 km (Dee et al., Reference Dee, Otake, Oliveira and Deen2009).
Cellular pathogenesis
PRRSV is believed to have a very restricted cell tropism in vivo and in vitro. In vivo, the virus mainly infects well-differentiated cells of the monocyte-macrophage lineage, in particular porcine alveolar macrophages (PAM), the primary target cells of virus and interstitial macrophages in other tissues such as heart, thymus, spleen and Peyer's patches, hepatic sinusoids, renal medullary interstitium and adrenal gland (Halbur et al., Reference Halbur, Miller, Paul, Meng, Huffman and Andrews1995; Halbur et al., Reference Halbur, Paul, Meng, Lum, Andrews and Rathje1996b; Duan et al., Reference Duan, Nauwynck and Pensaert1997; Beyer et al., Reference Beyer, Fichtner, Schirrmeier, Polster, Weiland and Wege2000). In addition to macrophages, PRRSV RNA and nucleocapsid protein were found by in situ hybridization (ISH) and immunohistochemistry, respectively, in testicular germ cells, endothelial cells in the heart, interdigitating cells in the thymus, dendritic cells in the spleen and Peyer's patches (Halbur et al., 1996a, Reference Halbur, Paul, Meng, Lum, Andrews and Rathje1996b; Sur et al., Reference Sur, Doster, Christian, Galeota, Wills, Zimmerman and Osorio1997). Immuno-gold-silver immunohistochemical staining was used in experimentally infected gnotobiotic pigs, to demonstrate PRRSV antigens in bronchiolar epithelial cells, arteriolar endothelial cells, monocytes as well as interstitial, alveolar and intravascular macrophages (Rossow et al., Reference Rossow, Benfield, Goyal, Nelson, Christopher-Hennings and Collins1996). PRRSV RNAs and antigens were also found in bronchiolar epithelial cells (Pol et al., Reference Pol, van Dijk, Wensvoort and Terpstra1991), epithelium-like cells of alveolar ducts (Magar et al., Reference Magar, Larochelle, Robinson and Dubuc1993), and pneumocytes (Pol et al., Reference Pol, van Dijk, Wensvoort and Terpstra1991; Cheon et al., Reference Cheon, Chae and Lee1997) in naturally infected pigs but were not found in these types of cells in the experimentally infected pig (Teifke et al., Reference Teifke, Dauber, Fichtner, Lenk, Polster, Weiland and Beyer2001). Tissues such as lung, lymphoid tissues, Peyer's patches and kidney were also preferred organ targets of PRRSV infection (Sur et al., Reference Sur, Cooper, Galeota, Hesse, Doster and Osorio1996; Haynes et al., Reference Haynes, Halbur, Sirinarumitr, Paul, Meng and Huffman1997).
PRRSV was originally isolated on primary cultures of PAM cells (Wensvoort et al., Reference Wensvoort, Terpstra, Pol, ter Laak, Bloemraad, de Kluyver, Kragten, van Buiten, den Besten, Wagenaar, Broekhuijsen, Moonen, Zetstra, de Boer, Tibben, de Jong, van't Veld, Groenland, van Genep, Voets, Verheijden and Braamskamp1991) and so far, these cells as well as blood monocytes (Voicu et al., Reference Voicu, Silim, Morin and Elazhary1994), remain the only porcine cells that can effectively be used for viral propagation. Two non-porcine permissive cell subclones of MA104 monkey kidney cell line, the MARC-145 and CL2621 cells (Benfield et al., Reference Benfield, Nelson, Collins, Harris, Goyal, Robison, Christianson, Morrison, Gorcyca and Chladek1992; Bautista et al., Reference Bautista, Goyal, Yoon, Joo and Collins1993; Kim et al., Reference Kim, Kwang, Yoon, Joo and Frey1993) are also routinely used for in vitro propagation of wild and vaccine PRRSV strains. Flow cytometric and fluorescence antibody (FA) analyses of PRRSV antigen expression revealed distinct primary and secondary infection phases in MARC-145 cells (Cafruny et al., Reference Cafruny, Duman, Wong, Said, Ward-Demo, Rowland and Nelson2006). PRRSV antigen is randomly expressed in a small amount of cells during the primary phase of infection (up to about 20–22 h post-infection (p.i.)), but the logarithmic infection phase (days 2–3 p.i.) is characterized by secondary spread to clusters of cells. The formation of secondary clusters of PRRSV-infected cells preceded the development of cytopathetic effect (CPE) in MARC-145 cells. The primary and secondary PRRSV infection phases are inhibited by colchicine and cytochalasin D, indicating a critical role of the cytoskeleton in viral permissiveness as well as cell-to-cell transmission (Cafruny et al., Reference Cafruny, Duman, Wong, Said, Ward-Demo, Rowland and Nelson2006). Cellular expression of actin also appeared to correlate with PRRSV resistance, suggesting a second role of the actin cytoskeleton as a potential barrier to cell-to-cell transmission (Cafruny et al., Reference Cafruny, Duman, Wong, Said, Ward-Demo, Rowland and Nelson2006).
How the virus enters cells was first reported in 1996 (Kreutz and Ackermann, Reference Kreutz and Ackermann1996). It was postulated that since the direct fusion of the PRRSV envelope with the cellular membrane was not observed at any time, PRRSV entry most likely occurs by receptor-mediated endocytosis. In 1998, this hypothesis was confirmed (Duan et al., Reference Duan, Nauwynck, Favoreel and Pensaert1998) and a PRRSV receptor was identified on PAM by generation of PAM-specific monoclonal antibodies (mAbs). Several viral receptors for PRRSV have been described, including heparin sulphate for binding, sialoadhesin for binding and internalization, and vimentin (Delputte et al., Reference Delputte, Vanderheijden, Nauwynck and Pensaert2002, Reference Delputte, Van Breedam, Barbe, Van Reeth and Nauwynck2007a,Reference Delputte, Van Breedam, Delrue, Oetke, Crocker and Nauwynckb; Delputte and Nauwynck, Reference Delputte and Nauwynck2004; Kim et al., Reference Kim, Fahad, Shanmukhappa and Kapil2006). However, additional factors are needed because the expression of both receptors in non-permissive cells results in virus internalization but not in virus uncoating and productive infection. Recently, a molecule that is expressed exclusively on cells of a monocytic lineage, the CD163, has been identified as a possible cellular receptor for PRRSV (Calvert et al., Reference Calvert, Slade, Shields, Jolie, Mannan, Ankenbauer and Welch2007; Van Gorp et al., Reference Van Gorp, Van Breedam, Delputte and Nauwynck2008; Patton et al., Reference Patton, Rowland, Yoo and Chang2009). Furthermore, susceptibility of macrophages to PRRSV infection was previously associated with high expression of CD163 (Lopez-Fuertes et al., Reference Lopez-Fuertes, Campos, Domenech, Ezquerra, Castro, Dominguez and Alonso2000). Both sialoadhesin and CD163 are involved in infection of macrophages with different PRRSV strains: sialoadhesin is confirmed as a PRRSV internalization receptor and CD163 is involved in PRRSV infection, probably during uncoating (Van Gorp et al., Reference Van Gorp, Van Breedam, Delputte and Nauwynck2008). Finally, non-permissive cells expressing both sialoadhesin and CD163 are clearly more susceptible to PRRSV infection and produce 10 to 100 more virus compared with cells expressing only CD163 (Van Gorp et al., Reference Van Gorp, Van Breedam, Delputte and Nauwynck2008).
The interaction between the viral glycoproteins and CD163 receptor was characterized using a co-immunoprecipitation (co-IP) assay using mono-specific Abs (Das et al., Reference Das, Dinh, Ansari, de Lima, Osorio and Pattnaik2010). It was found that the GP2a and GP4 proteins interacted with the CD163 molecule (Das et al., Reference Das, Dinh, Ansari, de Lima, Osorio and Pattnaik2010). The carboxy(C)-terminal 223 residues of CD163 molecule are not required for interactions with either GP2a or GP4, although these residues are required for conferring susceptibility to PRRSV infection in baby hamster kidney-21 (BHK-21) cells. It was postulated that GP4 is critical for mediating interglycoprotein interactions and, along with GP2a, serves as the viral attachment protein responsible for mediating interactions with CD163 for virus entry into susceptible host cell (Das et al., Reference Das, Dinh, Ansari, de Lima, Osorio and Pattnaik2010). The CD151 molecule was identified by RNA-ligand screening of a MARC-145 cell expression library to be a PRRSV 3′ UTR RNA-binding protein (Shanmukhappa et al., Reference Shanmukhappa, Kim and Kapil2007). The CD151 is a member of the tetraspanin superfamily, which has several cellular functions that include cell signaling, cell activation and platelet aggregation (Hasegawa et al., Reference Hasegawa, Nomura, Kishimoto, Yanagisawa and Fujita1998; Fitter et al., Reference Fitter, Sincock, Jolliffe and Ashman1999; Sincock et al., Reference Sincock, Fitter, Parton, Berndt, Gamble and Ashman1999). Transfection of CD151 rendered BHK-21, a non-susceptible cell line, susceptible to PRRSV infection. The transfection of siRNA against CD151 inhibited PRRSV infection of MARC-145 cells. Additionally, polyclonal anti-CD151 Ab completely blocked PRRSV infection of MARC-145 cells (Shanmukhappa et al., Reference Shanmukhappa, Kim and Kapil2007). These results suggest that CD151 plays a critical role in PRRSV infection in vitro.
PRRSV induces apoptosis both in vitro and in vivo (Suarez et al., Reference Suarez, Diaz-Guerra, Prieto, Esteban, Castro, Nieto and Ortin1996a; Sur et al., Reference Sur, Doster, Christian, Galeota, Wills, Zimmerman and Osorio1997, Reference Sur, Doster and Osorio1998; Sirinarumitr et al., Reference Sirinarumitr, Zhang, Kluge, Halbur and Paul1998; Choi and Chae, Reference Choi and Chae2002; Kim et al., Reference Kim, Benfield and Rowland2002; Labarque et al., Reference Labarque, Van Gucht, Nauwynck, Van Reeth and Pensaert2003; Miller and Fox, Reference Miller and Fox2004). PRRSV infection of both MARC-145 cells and PAM results in apoptosis which is characterized by morphological changes, DNA fragmentation and specific caspase activation. PRRSV seems to be able to induce apoptosis directly (in infected cells) or indirectly (in bystander non-infected cells) in infected animals. For example, GP5 protein of PRRSV induced apoptosis in GP5 expressing cells (Suarez et al., Reference Suarez, Diaz-Guerra, Prieto, Esteban, Castro, Nieto and Ortin1996a; Gagnon et al., Reference Gagnon, Lachapelle, Langelier, Massie and Dea2003). However, others have shown that PRRSV induces apoptosis mostly in uninfected bystander cells both in vitro and in vivo and that the majority of apoptotic cells in lung lavages, lungs and lymphoid tissues are not positive for PRRSV antigens and RNA (Suarez et al., Reference Suarez, Diaz-Guerra, Prieto, Esteban, Castro, Nieto and Ortin1996a; Sur et al., Reference Sur, Doster, Christian, Galeota, Wills, Zimmerman and Osorio1997, Reference Sur, Doster and Osorio1998; Sirinarumitr et al., Reference Sirinarumitr, Zhang, Kluge, Halbur and Paul1998; Choi and Chae, Reference Choi and Chae2002; Kim et al., Reference Kim, Benfield and Rowland2002; Labarque et al., Reference Labarque, Van Gucht, Nauwynck, Van Reeth and Pensaert2003; Miller and Fox, Reference Miller and Fox2004). Alveolar macrophages from PRRSV-infected pigs showed a significantly increased apoptotic rate (22–34%) compared to porcine circovirus 2 infected alveolar macrophages (3%) (Chang et al., Reference Chang, Jeng, Liu, Lin, Chang, Chia, Tsai and Pang2005). Given that only 5–10% of alveolar macrophages were PRRSV-infected, these authors suggested that TNF-α or GP5 released from PRRSV-infected cells induced apoptosis in non-infected cells. The same group demonstrated that increased FasL expression in PRRSV-infected macrophages caused apoptosis in co-cultured swine splenic lymphocytes (Chang et al., Reference Chang, Jeng, Lin, Liu, Chang, Tsai, Chia and Pang2007). Another report showed that the majority of apoptotic cells are characterized by both early and late apoptosis markers (Lee and Kleiboeker, Reference Lee and Kleiboeker2007). Ultimately, these authors demonstrated that PRRSV induced apoptosis through a mitochondria-mediated pathway. Two oppositely directed sets of reactions are switched on in PRRSV-infected macrophages in vitro: at first, the balance is driven toward anti-apoptosis, but finally, PRRSV-infected macrophages die by apoptosis (Costers et al., Reference Costers, Lefebvre, Delputte and Nauwynck2008). Both anti- and pro-apoptotic effects were observed in both PRRSV-infected macrophages and PRRSV-infected MARC-145 cells (Costers et al., Reference Costers, Lefebvre, Delputte and Nauwynck2008).
The nucleocapsid protein (N)
The unglycosylated N protein possesses 123 to 128 amino acids (aa) depending on the genotype of the strains (NA and EU, respectively) with a molecular weight of 15 kDa, and is encoded by ORF7 viral gene (Fig. 2). The N protein of PRRSV is multifunctional (Table 1). It is basic with an isoelectric point of 10.4 and is a serine phosphoprotein (Wootton et al., Reference Wootton, Rowland and Yoo2002). Given that EAV and coronavirus nucleocapsid proteins are also phosphoproteins (Zeegers et al., Reference Zeegers, Van der Zeijst and Horzinek1976; Laude et al., Reference Laude, Godet, Bernard, Gelfi, Duarte and Delmas1995), phosphorylation of the nucleocapsid protein appears to be a common feature of nidoviruses. Phosphorylation of the N protein occurs at position 120 (Fig. 3) in PRRSV-infected cells and also in ORF7 gene-transfected cells, where the N protein is synthesized in the absence of other viral components (Wootton et al., Reference Wootton, Rowland and Yoo2002). Evolutionary conservation of this post-translational modification suggests that phosphorylation of the N protein may be of significant biological importance for the virus and warrants further investigation. Multifunctional proteins are often regulated by phosphorylation, and this phenomenon is particularly common to positively charged, nucleic acid binding proteins that make up the nucleocapsids of the coronavirus (Yoo et al., Reference Yoo, Spaete, Geballe, Selby, Houghton and Han1992; Laude et al., Reference Laude, Godet, Bernard, Gelfi, Duarte and Delmas1995), hepatitis B virus (HBV; Roossinck and Siddiqui, Reference Roossinck and Siddiqui1987), influenza virus (Arrese and Portela, Reference Arrese and Portela1996; Yang and Zhang, Reference Yang and Zhang1999), rabies virus (McNabb and Courtney, Reference McNabb and Courtney1992; Yang and Zhang, Reference Yang and Zhang1999), herpes simplex virus (McNabb and Courtney, Reference McNabb and Courtney1992) and parvovirus (Maroto et al., Reference Maroto, Ramirez and Almendral2000). Since it is well documented that phosphorylation is involved in regulating the activities of proteins at multiple levels, including nucleic acid binding, oligomerization, and nuclear transport, phosphorylation may likewise affect these properties of the PRRSV N protein.
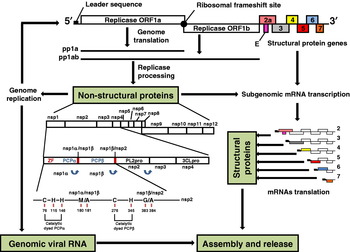
Fig. 2. Schematic representation of the PRRSV genome organization and replication. The replicase 1a and 1ab polyproteins (ppla and pp1ab) are expressed from the genomic viral RNA. The filled circle indicates the ribosomal frameshift site and the filled box indicates the leader sequence. The structural proteins are expressed from a nested set of sg mRNAs, RNAs 2–7, that contain a common 5′ leader sequence. The pp1a is predicted to be cleaved at eight sites to form nine nsps: nsp1α, nsp1β and nsp2 to nsp8. Proteolytic cleavage of the ORF1b gene portion of the pp1ab generates products nsp9 through nsp12. The processing of pp1a and pp1ab is mediated by accessory proteinases located in nsp1 (papain-like cysteine proteinases: PCPα and PCPβ) and nsp2 (chymotrypsin-like cysteine protease: PL2pro); and the main serine proteinase located in nsp4 (the serine/3C-like proteinase: 3CLpro). PCPα and PCPβ are responsible for the autoproteolytic release of nsp1α and nsp1β, respectively. In addition, nsp1 contains a zinc-finger motif (ZF) required for sg mRNA transcription. Predicted catalytic dyads of both PCP domains and the estimated positions of their respective nsp1α/nsp1β and nsp1β/nsp2 cleavage site are illustrated.

Fig. 3. PRRSV N protein schematic model of the NA isolates. The numbers identify the aa positions covered by each domain. I–V: five domains of antigenic importance (30–52, 37–52, 52–69, 69–112 and 112–123); 23-Cys: identifies the location of the conserved cysteine residue involved in the intermolecular disulphide bonds N protein homodimer formation; 120-Ser: identifies the location of a serine residue that is a phosphorylation target; NLS-1: nuclear localization signal −1 or cryptic NLS; NLS-2: nuclear localisation signal-2 or functional NLS; NoLS: nuclear localization signal sequence. This figure is adapted from Rowland and Yoo (Reference Rowland and Yoo2003).
Table 1. PRRSV structural and non-structural proteins characteristics and functions

ORF, open reading frames; EU, European PRRSV strains; NA, North American PRRSV strains.
The N protein is highly immunogenic in pigs (Meulenberg et al., Reference Meulenberg, Petersen-den Besten, De Kluyver, Moormann, Schaaper and Wensvoort1995a, Reference Meulenberg, Petersen-den Besten, de Kluyver, Moormann, Schaaper and Wensvoortb; Loemba et al., Reference Loemba, Mounir, Mardassi, Archambault and Dea1996) and in mice (Nelson et al., Reference Nelson, Christopher-Hennings, Drew, Wensvoort, Collins and Benfield1993; Drew et al., Reference Drew, Meulenberg, Sands and Paton1995; van Nieuwstadt et al., Reference van Nieuwstadt, Meulenberg, van Essen-Zanbergen, Petersen-den Besten, Bende, Moormann and Wensvoort1996; Rodriguez et al., Reference Rodriguez, Sarraseca, Garcia, Sanz, Plana-Duran and Ignacio Casal1997). Several groups have generated anti-N mAbs that recognize epitopes specific to or shared by NA and EU isolates (Nelson et al., Reference Nelson, Christopher-Hennings, Drew, Wensvoort, Collins and Benfield1993; Drew et al., Reference Drew, Meulenberg, Sands and Paton1995; Dea et al., Reference Dea, Gagnon, Mardassi and Milane1996; Meulenberg et al., Reference Meulenberg, van Nieuwstadt, van Essen-Zandbergen, Bos-de Ruijter, Langeveld and Meloen1998; Wootton et al., Reference Wootton, Nelson and Yoo1998). Based on the immunoreactivity of N protein deletion mutants with panels of N-specific mAbs, five domains of antigenic importance have been identified for a reference NA strain, four of them being localized at aa 30 to 52, 37 to 52, 69 to 112 and 112 to 123 (Fig. 3). Other mAbs revealed the presence of a common conformational antigenic site localized in the central region (aa 52–69) of the protein (Rodriguez et al., Reference Rodriguez, Sarraseca, Garcia, Sanz, Plana-Duran and Ignacio Casal1997; Wootton et al., Reference Wootton, Nelson and Yoo1998). Mutational analysis revealed that the C-terminus of N plays a critical role in the formation of the conformational epitopes, and a domain comprised of the 11 C-terminal-most aa (residues 112 to 123) (Fig. 3) was shown to be essential for binding of N-specific conformation-dependent mAbs (Meulenberg et al., Reference Meulenberg, van Nieuwstadt, van Essen-Zandbergen, Bos-de Ruijter, Langeveld and Meloen1998; Wootton et al., Reference Wootton, Nelson and Yoo1998, Reference Wootton, Koljesar, Yang, Yoon and Yoo2001). The region between aa 37 and 52 is well conserved among isolates of both genotypes and is the most hydrophilic region of the protein (Fig. 3).
None of the N-specific mAbs have been associated with virus neutralization. Four distinct antigenic domains were identified for the reference EU LV strain (Meulenberg et al., Reference Meulenberg, van Nieuwstadt, van Essen-Zandbergen, Bos-de Ruijter, Langeveld and Meloen1998). Three sites, designated A–C, contain linear epitopes, and these were mapped between aa 2–12, 25–30 and 40–46, respectively. However, the fourth region, designated domain D, contains conformation-dependent or discontinuous epitopes that are (partially) composed of aa 51–67 and 80–90 (Meulenberg et al., Reference Meulenberg, van Nieuwstadt, van Essen-Zandbergen, Bos-de Ruijter, Langeveld and Meloen1998). The early immunological response generated in PRRSV-infected pigs is directed mainly against the N protein, and this response, which can be detected as early as 1 week post-infection (Loemba et al., Reference Loemba, Mounir, Mardassi, Archambault and Dea1996), declines at a much lower rate than the one directed against the major structural proteins M and GP5 (Yoon et al., Reference Yoon, Zimmerman, Swenson, McGinley, Eernisse, Brevik, Rhinehart, Frey, Hill and Platt1995). Since the majority of Abs produced during PRRSV infection in pigs are directed against the N protein, for which major antigenic determinants are highly conserved, the N protein has been targeted as a suitable candidate for the detection of virus-specific Abs and diagnosis of the disease.
One of the fundamental roles of the viral capsid protein is to provide a protective enclosure for the viral genome during the extracellular phase of the virus life cycle. The N protein is the sole component of the viral capsid and interacts with itself through covalent and non-covalent interactions (Wootton and Yoo, Reference Wootton and Yoo2003). NA PRRSV N protein contains three highly conserved cysteine residues at aa positions 23, 75 and 90. Systematic mutation of these three cysteine residues, either alone or in combination, revealed that the cysteine at position 23 (Fig. 3) is involved in the formation of intermolecular disulphide bonds between N proteins (Wootton and Yoo, Reference Wootton and Yoo2003). Subsequently, the functional significance of the N cysteine residues for PRRSV infectivity was assessed using an infectious cDNA clone (Lee et al., Reference Lee, Calvert, Welch and Yoo2005). Each cysteine at positions 23, 75 and 90 was replaced with serine and the individual mutation was incorporated into the cDNA clone such that three independent cysteine mutants were constructed. When transfected, the wild-type (wt) and C75S clones induced cytopathic effects and produced infectious virus with indistinguishable plaque morphology. In contrast, the C23S mutation completely abolished infectivity of the clone, indicating that C23- mediated N protein homodimerization plays a critical role in PRRSV infectivity (Lee et al., Reference Lee, Calvert, Welch and Yoo2005). The C90S mutation also appeared to be lethal for virus infectivity. This is an unexpected observation since cysteine 90 is irrelevant to the N protein homodimerization and because cysteine 90 is absent from the EU PRRSV isolates. The EU isolates of PRRSV contain only two cysteines at positions 27 (involved in the formation of intermolecular disulphide bonds between N proteins) and 76. The N protein dimer (Fig. 1) was stable in the presence and absence of intermolecular disulphide linkages, indicating that non-covalent interactions also play a role in dimerization.
Using a series of N protein deletion mutants fused to glutathione S-transferase (GST) in a pull-down assay, aa 30 to 37 were shown to be essential for non-covalent N–N interactions (Fig. 3). Furthermore, since RNase A treatment markedly decreased N protein-binding affinity, it appears that at least in vitro, RNA may be involved in bridging N–N interactions. Upon translation in the cytoplasm, the N protein interacts with itself via non-covalent binding. As N is transported to the lumen of the endoplasmic reticulum (ER) and the Golgi complex, where its environment is oxidative and PRRSV maturation occurs, the N–N interaction becomes disulphide linked (Snijder and Meulenberg, Reference Snijder and Meulenberg1998; Wootton and Yoo, Reference Wootton and Yoo2003). Together, these findings demonstrate that the N protein possesses self-associative properties, and these likely provide the basis for PRRSV nucleocapsid assembly.
PRRSV, like several other RNA viruses, replicates in the cytoplasm (Benfield et al., Reference Benfield, Nelson, Collins, Harris, Goyal, Robison, Christianson, Morrison, Gorcyca and Chladek1992; Mardassi et al., Reference Mardassi, Wilson, Mounir and Dea1994b). However, a fraction of the N protein localizes in the nucleus and nucleolus during infection of PAM and MARC-145 cells (Rowland et al., Reference Rowland, Kervin, Kuckleburg, Sperlich and Benfield1999). Two conserved aa stretches, which are similar to sequences that resemble two classical types of nuclear localization signal (NLS), have been identified (Fig. 3) in the N protein at aa positions 10 to 13 and 41 to 47 (NLS-1 or cryptic NLS, and NLS-2 or functional NLS, respectively) (Rowland et al., Reference Rowland, Kervin, Kuckleburg, Sperlich and Benfield1999, Reference Rowland, Schneider, Fang, Wootton, Yoo and Benfield2003). Inactivation of NLS-1 by site-directed mutagenesis or deletion of the first 14 aa did not affect N protein localization to the nucleolus. The substitution of key lysine residues with uncharged aa in NLS-2 blocked nuclear/nucleolar localization. Site-directed mutagenesis identified the KKNKK sequence as forming the core localization domain within NLS-2. The NLS-2 was functional and sufficient for the translocation and accumulation of N in the nucleolus (Rowland et al., Reference Rowland, Kervin, Kuckleburg, Sperlich and Benfield1999, Reference Rowland, Schneider, Fang, Wootton, Yoo and Benfield2003; Rowland and Yoo, Reference Rowland and Yoo2003). Proteins that localize to the nucleolus typically possess a nucleolar localization signal (NoLS) motif. A region covering aa 41–72 (Fig. 3) was found to contain a NoLS sequence (Rowland et al., Reference Rowland, Schneider, Fang, Wootton, Yoo and Benfield2003; Yoo et al., Reference Yoo, Wootton, Li, Song and Rowland2003). The nucleolar localization of the N protein has also been reported for EAV (Tijms et al., Reference Tijms, van der Meer and Snijder2002) and for several members of the Coronaviridae (Wurm et al., Reference Wurm, Chen, Hodgson, Britton, Brooks and Hiscox2001; Chen et al., Reference Chen, Wurm, Britton, Brooks and Hiscox2002). The arterivirus and coronavirus N proteins, when expressed alone or fused to the red-shifted Enhanced green fluorescent protein (EGFP), localize to the nucleolus, demonstrating that translocation across the nuclear pore complex (NPC) and accumulation in the nucleolus are independent of other viral proteins (Hiscox et al., Reference Hiscox, Wurm, Wilson, Britton, Cavanagh and Brooks2001; Wurm et al., Reference Wurm, Chen, Hodgson, Britton, Brooks and Hiscox2001; Tijms et al., Reference Tijms, van der Meer and Snijder2002; Rowland and Yoo, Reference Rowland and Yoo2003).
Since the Golgi apparatus is thought to be the maturation site of arteriviruses, it has been postulated that the PRRSV N protein plays dual roles during virus infection; a virion structural role in the cytoplasm and a non-structural role in the nucleus and/or nucleolus. N protein NLS-null PRRSV-infected pigs had a significantly shorter mean duration of viremia than wt-infected pigs and increased production of neutralizing Abs in infected pigs (Lee et al., Reference Lee, Hodgins, Calvert, Welch, Jolie and Yoo2006a, Reference Lee, Hodgins, Calvert, Welch, Jolie and Yoob). However, MARC-145 cells infected with the NLS-null mutant virus produced cytopathology indistinguishable from that of wt virus, but with lower viral titers and smaller sized plaques (Lee et al., Reference Lee, Hodgins, Calvert, Welch, Jolie and Yoo2006a, Reference Lee, Hodgins, Calvert, Welch, Jolie and Yoob). Furthermore, N protein nuclear localization is associated with virulence of PRRSV since modification in the NLS causes attenuation of the virus (Lee et al., Reference Lee, Hodgins, Calvert, Welch, Jolie and Yoo2006a, Reference Lee, Hodgins, Calvert, Welch, Jolie and Yoob; Pei et al., Reference Pei, Hodgins, Lee, Calvert, Welch, Jolie, Keith and Yoo2008) suggesting that N protein localization in the nucleus may play a role in viral pathogenesis. The precise mechanism of N protein trafficking within a cell and to the nucleolus is unknown, although N protein has been shown to interact with the small nucleolar RNA (snoRNA)-associated protein fibrillarin and thus may potentially localize to the nucleolus via this interaction (Yoo et al., Reference Yoo, Wootton, Li, Song and Rowland2003). Using an in vitro pull-down assay, the N protein was able to bind importin-α and importin-β nuclear transport proteins (Rowland and Yoo, Reference Rowland and Yoo2003). The formation of an N protein/importin complex using in vitro translated N demonstrates that N protein binding is independent of other viral proteins.
The GST-bead pull-down assay was used to determine if the principal export shuttle protein, the chromosome region maintenance 1 (CRM1), could bind N protein (Rowland and Yoo, Reference Rowland and Yoo2003). Radiolabeled N protein, prepared from virus infected cells or by in vitro translation, was incubated with the GST-human CRM1 fusion protein immobilized on glutathione–Sepharose beads. The results showed that CRM1 indeed bound to PRRSV N protein (Rowland and Yoo, Reference Rowland and Yoo2003). By contrast, Tijms et al. (Reference Tijms, van der Meer and Snijder2002) reported that EAV N protein localized to all nucleoli when cells were treated with leptomycin B (LMB), an inhibitor of CRM1-dependent export. In conclusion, the N protein nuclear transport is importin-α/β-based and CRM1-dependent. It is interesting that a virion structural protein from a cytoplasm-replicating RNA virus interacts with a host cell transcription factor. It was shown previously that the PRRSV N protein can interact with fibrillarin and co-localizes with fibrillarin in the nucleolus (Yoo et al., Reference Yoo, Wootton, Li, Song and Rowland2003). For NA N protein, 8 aa (IAQQNQSR) at positions 30 to 37 were identified as the binding domain with fibrillarin. Interestingly, this string of aa was previously mapped as the region that participated in the formation of the N–N interaction (Wootton and Yoo, Reference Wootton and Yoo2003). This region corresponds to a relatively hydrophilic region of N and forms part of the conformational epitope recognized by mAb SDOW17 (Wootton et al., Reference Wootton, Koljesar, Yang, Yoon and Yoo2001). The interaction of viral capsid with the cellular transcription factor implicates a possible regulation of host cell gene expression by the N protein during PRRSV infection (Song et al., Reference Song, Lu, Bienzle, Liu and Yoo2009). Localization of the virus capsid proteins and replication proteins in the nucleus and/or nucleolus appears to be an emerging feature of positive strand RNA viruses (Song et al., Reference Song, Lu, Bienzle, Liu and Yoo2009). The reason why positive strand RNA virus capsid proteins localize in the nucleolus is unknown, and several hypotheses have been proposed including: (i) as part of a cellular defense mechanism to sequester viral proteins away from sites of virus replication and assembly; (ii) to recruit nucleolar proteins to facilitate virus replication; (iii) to usurp cellular processes by disrupting the nucleolar proteome; or (iv) only because the viral protein contains motifs which mimic NoLSs (Hiscox, Reference Hiscox2002, Reference Hiscox2003, Reference Hiscox2007; Rowland and Yoo, Reference Rowland and Yoo2003; Weidman et al., Reference Weidman, Sharma, Raychaudhuri, Kundu, Tsai and Dasgupta2003; Uchil et al., Reference Uchil, Kumar and Satchidanandam2006).
To summarize, the N protein is the sole component of the PRRSV capsid and is a highly immunogenic protein which makes it a suitable candidate for the detection of virus-specific Abs and diagnosis of disease. The PRRSV N protein plays dual roles during virus infection; a virion structural function and a non-structural role in the nucleus/nucleolus, indicating that N protein localization in these compartments may play a fundamental role in viral pathogenesis such as in the regulation of cell genes expression. Further studies need to be conducted to elucidate the role of the N protein in the nucleus/nucleolus and may provide new interesting insights on cell pathogenesis of PRRSV.
The membrane envelope protein (M)
The M protein is an 18 to 19 kDa class III membrane protein composed of 174 and 173 aa for NA and EU genotypes, respectively, and is encoded by ORF6 viral gene (Fig. 2). Its membrane structure consists of a core of three successive membrane-spanning domains preceded by an ectodomain of 13–18 aa and followed by a C-terminal endodomain of 81–87 aa (de Vries et al., Reference de Vries, Chirnside, Horzinek and Rottier1992; Faaberg and Plagemann, Reference Faaberg and Plagemann1995; Meulenberg et al., Reference Meulenberg, Petersen-den Besten, De Kluyver, Moormann, Schaaper and Wensvoort1995a, Reference Meulenberg, Hulst, de Meijer, Moonen, den Besten, de Kluyver, Wensvoort and Moormannb). As demonstrated experimentally for the closely related M protein of coronaviruses (Vennema et al., Reference Vennema, Godeke, Rossen, Voorhout, Horzinek, Opstelten and Rottier1996; de Haan et al., Reference de Haan, Kuo, Masters, Vennema and Rottier1998), the arteriviral M protein is likely to play a key role in virus assembly and budding (Table 1). The M protein is unglycosylated and is the most conserved structural protein of arteriviruses and PRRSV (Meulenberg et al., Reference Meulenberg, Hulst, de Meijer, Moonen, den Besten, de Kluyver, Wensvoort and Moormann1993b; Mardassi et al., Reference Mardassi, Mounir and Dea1995a, Reference Mardassi, Mounir and Deab). In the virion, the GP5 and M proteins are found as a disulphide-linked heterodimer (Fig. 1), which is essential for virus infectivity for arteriviruses (Faaberg et al., Reference Faaberg, Even, Palmer and Plagemann1995a; Mardassi et al., Reference Mardassi, Massie and Dea1996; Delputte et al., Reference Delputte, Vanderheijden, Nauwynck and Pensaert2002; Snijder et al., Reference Snijder, Dobbe and Spaan2003). These disulphide-linked complexes are formed in the ER of infected cells preceding or during virus assembly (de Vries et al., Reference de Vries, Post, Raamsman, Horzinek and Rottier1995; Faaberg et al., Reference Faaberg, Even, Palmer and Plagemann1995a; Mardassi et al., Reference Mardassi, Massie and Dea1996; Dobbe et al., Reference Dobbe, van der Meer, Spaan and Snijder2001).
The GP5–M heterodimerization is essential for virus infectivity and is a key step in the assembly of EAV virion (Snijder et al., Reference Snijder, Dobbe and Spaan2003). The disulphide bond between PRRSV GP5 and M proteins presumably occurs between cysteine residues at positions 50 and 8 (EU isolates), respectively (Verheije et al., Reference Verheije, Welting, Jansen, Rottier and Meulenberg2002). Mutating either one of these residues resulted in a complete block of particle production (Verheije et al., Reference Verheije, Welting, Jansen, Rottier and Meulenberg2002), indicating that the covalent association of GP5 and M is crucial for virus assembly. Using a reverse genetics approach it was shown that the two major envelope proteins, the GP5 and M proteins are essential for the formation of EAV particles (Wieringa et al., Reference Wieringa, de Vries, van der Meulen, Godeke, Onderwater, van Tol, Koerten, Mommaas, Snijder and Rottier2004). Noteworthy, the disulphide-linked ectodomains of the LDV heterodimer VP-3P-M protein (VP-3P is the GP5 homolog protein of LDV) have been proposed to stabilize the virus attachment site for interaction with a host cell receptor (Faaberg et al., Reference Faaberg, Palmer, Even, Anderson and Plagemann1995b).
The development of neutralizing Abs was reported in mice inoculated with a Mycobacterium bovis bacille Calmette–Guérin (BCG) expressing the M protein (Bastos et al., Reference Bastos, Dellagostin, Barletta, Doster, Nelson and Osorio2002). More recently, three replication-defective recombinant adenoviruses were developed as potential vaccines against PRRSV and evaluated in a mouse model (Jiang et al., Reference Jiang, Jiang, Li, Tang, Wang and Ma2006a). Three groups of BALB/c mice were inoculated subcutaneously twice at 2-week intervals with the recombinant adenovirus expressing PRRSV GP5 (rAd-GP5), M (rAd-M), or M–GP5 fusion protein (rAd-M–GP5). The results showed that the mice inoculated with recombinant adenoviruses developed PRRSV-specific Abs and a cellular immune response 2 weeks after the second inoculation (Jiang et al., Reference Jiang, Jiang, Li, Tang, Wang and Ma2006a). However, only mice immunized with rAd-M–GP5 developed significantly higher titers of neutralizing Abs to PRRSV and produced stronger lymphocyte proliferation responses compared to mice immunized with rAd-M or rAd-GP5 (Jiang et al., Reference Jiang, Jiang, Li, Tang, Wang and Ma2006a). In conclusion, the presence of M protein increases the immune response against GP5 by increasing the cellular immune response and the production of neutralizing Abs.
Using Pepscan technology and sera of 15 experimentally NA PRRSV infected pigs, the B-cell linear epitopes were identified in some of the nsps and all of the structural proteins (de Lima et al., Reference de Lima, Pattnaik, Flores and Osorio2006). The highest degree of immunogenicity and conservation was exhibited by two epitopes in the C-terminal end of the M protein (ORF6). These immunoreactive peptides are located at position 151–174 which corresponds to the C-terminus end of the endodomain of the M protein. In order to confirm the results shown by sequence alignment, the reactivity of both synthetic peptides was further tested with sera from pigs experimentally infected with several other NA PRRSV strains and all serum samples were confirmed positive. It was found that the peptide containing the residues A161VKQGVVNLVKYAK174 can be particularly useful for diagnostic purposes and is an attractive candidate as a negative serological marker in a PRRSV vaccine (de Lima et al., Reference de Lima, Pattnaik, Flores and Osorio2006). An earlier study (Oleksiewicz et al., Reference Oleksiewicz, Botner and Normann2002), using phage-display technology, also reported the identification of one epitope localized in the large putative endodomain of the M protein (aa 138–159) of an EU PRRSV strain that was recognized by sera collected very late in infection.
The major glycosylated envelope viral protein (GP5)
One of the most variable regions of the PRRSV genome is the ORF5 gene (Fig. 2), which encodes the GP5 glycoprotein (Mardassi et al., Reference Mardassi, Mounir and Dea1995a; Meng et al., Reference Meng, Paul, Halbur and Morozov1995b; Andreyev et al., Reference Andreyev, Wesley, Mengeling, Vorwald and Lager1997). The GP5 is a transmembrane (TM) protein of approximately 25 kDa (Meulenberg et al., Reference Meulenberg, Petersen-den Besten, De Kluyver, Moormann, Schaaper and Wensvoort1995a; Dea et al., Reference Dea, Gagnon, Mardassi, Pirzadeh and Rogan2000a). The GP5 protein contains an approximately 30 aa N-terminal putative signal sequence which is assumed to be cleaved (Mardassi et al., Reference Mardassi, Mounir and Dea1995a; Meng et al., Reference Meng, Paul, Halbur and Morozov1995b; Meulenberg et al., Reference Meulenberg, Petersen-den Besten, De Kluyver, Moormann, Schaaper and Wensvoort1995a; Murtaugh et al., Reference Murtaugh, Elam and Kakach1995), followed by an ectodomain of approximately 35 residues with a variable number of potential N-glycosylation sites, a long hydrophobic region of about 60 residues that is presumed to span the membrane one to three times, and a hydrophilic C-terminus of approximately 70 aa (Meulenberg et al., Reference Meulenberg, Petersen-den Besten, De Kluyver, Moormann, Schaaper and Wensvoort1995a). Some authors have suggested that it possesses two membrane-spanning motifs between residues 65–130 and 170–190 (Dea et al., Reference Dea, Gagnon, Mardassi, Pirzadeh and Rogan2000a), while others have proposed that the protein may span the membrane three times (Meulenberg, Reference Meulenberg2000). GP5 is one of the most variable (Table 1) structural proteins in the PRRSV genome (Mardassi et al., Reference Mardassi, Mounir and Dea1995a; Meng et al., Reference Meng, Paul, Halbur and Morozov1995b) with the highest degree of diversity within one genotype: 89–94% identity among NA isolates and 87.1–99.25% identity among EU isolates (Suarez et al., Reference Suarez, Zardoya, Martin, Prieto, Dopazo, Solana and Castro1996b; Andreyev et al., Reference Andreyev, Wesley, Mengeling, Vorwald and Lager1997). It is noteworthy that other parts of the genome also possess considerable degree of variability, such as ORF3 gene and the nsp2 protein (Oleksiewicz et al., Reference Oleksiewicz, Botner, Toft, Grubbe, Nielsen, Kamstrup and Storgaard2000; Fang et al., Reference Fang, Kim, Ropp, Steen, Christopher-Hennings, Nelson and Rowland2004). Interestingly, it was hypothesized that PRRSV exists during natural infection as a quasi-species distribution of related genotypes and that this variation could account for the inability of traditional approaches such as vaccination to control PRRS adequately. It was also found that multiple variants of PRRSV can exist simultaneously on farms and within individual animals during natural infection (Goldberg et al., Reference Goldberg, Lowe, Milburn and Firkins2003). Nonetheless, the existence of quasi-species could be responsible for the emergence of viruses with novel clinical and/or antigenic properties and reduce the efficiency of vaccination.
The GP5 protein of PRRSV is one of the most important structural proteins (Table 1) exposed on the surface of the virion and contains epitopes involved in virus neutralization and protection (Pirzadeh and Dea, Reference Pirzadeh and Dea1997, Reference Pirzadeh and Dea1998; Gonin et al., Reference Gonin, Pirzadeh, Gagnon and Dea1999; Wissink et al., Reference Wissink, van Wijk, Kroese, Weiland, Meulenberg, Rottier and van Rijn2003; Ansari et al., Reference Ansari, Kwon, Osorio and Pattnaik2006). mAbs against GP5 are able to neutralize virus infectivity in cell culture (Pirzadeh and Dea, Reference Pirzadeh and Dea1997; Zhang et al., Reference Zhang, Sharma and Paul1998; Gonin et al., Reference Gonin, Pirzadeh, Gagnon and Dea1999; Weiland et al., Reference Weiland, Wieczorek-Krohmer, Kohl, Conzelmann and Weiland1999). In vivo, most of the neutralizing Abs in infected animals are predominantly directed against GP5 (Gonin et al., Reference Gonin, Pirzadeh, Gagnon and Dea1999). Pigs vaccinated with genetically engineered vaccines that express the GP5 protein produced neutralizing Abs against PRRSV (Pirzadeh and Dea, Reference Pirzadeh and Dea1998; Kheyar et al., Reference Kheyar, Jabrane, Zhu, Cleroux, Massie, Dea and Gagnon2005; Jiang et al., Reference Jiang, Xiao, Fang, Yu, Song, Niu and Chen2006b) and the pigs could be partially protected against generalized viremia and lung lesions development following PRRSV challenge (Pirzadeh and Dea, Reference Pirzadeh and Dea1998; Xue et al., Reference Xue, Zhao, Zhou, Qiu, Wang, Wu, Tian and Tong2004; Qiu et al., Reference Qiu, Tian, Tong, Zhou, Ni, Luo and Cai2005). Pregnant gilts treated with hyperimmune serum from convalescent pigs are fully protected from reproductive failure upon homologous challenge which suggests that IgG can provide protective immunity in vivo (Osorio et al., Reference Osorio, Galeota, Nelson, Brodersen, Doster, Wills, Zuckermann and Laegreid2002). On the other hand, others have shown that GP5 Abs alone do not provide full protection against PRRS disease even after homologous challenge (Plana-Duran et al., Reference Plana-Duran, Bastons, Urniza, Vayreda, Vila and Mane1997a; Pirzadeh and Dea, Reference Pirzadeh and Dea1998). Consequently, GP5 is not the only determinant of protective immunity, and is particularly unlikely to be the determinant of cross protection in the case of heterologous challenge since it is highly variable.
Hydropathy profiles of the GP5 protein predict that it possesses a signal sequence (aa 1–32), an ectodomain (aa 33–63), a TM region (aa 64–134) and an endodomain (aa 135–200), although there are some differences in the length of the predicted signal sequence between the NA and EU strains of PRRSV (Fig. 4) (Meng et al., Reference Meng, Paul, Halbur and Morozov1995b; Meulenberg et al., Reference Meulenberg, Petersen-den Besten, De Kluyver, Moormann, Schaaper and Wensvoort1995a; Andreyev et al., Reference Andreyev, Wesley, Mengeling, Vorwald and Lager1997; Yang et al., Reference Yang, Kwang and Laegreid1998; Dea et al., Reference Dea, Gagnon, Mardassi, Pirzadeh and Rogan2000a; Stadejek et al., Reference Stadejek, Stankevicius, Storgaard, Oleksiewicz, Belak, Drew and Pejsak2002). Comparison of the nt and aa sequences of the GP5 protein of a large number of PRRSV strains from NA and EU strains identified a hypervariable region (aa 32–40), two variable regions (aa 57–70 and 121–130), three conserved regions (aa 41–56, 71–120 and 131–200) and multiple potential N-glycosylation sites (Faaberg and Plagemann, Reference Faaberg and Plagemann1995; Meng et al., Reference Meng, Paul, Halbur and Morozov1995b; Plagemann, Reference Plagemann, Fields, Knipe and Howley1996, Reference Plagemann2004; Andreyev et al., Reference Andreyev, Wesley, Mengeling, Vorwald and Lager1997; Yang et al., Reference Yang, Kwang and Laegreid1998; Key et al., Reference Key, Haqshenas, Guenette, Swenson, Toth and Meng2001; Stadejek et al., Reference Stadejek, Stankevicius, Storgaard, Oleksiewicz, Belak, Drew and Pejsak2002).

Fig. 4. Functional and structural domains of the PRRSV GP5 protein of NA isolates. The numbers identify the aa positions covered by each domain. Conserved (black) and non-conserved (gray) glycolysation sites: N30, N34, N44 and N51; the conserved cysteine residue involved in formation of the disulphide bond between GP5 and M is illustrated: 48-Cys; signal sequence: 1–32; ectodomain: 33–63; transmembrane region: 64–134; endodomain: 135–200; major neutralizing epitope: 36–52; hypervariable immunodominant non-neutralizing epitope A (decoy epitope): 27–30; conserved non-immunodominant neutralizing epitope B: 37–45; variable regions 57–70 and 121–130; conserved regions: 41–56, 71–120 and 131–200.
The first potential N-glycosylation site is located in the hypervariable region (N32 or N33 or N34) of the GP5 ectodomain, and it is present in many but not all NA and EU strains of PRRSV (Fig. 4). The second and third potential N-glycosylation sites are located at either aa residues N44 and N51 (NA strains of PRRSV) or N46 and N53 (EU strains of PRRSV), and are highly conserved among field strains of PRRSV. In addition to these three N-glycosylation sites some NA isolates (U.S. ATCC VR-2332 and Quebec IAF-Klop reference strains) have a fourth potential N-glycosylation site at aa residue N30 (Dea et al., Reference Dea, Gagnon, Mardassi, Pirzadeh and Rogan2000a; Key et al., Reference Key, Haqshenas, Guenette, Swenson, Toth and Meng2001; Plagemann, Reference Plagemann2004).
Using peptide mapping it was shown that the major neutralization epitope of PRRSV is located in the middle of the GP5 ectodomain (aa 36–52) (Plagemann et al., Reference Plagemann, Rowland and Faaberg2002). Using swine polyclonal antisera and mouse mAbs, the non-neutralizing epitope (epitope A) and the neutralizing epitope (epitope B) in the ectodomain of the GP5 protein of PRRSV were identified (Ostrowski et al., Reference Ostrowski, Galeota, Jar, Platt, Osorio and Lopez2002). Epitope B was recognized by a neutralizing mAb and high SN-titered swine antisera. Neutralization epitope B includes aa 37–45 of GP5 and is conserved among PRRSV isolates, but it is not immunodominant (Fig. 4). In contrast, the non-neutralizing epitope A includes aa residues 27–30, and is hypervarible and immunodominant (Fig. 4). Interestingly, PRRSV-infected pigs first develop Abs against epitope A and neutralizing Abs against epitope B only appear later. Thus, the immunodominant epitope A may act as a decoy and so delay the neutralizing Ab response against epitope B, although the true significance of this potential mechanism of immune evasion of field strains of PRRSV remains uncertain (Plagemann, Reference Plagemann2004). The presence of N-glycans in and around epitope B may also reduce the immunogenicity of this critical neutralization determinant in the GP5 protein, consistent with the low immunogenicity of PRRSV virions in mice and their failure to develop strong neutralizing Ab response to the virus. Using site-directed mutagenesis and pepscan analysis it was shown that the aa residue at position 24 of the GP5 signal sequence of an EU strain of PRRSV was critical for neutralizing mAb recognition; specifically, the presence of a proline residue at position 24 promotes cleavage of the signal peptide at residues 28 and 29 rather than 32 and 33 (Wissink et al., Reference Wissink, van Wijk, Kroese, Weiland, Meulenberg, Rottier and van Rijn2003). The authors also demonstrated a second neutralization epitope recognized by mAbs that includes aa 29–35 of GP5. These data suggest that the major neutralization determinants of PRRSV are located in the N-terminal portion of the GP5 protein, and that inherent properties of the protein such as ‘decoy’ epitopes and heterogeneous glycosylation that obscures the critical neutralization site(s) can impede and decrease the humoral immune response against PRRSV GP5.
Several studies provide evidence that glycosylation of GP5 of PRRSV plays an important role in escaping or minimizing virus-neutralizing Ab response by a N-glycan-shielding mechanism (Johnson et al., Reference Johnson, Lifson, Lang, Johnson and Desrosiers2003; Wei et al., Reference Wei, Decker, Wang, Hui, Kappes, Wu, Salazar-Gonzalez, Salazar, Kilby, Saag, Komarova, Nowak, Hahn, Kwong and Shaw2003). This N-glycan neutralization shielding is also observed in LDV (Chen et al., Reference Chen, Li and Plagemann2000) and other viruses such as HBV (Lee et al., Reference Lee, Chen, Lu, Tung, Chou, Wang, Chen, Hung, Huang and Chen2003), simian immunodeficiency virus (Reitter and Desrosiers, Reference Reitter and Desrosiers1998), influenza virus (Skehel et al., Reference Skehel, Stevens, Daniels, Douglas, Knossow, Wilson and Wiley1984) and human immunodeficiency virus (HIV) (Wei et al., Reference Wei, Decker, Wang, Hui, Kappes, Wu, Salazar-Gonzalez, Salazar, Kilby, Saag, Komarova, Nowak, Hahn, Kwong and Shaw2003). Biochemical studies indicate that the mature GP5 contains high-mannose-type sugar moieties at all three N-glycosylation sites (N34, N44 and N51) (Fig. 4). The mutation involving residue N44 did not result in infectious progeny production, indicating that N44 is the most critical aa residue for infectivity. Viruses carrying mutations at N34, N51 and N34/51 grew to lower titers than the wt PRRSV (Ansari et al., Reference Ansari, Kwon, Osorio and Pattnaik2006). All mutated viruses exhibited enhanced sensitivity to neutralization by swine sera. Furthermore, inoculation of pigs with the mutant viruses induced significantly higher levels of neutralizing Abs against the mutants as well as the wt PRRSV, suggesting that the loss of N-glycan residues in the ectodomain of GP5 enhances both the sensitivity of these viruses to in vitro neutralization and the immunogenicity of the nearby neutralization epitope (Ansari et al., Reference Ansari, Kwon, Osorio and Pattnaik2006). Similar results have been obtained with GP5 of the EU reference strain LV and demonstrate that the N-glycan moiety of the N46 glycosylation site (which corresponds to the N44 in the NA strains) is required for virus particle production (Wissink et al., Reference Wissink, Kroese, Maneschijn-Bonsing, Meulenberg, van Rijn, Rijsewijk and Rottier2004). Interestingly, the authors also found that the N-linked glycans normally located at N53 of GP5 (which corresponds to N51 for NA strains) and present on the GP2a protein are not essential for particle formation. Furthermore, the lack of N-linked oligosaccharides on GP2a and at N53 of GP5 did not significantly affect the infectivity of the viruses compared to the GP5 N46 mutant (Wissink et al., Reference Wissink, Kroese, Maneschijn-Bonsing, Meulenberg, van Rijn, Rijsewijk and Rottier2004). In conclusion, preventing glycosylation of the GP5 protein at position N44 and N46, respectively, for NA and EU strains, had dramatic effects on virion production and virus infectivity (Wissink et al., Reference Wissink, Kroese, Maneschijn-Bonsing, Meulenberg, van Rijn, Rijsewijk and Rottier2004). The N-linked glycosylation, in general, is important for correct folding, targeting and biological activity of proteins (Helenius, Reference Helenius1994; Helenius and Aebi, Reference Helenius and Aebi2001, Reference Helenius and Aebi2004).
In many enveloped viruses, the envelope proteins are modified by the addition of sugar moieties and the N-linked glycosylation of envelope protein plays diverse functions such as receptor binding, membrane fusion, penetration into cells and virus budding (Doms et al., Reference Doms, Lamb, Rose and Helenius1993; Braakman and van Anken, Reference Braakman and van Anken2000). Furthermore, it has become evident that glycosylation of viral envelope proteins is a major mechanism for viral immune evasion and virus persistence which has been used by several enveloped viruses to escape, block, or minimize the virus-neutralizing Ab response. Examples of this effect have now been reported for PRRSV and several other viruses such as arterivirus LDV (Chen et al., Reference Chen, Li and Plagemann2000), simian immunodeficiency virus (Reitter and Desrosiers, Reference Reitter and Desrosiers1998), HBV (Lee et al., Reference Lee, Chen, Lu, Tung, Chou, Wang, Chen, Hung, Huang and Chen2003) and influenza virus (Skehel et al., Reference Skehel, Stevens, Daniels, Douglas, Knossow, Wilson and Wiley1984). In addition to the GP5 N-glycan neutralization shielding, the GP5 seems to be involved in a phenomenon, named Ab-dependent enhancement (ADE), which favors the infection of macrophages (Cancel-Tirado et al., Reference Cancel-Tirado, Evans and Yoon2004). ADE facilitates the attachment and internalization of several viruses into macrophages and monocytes, through Fc receptor mediated endocytosis using specific Abs (Cancel-Tirado and Yoon, Reference Cancel-Tirado and Yoon2003). The PRRSV ADE mechanism has not been extensively studied, nonetheless, some authors have reported that vaccination with E. coli expressed GP5 has increased the histopathological lung lesions in PRRSV infected animals possibly through an ADE mechanism (Pirzadeh and Dea, Reference Pirzadeh and Dea1998).
GP5 forms a heterodimeric complex with the M protein (Fig. 1) via disulphide bound (Mardassi et al., Reference Mardassi, Massie and Dea1996; Snijder et al., Reference Snijder, Dobbe and Spaan2003). The disulphide bond between GP5 and M occurs between Cys50 and Cys8 (EU isolates) of the respective proteins (Verheije et al., Reference Verheije, Welting, Jansen, Rottier and Meulenberg2002). Mutating either one of these residues resulted in a complete block of particle production (Verheije et al., Reference Verheije, Welting, Jansen, Rottier and Meulenberg2002), indicating that the covalent association of GP5 and M is crucial for virus assembly (Verheije et al., Reference Verheije, Welting, Jansen, Rottier and Meulenberg2002). The PRRSV GP5–M complexes were shown to interact with heparin (Delputte et al., Reference Delputte, Vanderheijden, Nauwynck and Pensaert2002), suggesting a role in attachment of virus to alveolar macrophages. Thereafter, it has been proposed that the GP5 is involved in the entry of virus into the host cells, presumably by interacting with the macrophages host cell receptor sialoadhesin (Delputte and Nauwynck, Reference Delputte and Nauwynck2004). The role of GP5 in receptor recognition is supported by the presence of a major neutralization epitope in the N-terminal ectodomain (Ostrowski et al., Reference Ostrowski, Galeota, Jar, Platt, Osorio and Lopez2002), implying a central role for the GP5 ectodomain in the infection process.
The GP5 protein is involved in the apoptosis phenomenon induce by PRRSV (Suarez et al., Reference Suarez, Diaz-Guerra, Prieto, Esteban, Castro, Nieto and Ortin1996a; Gagnon et al., Reference Gagnon, Lachapelle, Langelier, Massie and Dea2003). The apoptosis inducing region of GP5 has been mapped to the first N-terminal 119 aa (Fernandez et al., Reference Fernandez, Suarez, Castro, Tabares and Diaz-Guerra2002). During apoptosis in PRRSV infected MARC-145 cells and in MARC-145 cells expressing the GP5 protein, specific apoptotic enzymes are activated, like caspase-3 which is a hallmark of apoptosis pathways induction (Gagnon et al., Reference Gagnon, Lachapelle, Langelier, Massie and Dea2003).
MARC-145 and HeLa cells were transfected with ORF4 and ORF5 of PRRSV and differential gene expressions were studied using microarray chips embedded with 1718 human-expressed sequence tags (Lee et al., Reference Lee, Bachand, Murtaugh and Yoo2004). Genes associated with protein degradation, protein synthesis and transport and various other biochemical pathways were found to be regulated. In GP5-expressing HeLa cells, actin-related protein 1 (ARP1) homologs A and B were up-regulated (Lee et al., Reference Lee, Bachand, Murtaugh and Yoo2004). These genes encode a subunit of dynactin that binds to both microtubules and cytoplasmic dynein, and is involved in ER-to-Golgi transport (Lees-Miller et al., Reference Lees-Miller, Helfman and Schroer1992). Thus, it is possible that the GP5 may play a role in the intracellular transport of viral and cellular components. The bisphosphoglycerate mutase (BPGM) gene was down-regulated by GP5 by more than six-fold (Lee et al., Reference Lee, Bachand, Murtaugh and Yoo2004). In humans, deficiency of BPGM is associated with anemia (Jacobasch and Rapoport, Reference Jacobasch and Rapoport1996). RASSF2 was also down-regulated by GP5. RASSF2 is a new member of the RASSF1 family and shares the properties of Ras effector/tumor suppressors (Vos et al., Reference Vos, Ellis, Elam, Ulku, Taylor and Clark2003). Similarly, cyclin D3 gene expression was suppressed. D-type cyclins are the key regulators along with cyclin E for cell cycle progression from G1 to S phase. Complexes formed between cyclin D or cyclin E and their kinase partners are involved in phosphorylation of retinoblastoma protein, which ultimately leads to activation of E2F transcription factor and progression to the S phase of the cell cycle. Another study demonstrated a clear reduction of cyclin D3 and cell cycle arrest in G0/G1 phase in cells infected with mouse hepatitis coronavirus, a member of the nidoviruses (Chen and Makino, Reference Chen and Makino2004). In addition, a consistent increase in the expression of cathepsin genes was observed in GP5 expressing cells (Lee et al., Reference Lee, Bachand, Murtaugh and Yoo2004). Cathepsin is involved in protein degradation. Although a specific role of cathepsin during virus replication remains to be determined, the up-regulation of genes encoding proteases may represent a cellular defense against expression of foreign proteins.
In summary, the GP5 protein is one of the most variable proteins of PRRSV and is an important virus structural protein exposed on the surface of the virion that is involved in apoptosis, cell recognition and binding, Ab-dependent virus neutralization, and immune protection in immunized or infected animals. Noteworthy, GP5 is not the only determinant of protective immunity and is particularly unlikely to be important with regard to cross protection immunity which is needed because of the broad spectrum of PRRSV strain heterogeneity. The N-glycosylation of GP5 decreases significantly the immunogenicity of the nearby enclosed neutralizing epitope and provides a way to evade the neutralizing Ab immune response. Other viral epitopes, which are able to induce neutralizing Abs but with a significantly lesser extent compared to GP5, appear to reside on other PRRSV proteins such as M, GP2a, GP3 and GP4. GP5 immunized animals develop a partially protective immune response. GP5 fusion proteins (such as M–GP5 and GP3–GP5, GP4–GP5, or GP3–GP4–GP5) increase the protective immune response in vaccinated animals compared to GP5 alone. Thus, for vaccine development, GP5 should be optimized in its ability to induce neutralizing Abs by a fusion with other viral proteins or by reducing its N-glycosylation state. Nonetheless, all data suggest that other viral proteins should be further investigated, such as the nsp proteins (see the nsp proteins section below), if we hope to develop an efficient method to increase significantly the protection of swine against PRRSV infection.
Minor PRRSV structural proteins
Three N-glycosylated minor envelope proteins (GP2a, GP3 and GP4) are translated from ORF2a, 3 and 4 and are found in virions as heterotrimers formed by disulphide linkage (Figs 1 and 2) (Wissink et al., Reference Wissink, Kroese, van Wijk, Rijsewijk, Meulenberg and Rottier2005). The ORF2b gene, which is completely embedded in ORF2a, encodes another non-glycosylated minor protein named E protein (Fig. 2) (Wu et al., Reference Wu, Fang, Farwell, Steffen-Bien, Rowland, Christopher-Hennings and Nelson2001).
The ORF2 gene (Fig. 2) encodes two minor structural proteins (GP2a and E) (Snijder et al., Reference Snijder, van Tol, Pedersen, Raamsman and de Vries1999). It was shown that NA PRRSV ORF2 contains a small 219 nt ‘ORF2b’ gene and its translation is initiated 5 nt downstream of the initiation codon of the large ORF2, now named ‘ORF2a’. Using a baculovirus expression construct, it was shown that the full-length ORF2 gene expressed both GP2a and E in insect cells and that ORF2b is in a better context for translation and may be preferentially expressed compared to ORF2a (Wu et al., Reference Wu, Fang, Farwell, Steffen-Bien, Rowland, Christopher-Hennings and Nelson2001).
The minor glycosylated envelope viral protein GP2a
The 29 to 30 kDa GP2a is a putative class I integral membrane protein with an N-terminal signal sequence and a C-terminal membrane anchor domain (Meulenberg et al., Reference Meulenberg, Petersen-den Besten, De Kluyver, Moormann, Schaaper and Wensvoort1995a). The GP2a of the NA and EU strains possesses 256 and 249 aa, respectively (Table 1; Meulenberg et al., Reference Meulenberg, Petersen-den Besten, De Kluyver, Moormann, Schaaper and Wensvoort1995a). The GP2a of EU and NA strains of PRRSV contains two distinctive hydrophobic peaks and shares two highly conserved putative N-linked glycosylation sites (Meng et al., Reference Meng, Paul, Halbur and Morozov1995b; Meulenberg et al., Reference Meulenberg, Petersen-den Besten, De Kluyver, Moormann, Schaaper and Wensvoort1995a, Reference Meulenberg, Petersen den Besten, de Kluyver, van Nieuwstadt, Wensvoort and Moormann1997; Morozov et al., Reference Morozov, Meng and Paul1995; Wissink et al., Reference Wissink, Kroese, Maneschijn-Bonsing, Meulenberg, van Rijn, Rijsewijk and Rottier2004). The significance of the N-glycosylation of the GP2a protein for virus production and viral infectivity has been investigated with the EU LV strain (Wissink et al., Reference Wissink, Kroese, Maneschijn-Bonsing, Meulenberg, van Rijn, Rijsewijk and Rottier2004). Analysis of GP2a protein of virus mutants showed that the GP2a N-linked glycans are not essential for virus particle formation (Wissink et al., Reference Wissink, Kroese, Maneschijn-Bonsing, Meulenberg, van Rijn, Rijsewijk and Rottier2004). Antigenicity of GP2a is largely unexplored and there are no data for the NA strains. Two GP2a linear epitopes were immunoreactive with 9/15 swine sera (de Lima et al., Reference de Lima, Pattnaik, Flores and Osorio2006). The reactive peptides comprise regions at aa positions 41–55 and 121–135. Using phage-displayed peptides, three weakly antigenic B-cell epitopes were identified in GP2a at positions 36–51, 117–139 and 120–142 (Oleksiewicz et al., Reference Oleksiewicz, Botner and Normann2002). When ORF2 and ORF4 genes (which encode for minor structural glycoproteins GP2a/E and GP4, respectively) were individually deleted from the viral genome of a NA strain, both PRRSV deletion mutants were non-viable in MARC-145 and PAM cells, indicating that both genes are essential for virus replication (Welch et al., Reference Welch, Jolie, Pearce, Koertje, Fuog, Shields, Yoo and Calvert2004).
The minor non-glycosylated envelope viral protein E
The E protein is an unglycosylated small hydrophobic protein which is membrane-associated and believed to be present in all arteriviruses (Snijder et al., Reference Snijder, van Tol, Pedersen, Raamsman and de Vries1999; Wu et al., Reference Wu, Fang, Farwell, Steffen-Bien, Rowland, Christopher-Hennings and Nelson2001). The PRRSV E protein is translated from the internal ORF2b starting from the +6 nt position in mRNA2 (Fig. 2) (Snijder et al., Reference Snijder, van Tol, Pedersen, Raamsman and de Vries1999). The E protein of the NA and EU strains possesses 73 and 70 aa, respectively (Table 1; Wu et al., Reference Wu, Fang, Farwell, Steffen-Bien, Rowland, Christopher-Hennings and Nelson2001). The E protein is a 10-kDa protein with a central hydrophobic domain and a hydrophilic C-terminus containing a cluster of basic residues (Snijder et al., Reference Snijder, van Tol, Pedersen, Raamsman and de Vries1999). In addition, the E protein possesses a potential N-terminal N-myristoylation site and a potential casein kinase II phosphorylation site (Snijder et al., Reference Snijder, van Tol, Pedersen, Raamsman and de Vries1999). The E protein of PRRSV is incorporated into the envelope virion structure such as its EAV homologous protein (Wu et al., Reference Wu, Fang, Rowland, Lawson, Christopher-Hennings, Yoon and Nelson2005) (Fig. 1).
Functions of the E protein, other than its structural function, are unknown. Reverse genetics, using an infectious EAV clone, demonstrated that the E protein is required for the production of infectious virions (Snijder et al., Reference Snijder, van Tol, Pedersen, Raamsman and de Vries1999). A covalent association of the E protein with the GP2b–GP3–GP4 heterotrimers exists in EAV, suggesting a possible role of the heteromultimeric complex in the virus entry process. The absence of the E protein entirely prevented the incorporation of the GP2b–GP3–GP4 proteins into viral particles (Wieringa et al., Reference Wieringa, de Vries, van der Meulen, Godeke, Onderwater, van Tol, Koerten, Mommaas, Snijder and Rottier2004). Several studies using an EU PRRSV isolate showed that the E protein is incorporated into the virions and associated covalently with GP2a–GP3–GP4 heterotrimers (Fig. 1), suggesting a critical role for the heteromultimeric complex (Wissink et al., Reference Wissink, Kroese, van Wijk, Rijsewijk, Meulenberg and Rottier2005). The PRRSV E protein possesses cysteines at positions 49 and 54 that are highly conserved among NA isolates. The importance of the cysteine residues of the E protein was determined using a NA PRRSV infectious cDNA clone. Each cysteine was substituted by a serine. When the mutated PRRSV infectious cDNA clones were transfected into MARC-145 cells, all cysteine mutant clones induced PRRSV-specific cytopathic effects and produced infectious progeny virus. The data indicate that cysteine residues in the E protein are not essential for PRRSV replication (Lee and Yoo, Reference Lee and Yoo2005). In addition, the authors showed that the N and E proteins of PRRSV do not form cysteine-linked covalent linkages but form cysteine-independent non-covalent associations. Therefore, the authors postulated that the interaction between N and E may be initiated following the binding of viral RNA to the N protein. Subsequently, the N–RNA interaction may promote the N protein association with E, providing stable assembly of the core structure in the virion. It is noteworthy that the cysteines of E protein are not well conserved among arteriviruses (Snijder et al., Reference Snijder, van Tol, Pedersen, Raamsman and de Vries1999). The EU strains contain only a single cysteine at position 51, while E proteins of LDV, EAV and SHFV contain a single cysteine residue at different positions (Snijder et al., Reference Snijder, van Tol, Pedersen, Raamsman and de Vries1999).
Interestingly, a single NA PRRSV mutation at arginine 51 in the basic region of E is lethal for PRRSV, supporting the premise that the N–E non-covalent interaction may be a requirement for PRRSV replication (Lee and Yoo, Reference Lee and Yoo2005). On the other hand, studies with an infectious cDNA clone E gene-knockout PRRSV mutant showed that the E protein was essential for virus infectivity but dispensable for virus particle formation (Lee and Yoo, Reference Lee and Yoo2006). The E deleted non-infectious virus particles were able to enter cells but unable to continue further steps of replication. It is therefore tempting to speculate that PRRSV may contain a viral ion channel protein to promote the uncoating process in the endosome during the early stage of infection. Furthermore, two ion channel blockers greatly affected PRRSV replication at early stages of infection, suggesting that ion channel activity was essential for virus uncoating (Lee and Yoo, Reference Lee and Yoo2006). In addition expression of the E protein enhanced membrane permeability of hygromycin B in bacterial cells. Interestingly, the coronavirus E protein has been shown to modify membrane permeability (Liao et al., Reference Liao, Lescar, Tam and Liu2004; Madan et al., Reference Madan, Garcia, Sanz and Carrasco2005) and to form cation-selective ion channels in an artificial membrane (Wilson et al., Reference Wilson, McKinlay, Gage and Ewart2004). Results from another study support the hypothesis that the PRRSV E protein may function as an ion channel for virus uncoating (Lee and Yoo, Reference Lee and Yoo2006).
Recently, E protein myristoylation was explored using reverse genetics (Du et al., Reference Du, Zuckermann and Yoo2010). The E protein contains the consensus 1MGxxxS6 motif for its myristoylation, and in the presence of 2-hydroxymyristic acid, the virus TCID50 titer decreased by 2.5 log10 and the level of viral RNA was reduced significantly. Thus, E protein myristoylation is non-essential for PRRSV infectivity but promotes growth of the virus (Du et al., Reference Du, Zuckermann and Yoo2010).
The minor glycosylated envelope viral protein GP3
The GP3 protein is encoded by the ORF3 (Fig. 2) gene and is one of the most variable PRRSV proteins, showing approximately 54–60% aa identity between the NA and EU genotypes (Dea et al., Reference Dea, Gagnon, Mardassi, Pirzadeh and Rogan2000a). The GP3 is comprised of 254 and 265 aa for NA and EU isolates, respectively (Table 1; Mardassi et al., Reference Mardassi, Mounir and Dea1995a; Meng et al., Reference Meng, Paul, Halbur and Morozov1995b; Meulenberg et al., Reference Meulenberg, Petersen-den Besten, De Kluyver, Moormann, Schaaper and Wensvoort1995a; Murtaugh et al., Reference Murtaugh, Elam and Kakach1995). Several studies have reported that the most variable region of GP3 is located at the first 35 aa, which is 29% identical among EU and NA isolates (Katz et al., Reference Katz, Shafer, Eernisse, Landgraf and Nelson1995; Mardassi et al., Reference Mardassi, Mounir and Dea1995a; Murtaugh et al., Reference Murtaugh, Elam and Kakach1995). The 45 to 50 kDa molecular weight GP3 protein is highly glycosylated (Table 1), possesses seven potential N-linked oligosaccharides, and has a single N-terminal hydrophobic domain (Meulenberg et al., Reference Meulenberg, Petersen-den Besten, De Kluyver, Moormann, Schaaper and Wensvoort1995a). The glycosylation sites are highly conserved among NA and EU PRRSV stains (Mardassi et al., Reference Mardassi, Mounir and Dea1995a; Meulenberg et al., Reference Meulenberg, Petersen-den Besten, De Kluyver, Moormann, Schaaper and Wensvoort1995a; Gonin et al., Reference Gonin, Mardassi, Gagnon, Massie and Dea1998; Wissink et al., Reference Wissink, Kroese, Maneschijn-Bonsing, Meulenberg, van Rijn, Rijsewijk and Rottier2004).
The GP3 protein seems to take a special position among the arterivirus glycoproteins. Since its membrane topology has not been resolved and seems to be strain dependent (Mardassi et al., Reference Mardassi, Gonin, Gagnon, Massie and Dea1998; Hedges et al., Reference Hedges, Balasuriya and MacLachlan1999; Wieringa et al., Reference Wieringa, de Vries, Raamsman and Rottier2002), its virion structural nature is also controversial (Fig. 1). For LDV, the GP3 protein was reported to be a non-structural, soluble glycoprotein that is secreted from infected cells (Faaberg and Plagemann, Reference Faaberg and Plagemann1997). In contrast, the GP3 protein of EAV was clearly demonstrated to be incorporated into virions (Wieringa et al., Reference Wieringa, de Vries, Raamsman and Rottier2002). For PRRSV, both situations have been described (Meulenberg et al., Reference Meulenberg, Petersen-den Besten, De Kluyver, Moormann, Schaaper and Wensvoort1995a; Van Nieuwstadt et al., Reference van Nieuwstadt, Meulenberg, van Essen-Zanbergen, Petersen-den Besten, Bende, Moormann and Wensvoort1996; Gonin et al., Reference Gonin, Mardassi, Gagnon, Massie and Dea1998; Mardassi et al., Reference Mardassi, Gonin, Gagnon, Massie and Dea1998; Wissink et al., Reference Wissink, Kroese, van Wijk, Rijsewijk, Meulenberg and Rottier2005; de Lima et al., Reference de Lima, Ansari, Das, Ku, Martinez-Lobo, Pattnaik and Osorio2009). The GP3 protein of the prototype EU LV strain was found to be incorporated into virions (Meulenberg et al., Reference Meulenberg, Petersen-den Besten, De Kluyver, Moormann, Schaaper and Wensvoort1995a; van Nieuwstadt et al., Reference van Nieuwstadt, Meulenberg, van Essen-Zanbergen, Petersen-den Besten, Bende, Moormann and Wensvoort1996) and membrane-associated as heterotrimers with GP2a and GP4 (Wissink et al., Reference Wissink, Kroese, van Wijk, Rijsewijk, Meulenberg and Rottier2005). However, the GP3 protein of the IAF-Klop reference strain (a typical NA isolate) was non-structural and secreted from PRRSV infected cells (Gonin et al., Reference Gonin, Mardassi, Gagnon, Massie and Dea1998; Mardassi et al., Reference Mardassi, Gonin, Gagnon, Massie and Dea1998). This dual nature of GP3 is reminiscent of viral glycoproteins in several other viruses such as the glycoprotein G (gG) of alphaherpesviruses (Murata et al., Reference Murata, Goshima, Takakuwa and Nishiyama2002; Bryant et al., Reference Bryant, Davis-Poynter, Vanderplasschen and Alcami2003) and the Erns glycoprotein of bovine viral diarrhea virus (BVDV) (Iqbal et al., Reference Iqbal, Poole, Goodbourn and McCauley2004). The gG protein of alphaherpesviruses is found in the virus envelope and, in some alphaherpesviruses, is also secreted after proteolytic processing. The secreted forms of gG were reported to have chemokine binding activity, thereby inhibiting the biological activity of the chemokines in vitro (Bryant et al., Reference Bryant, Davis-Poynter, Vanderplasschen and Alcami2003). The BVDV Erns protein, in addition to being a virion protein, is also secreted from infected cells into the extracellular environment. This secreted form was recently shown to block the double-stranded RNA interferon (IFN)-inducing signal (Iqbal et al., Reference Iqbal, Poole, Goodbourn and McCauley2004).
According to several reports, GP3 is highly antigenic (Katz et al., Reference Katz, Shafer, Eernisse, Landgraf and Nelson1995; Faaberg and Plagemann, Reference Faaberg and Plagemann1997; Gonin et al., Reference Gonin, Mardassi, Gagnon, Massie and Dea1998; Hedges et al., Reference Hedges, Balasuriya and MacLachlan1999). Although GP3 Ab is present at a very low level in pigs, it reportedly plays a role in clearing the viral infection (Plana Duran et al., Reference Plana Duran, Climent, Sarraseca, Urniza, Cortes, Vela and Casal1997b) and may be involved in viral neutralization along with the GP5 and M proteins (Cancel-Tirado et al., Reference Cancel-Tirado, Evans and Yoon2004). Using a phage display library, two epitopes spanning aa regions 60–85 and 243–250 of a EU GP3 protein were identified (Oleksiewicz et al., Reference Oleksiewicz, Botner, Toft, Normann and Storgaard2001b, Reference Oleksiewicz, Botner and Normann2002). Two other GP3 epitopes of an NA strain were found and located at aa 73–87 and 66–81 (Zhou et al., Reference Zhou, An, He, Liu, Qiu, Wang and Tong2006). Further analyses revealed that the two epitopes are not conserved among all NA-type isolates. After analyzing the degree of antigenic variability in a series of EU isolates using mAbs, it was determined that the C-terminus antigenicity of GP3 varied greatly among the different EU isolates (Drew et al., Reference Drew, Meulenberg, Sands and Paton1995). Two replication-defective recombinant adenoviruses expressing GP3 (rAd-GP3) or truncated GP3 (rAd-tGP3, a hydrophobic region of aa 2–64 was deleted) were constructed and their immunogenicity was tested in mice (Jiang et al., Reference Jiang, Jiang, Li, Wang and Du2007). The mice immunized with recombinant adenoviruses developed PRRSV-specific neutralizing Abs and cellular immune response, including T-cell proliferation responses and cytotoxic T-cell responses, by 2 weeks post-primary immunization. Moreover, the levels of immune responses of mice immunized with rAd-tGP3 were significantly higher than those of mice with rAd-GP3.
The minor glycosylated envelope viral protein GP4
The 31 to 35 kDa molecular weight GP4 protein is a putative class I integral membrane protein with an N-terminal signal sequence and a C-terminal membrane anchor (Meulenberg et al., Reference Meulenberg, Petersen-den Besten, De Kluyver, Moormann, Schaaper and Wensvoort1995a). The GP4 protein is highly glycosylated during transport through the ER–Golgi complex (van Nieuwstadt et al., Reference van Nieuwstadt, Meulenberg, van Essen-Zanbergen, Petersen-den Besten, Bende, Moormann and Wensvoort1996). Four putative N-linked glycosylation sites which are conserved among the NA and EU PRRSV strains are found within the protein (Meulenberg et al., Reference Meulenberg, Petersen-den Besten, De Kluyver, Moormann, Schaaper and Wensvoort1995a; van Nieuwstadt et al., Reference van Nieuwstadt, Meulenberg, van Essen-Zanbergen, Petersen-den Besten, Bende, Moormann and Wensvoort1996; Wissink et al., Reference Wissink, Kroese, Maneschijn-Bonsing, Meulenberg, van Rijn, Rijsewijk and Rottier2004). The aa sequence shows that GP4 contains a putative N-terminal signal sequence at positions 1–22, and an additional hydrophobic sequence at positions 162–178 at the C-terminus. The GP4 protein is a minor structural protein consisting of 178 and 183 aa (Table 1) for NA and EU strains, respectively (Murtaugh et al., Reference Murtaugh, Elam and Kakach1995) and like GP5, is able to induce neutralizing Abs to a lesser extent (Meulenberg et al., Reference Meulenberg, Petersen den Besten, de Kluyver, van Nieuwstadt, Wensvoort and Moormann1997; Weiland et al., Reference Weiland, Wieczorek-Krohmer, Kohl, Conzelmann and Weiland1999). The GP4 protein of LV possesses neutralizing epitopes which are not conserved among NA and EU strains (Meulenberg et al., Reference Meulenberg, Petersen den Besten, de Kluyver, van Nieuwstadt, Wensvoort and Moormann1997; Weiland et al., Reference Weiland, Wieczorek-Krohmer, Kohl, Conzelmann and Weiland1999). The neutralizing domain of the GP4 of the LV strain has been mapped to a hydrophilic exposed region, adjacent to its N-terminal region (aa position 40–79) which appears to be highly variable among the EU strains (Meulenberg et al., Reference Meulenberg, Petersen den Besten, de Kluyver, van Nieuwstadt, Wensvoort and Moormann1997; Weiland et al., Reference Weiland, Wieczorek-Krohmer, Kohl, Conzelmann and Weiland1999). Approximately 65% of PRRSV-positive sera obtained from affected pig farms in the US and Canada reacted positively by immunoblotting against the E. coli-expressed recombinant ORF4 protein (Kwang et al., Reference Kwang, Kim and Joo1994; Gonin et al., Reference Gonin, Pirzadeh, Gagnon and Dea1999). However, there was no correlation between virus neutralization titers of convalescent pig sera and the presence of anti-GP4 Abs (Gonin et al., Reference Gonin, Pirzadeh, Gagnon and Dea1999). Using Pepscan technology de Lima et al. (Reference de Lima, Pattnaik, Flores and Osorio2006) detected the B-cell linear epitope in NA GP4 (aa 51–65) and using phage display libraries Oleksiewicz et al. (2001b and 2005) detected a single linear epitope in EU GP4 (aa 59–71).
Transfection of PRRSV permissive cells with an ORF4 deleted infectious cDNA clone revealed that the ORF4 gene is essential for virus replication (Welch et al., Reference Welch, Jolie, Pearce, Koertje, Fuog, Shields, Yoo and Calvert2004). The experiments conducted by Lee et al. (Reference Lee, Bachand, Murtaugh and Yoo2004) demonstrated that, like GP5, GP4 is able to alter cellular mRNA synthesis. In cells expressing GP4, the majority of differentially expressed genes were involved in synthesis, transport and biochemical pathways (Lee et al., Reference Lee, Bachand, Murtaugh and Yoo2004). This observation implies that GP4 may utilize or change host cell machinery to transport viral or cellular components to the cell surface.
In summary, the minor structural proteins are essential for virus infectivity and are incorporated into virions as a multimeric complex. Recently they became the point of interest of several PRRSV investigators. Therefore, new interesting findings regarding the function of the minor viral structural proteins should be expected. The minor structural proteins multimeric complex was shown to interact with the cell receptor CD163 with or without the involvement of M-GP5 heterodimers. Thus, this latest finding could lead us to formulate new important questions such as (1) can minor structural proteins multimeric complex target the virus to a certain cell type and organ?; or (2) is the multimeric complex involved in the receptor-mediated endocytosis virus entry?
Viral structural protein interactions
While the major viral structural proteins are essential for particle formation, the minor viral structural proteins are essential for virus infectivity (Wissink et al., Reference Wissink, Kroese, van Wijk, Rijsewijk, Meulenberg and Rottier2005). Furthermore, the minor structural proteins are incorporated into virions as a multimeric complex (Fig. 1). Interestingly, the absence of any of the four proteins appears to affect the incorporation of the other three. Thus, no GP3 was detected in viral particles when the expression of either GP2a or E or GP4 was prevented, and no detectable GP4 was incorporated in the absence of GP2a, E and GP3. Similar observations were made with EAV (Wieringa et al., Reference Wieringa, de Vries, van der Meulen, Godeke, Onderwater, van Tol, Koerten, Mommaas, Snijder and Rottier2004). From these results, three main conclusions could be drawn. First, each of the membrane viral proteins is essential for the production of infectious virus. Second, the GP5 and M protein are both crucial for particle formation, consistent with a role as the fundamental building blocks of the viral envelope. Third, while not required for particle assembly, the minor glycoproteins GP2a, GP3 and GP4, and probably also the minor envelope protein E, interact with each other. This interaction is likely to be of critical importance for their incorporation as heteromultimeric complexes into PRRSV particles and essential for rendering these particles infectious (Wissink et al., Reference Wissink, Kroese, van Wijk, Rijsewijk, Meulenberg and Rottier2005). Noteworthy, contradictory reports regarding the presence or absence of GP3 in viral particles seems to be a strain-dependent phenomenon and its absence does not seem to abolish the formation of infectious viruses (Wissink et al., Reference Wissink, Kroese, van Wijk, Rijsewijk, Meulenberg and Rottier2005).
Recently, a strong interaction was found between GP4 and GP5 proteins, although weak interactions among the other minor envelope glycoproteins and GP5 have been detected (Das et al., Reference Das, Dinh, Ansari, de Lima, Osorio and Pattnaik2010). Both GP2a and GP4 proteins interact with all the other viral glycoproteins resulting in the formation of multiprotein complex. In addition, the multimeric complexes of the minor glycoproteins, with or without the M and GP5 proteins, are involved in direct interaction with the cell surface receptor (Das et al., Reference Das, Dinh, Ansari, de Lima, Osorio and Pattnaik2010). It appears that GP4 is a critical viral envelope protein that not only mediates interactions with other viral envelope glycoproteins but also along with GP2a, mediates interactions with CD163 for virus entry. More specifically, only the GP2a and GP4 proteins interact with CD163. Since the minor envelope proteins are not required for particle formation (Wissink et al., Reference Wissink, Kroese, van Wijk, Rijsewijk, Meulenberg and Rottier2005) and there are only few large multimeric glycoprotein complexes present on the virion envelope (Spilman et al., Reference Spilman, Welbon, Nelson and Dokland2009), these results suggest that the major function of the minor glycoprotein complex on the virion envelope is to interact specifically with the cell surface receptor for virus entry (Das et al., Reference Das, Dinh, Ansari, de Lima, Osorio and Pattnaik2010).
Several research groups have been involved in the study of the immune response triggered by each of the major structural proteins (GP5 and M) of PRRSV (Plana-Duran et al., Reference Plana-Duran, Bastons, Urniza, Vayreda, Vila and Mane1997a, Reference Plana Duran, Climent, Sarraseca, Urniza, Cortes, Vela and Casalb; Pirzadeh and Dea, Reference Pirzadeh and Dea1998; Bastos et al., Reference Bastos, Dellagostin, Barletta, Doster, Nelson and Osorio2002, Reference Bastos, Dellagostin, Barletta, Doster, Nelson, Zuckermann and Osorio2004; Qiu et al., Reference Qiu, Tian, Tong, Zhou, Ni, Luo and Cai2005; Jiang et al., Reference Jiang, Xiao, Fang, Yu, Song, Niu and Chen2006b) but only a little information is available on the characterization of the minor structural proteins, such as GP3 and GP4. A recent study (Jiang et al., Reference Jiang, Jiang, Wang, Li and Du2008) reports the tandem cloning and expression of two or three proteins of PRRSV, the GP3, GP4, or GP5, in replication-defective human adenovirus type 5 (Ad5) vectors. Mice inoculated with the recombinant Ad5 expressing GP3–GP5, GP4–GP5, or GP3–GP4–GP5 of PRRSV developed a specific immune response against the viral proteins, and this response was significantly increased against GP5 when GP3 and/or GP4 were present in the adenovirus vector compared to GP5 alone. These results indicate that fusion expression of GP3 and/or GP4 with GP5 may be an alternative strategy to design more effective vaccines.
Non-structural PRRSV proteins
RNA synthesis of the positive-stranded RNA viruses that belong to the order Nidovirales involves the replication of the genome-length RNA (RNA1) and the synthesis of sg mRNAs. Both processes are mediated by a ‘replication/transcription complex’ (RTC) composed of virus-encoded nsps and presumably also host factors (Gorbalenya et al., Reference Gorbalenya, Enjuanes, Ziebuhr and Snijder2006). Following virus entry and release of the genome into the cytoplasm, the nidovirus life cycle (Fig. 2) starts with the expression of the large replicase gene that consists of ORF1a and ORF1b. The replicase-associated genes, ORF1a and ORF1b, are located on the 5′ end of the genome (representing almost 75% of the viral genome) and encode for the polyproteins pp1a and pp1ab. The pp1a is encoded by ORF1a, and the synthesis of pp1ab occurs through a ribosomal frame shift at the ORF1a-1b junction (Brierley, Reference Brierley1995; Snijder and Meulenberg, Reference Snijder and Meulenberg1998). The pp1a is predicted to be cleaved at eight (Fig. 2) sites to form nine nsps: nsp1α, nsp1β and nsp2 to nsp8 (den Boon et al., Reference den Boon, Faaberg, Meulenberg, Wassenaar, Plagemann, Gorbalenya and Snijder1995; Snijder and Meulenberg, Reference Snijder and Meulenberg1998). Proteolytic cleavage of the ORF1b gene portion of the pp1ab generates (Fig. 2) products nsp9 through nsp12 (van Dinten et al., Reference van Dinten, Wassenaar, Gorbalenya, Spaan and Snijder1996). The products derived from pp1a possess proteolytic activities (Table 1) and are responsible for processing the other nsp cleavage products; whereas nsp9 to nsp12 are involved in virus transcription and replication (van Dinten et al., Reference van Dinten, Wassenaar, Gorbalenya, Spaan and Snijder1996; Snijder and Meulenberg, Reference Snijder and Meulenberg1998). These polyproteins are immediately translated upon virus entry and then proteolytically processed by viral encoded proteinases into intermediate precursors and at least 12 mature nsps, which appear to be responsible for forming membrane-bound replication complexes, called virus-induced double-membrane vesicles and which are sites for viral RNA synthesis (Snijder and Meulenberg, Reference Snijder and Meulenberg1998). The processing of pp1a and pp1ab is believed to be mediated by accessory proteinases (Fig. 2), located in nsp1 and nsp2, and the main serine proteinase located in nsp4, the 3C-like proteinase (3CLpro) (Ziebuhr et al., Reference Ziebuhr, Snijder and Gorbalenya2000). The catalytic sites of these enzymes and the corresponding cleavage sites of pp1a are well conserved among arteriviruses (Ziebuhr et al., Reference Ziebuhr, Snijder and Gorbalenya2000). Processing of pp1a in PRRSV begins with the N-terminal nsp1, which is automatically cleaved by papain-like cysteine proteases (PCPα and PCPβ) which are contained in nsp1 (Fig. 2) (Kroese et al., Reference Kroese, Zevenhoven-Dobbe, Bos-de Ruijter, Peeters, Meulenberg, Cornelissen and Snijder2008), while the PCPα domain is inactive in EAV (Snijder et al., Reference Snijder, Wassenaar and Spaan1993; den Boon et al., Reference den Boon, Faaberg, Meulenberg, Wassenaar, Plagemann, Gorbalenya and Snijder1995). Arterivirus nsp2 contains a predicted cysteine proteinase (PL2pro) in its N terminus (Fig. 2) that cleaves the pp1a at the nsp2–nsp3 junction in EAV (Snijder et al., Reference Snijder, Wassenaar, Spaan and Gorbalenya1995b; Snijder, Reference Snijder1998). The 3CLpro (Fig. 2) is responsible for processing the remainder of ORF1a and ORF1ab into several subunits (nsp3 to nsp12) (van Dinten et al., Reference van Dinten, Rensen, Gorbalenya and Snijder1999; Ziebuhr et al., Reference Ziebuhr, Snijder and Gorbalenya2000).
The non-structural polypeptides encoded in Nidovirales ORF1b regions have been predicted to have essential roles (Table 1) in virus replication and gene expression (Gorbalenya et al., Reference Gorbalenya, Koonin, Donchenko and Blinov1989; Meulenberg et al., Reference Meulenberg, de Meijer and Moormann1993a; van Dinten et al., Reference van Dinten, Wassenaar, Gorbalenya, Spaan and Snijder1996; Allende et al., Reference Allende, Lewis, Lu, Rock, Kutish, Ali, Doster and Osorio1999; Nelsen et al., Reference Nelsen, Murtaugh and Faaberg1999). Evidence for the significant role of these proteins in arteriviruses has been derived using an infectious clone of EAV (van Dinten et al., Reference van Dinten, den Boon, Wassenaar, Spaan and Snijder1997, Reference van Dinten, Rensen, Gorbalenya and Snijder1999, Reference van Dinten, Van Tol, Gorbalenya and Snijder2000; van Marle et al., Reference van Marle, van Dinten, Spaan, Luytjes and Snijder1999). Putative RNA-dependent RNA polymerase and NTPase/RNA helicase motifs are associated with nsp9 and nsp10, respectively. The nsp10 also contains a putative zinc finger region, which is implicated in sg mRNA synthesis and genome replication. Thus, it can be assumed that the reduction of ORF1ab transcripts and nsp10 expression might affect negatively expression of the other viral proteins. The nsp11 contains a region with unknown function, but is conserved in all nidoviruses which suggests a crucial role for this viral protein (Gorbalenya et al., Reference Gorbalenya, Koonin, Donchenko and Blinov1989; den Boon et al., Reference den Boon, Snijder, Chirnside, de Vries, Horzinek and Spaan1991; Godeny et al., Reference Godeny, Chen, Kumar, Methven, Koonin and Brinton1993; Meulenberg et al., Reference Meulenberg, de Meijer and Moormann1993a; van Dinten et al., Reference van Dinten, Wassenaar, Gorbalenya, Spaan and Snijder1996).
The nsp1 is a multifunctional protein (Table 1) containing two papain-like cysteine proteases and a zinc-finger motif required for sg mRNA transcription (den Boon et al., Reference den Boon, Faaberg, Meulenberg, Wassenaar, Plagemann, Gorbalenya and Snijder1995; Tijms et al., Reference Tijms, van Dinten, Gorbalenya and Snijder2001; Tijms and Snijder, Reference Tijms and Snijder2003; Oleksiewicz et al., Reference Oleksiewicz, Snijder and Normann2004; Kroese et al., Reference Kroese, Zevenhoven-Dobbe, Bos-de Ruijter, Peeters, Meulenberg, Cornelissen and Snijder2008). Intracellular concentrations of nsp1 may be higher than other nsp, due to translation from heteroclite RNAs (Yuan et al., Reference Yuan, Murtaugh and Faaberg2000, Reference Yuan, Murtaugh, Schumann, Mickelson and Faaberg2004). Its papain-like autoprotease domain releases nsp1 from the replicase polyproteins, a cleavage essential for viral RNA synthesis. Several mutations in the putative N-terminal zinc finger domain of nsp1 selectively abolished transcription, while replication was either not affected or even increased. Other nsp1 mutations did not significantly affect either replication or transcription but still dramatically reduced the production of infectious progeny. Thus, nsp1 is involved in at least three consecutive key processes in the EAV life cycle: ORF1ab polyprotein processing, transcription and virion biogenesis (Tijms et al., Reference Tijms, Nedialkova, Zevenhoven-Dobbe, Gorbalenya and Snijder2007). Both N-terminal cleavage products of nsp1, the nsp1α and the nsp1β, of the replicase polyproteins of PRRSV contain papain-like autoproteinase domains, which have been named PCPα and PCPβ, respectively. To assess their role in the PRRSV life cycle, substitutions and deletions of the presumed catalytic cysteine and histidine residues of PCPα and PCPβ were made in a PRRSV infectious cDNA clone. Mutations that inactivated PCPα activity completely blocked sg mRNA synthesis, but did not affect genome replication. In contrast, mutants in which PCPβ activity was blocked proved to be non-viable and no sign of viral RNA synthesis was detected, indicating that the correct processing of the nsp1β/nsp2 cleavage site is essential for PRRSV genome replication. In conclusion, the data show that a productive PRRSV life cycle depends on the correct processing of both the nsp1α /nsp1β and nsp1β/nsp2 junctions (Kroese et al., Reference Kroese, Zevenhoven-Dobbe, Bos-de Ruijter, Peeters, Meulenberg, Cornelissen and Snijder2008). Cleavage sites between nsp1α/nsp1β and nsp1β/nsp2 were identified by protein microsequencing analysis (Chen et al., Reference Chen, Sun, Zhou, Guan, Christopher-Hennings, Nelson and Fang2010). The cleavage site between nsp1α/nsp1β is located at position 180M/181A (Fig. 2) and is conserved among different NA PRRSV strains (Chen et al., Reference Chen, Sun, Zhou, Guan, Christopher-Hennings, Nelson and Fang2010). The cleavage site between nsp1β/nsp2 is located at position 383G/384A (Fig. 2) and is well conserved among different PRRSV isolates (Chen et al., Reference Chen, Sun, Zhou, Guan, Christopher-Hennings, Nelson and Fang2010). The replicase polyproteins of PRRSV are predicted to be cleaved into 13 nsps by the nsp4 main proteinase and three accessory proteinases residing in nsp1α, nsp1β and nsp2 (Ziebuhr et al., Reference Ziebuhr, Snijder and Gorbalenya2000; van Aken et al., Reference van Aken, Benckhuijsen, Drijfhout, Wassenaar, Gorbalenya and Snijder2006a, Reference van Aken, Snijder and Gorbalenyab, Reference van Aken, Zevenhoven-Dobbe, Gorbalenya and Snijderc). PCPα directs the release of nsp1α, whereas the liberation of nsp1β depends on the activities of PCPα and a second proteinase, PCPβ. A third cysteine proteinase, residing in the N-terminal domain of nsp2, is responsible for the cleavage of the nsp2/nsp3 site (den Boon et al., Reference den Boon, Faaberg, Meulenberg, Wassenaar, Plagemann, Gorbalenya and Snijder1995; Snijder et al., Reference Snijder, Wassenaar, Den Boon and Spaan1995a, Reference Snijderb; Snijder, Reference Snijder1998). Viral papain-like proteinases are characterized by a catalytic dyad that consists of a nucleophilic cysteine residue and a downstream histidine (Fig. 2).
Among the nsps, the nsp2 multidomain is the largest PRRSV replicative protein and is predicted to possess approximately 980 aa (Allende et al., Reference Allende, Lewis, Lu, Rock, Kutish, Ali, Doster and Osorio1999; Ziebuhr et al., Reference Ziebuhr, Snijder and Gorbalenya2000). Three major domains were discerned through the alignment of arterivirus nsp2 proteins: (1) an N-terminal cysteine proteinase domain (PL2), (2) a functionally unspecified middle region and (3) a hydrophobic TM region near the C-terminus (Ziebuhr et al., Reference Ziebuhr, Snijder and Gorbalenya2000; Han et al., Reference Han, Wang and Faaberg2006). The C-terminal end of nsp2 forms a non-covalent interaction with nsp3, which together form a scaffolding complex that supports the formation of double membrane vesicles and virus replication complexes (Snijder et al., Reference Snijder, Wassenaar and Spaan1994, Reference Snijder, van Tol, Roos and Pedersen2001; Snijder and Meulenberg, Reference Snijder and Meulenberg1998; Ziebuhr et al., Reference Ziebuhr, Snijder and Gorbalenya2000). The PL2 of PRRSV nsp2 (Fig. 2) is predicted to act on the potential substrates at the nsp2–nsp3 junction and two potential cleavage sites have been proposed: 981G/G and 1196G/G/G (Allende et al., Reference Allende, Lewis, Lu, Rock, Kutish, Ali, Doster and Osorio1999; Ziebuhr et al., Reference Ziebuhr, Snijder and Gorbalenya2000), according to the knowledge obtained from nsp2 of EAV, which prefers G/G dipeptides (Snijder et al., Reference Snijder, Wassenaar, Spaan and Gorbalenya1995b; Snijder, Reference Snijder1998). The function of PRRSV nsp2 in the virus life cycle is poorly understood, while EAV nsp2 is involved in the generation of double-membrane vesicles together with nsp3, and functions as a co-factor with nsp4 serine protease to process the other cleavage products (Snijder et al., Reference Snijder, Wassenaar and Spaan1994, Reference Snijder, Wassenaar, Den Boon and Spaan1995a, Reference Snijderb; Wassenaar et al., Reference Wassenaar, Spaan, Gorbalenya and Snijder1997). Although PRRSV nsp2 possesses potential enzymatic function, it is highly heterogeneous and variable (Table 1). It is well documented that PRRSV nsp2 accounts for the major genetic differences (with GP5 major structural protein) between NA and EU PRRSV strains, sharing less than 40% similarity at the aa level (Allende et al., Reference Allende, Lewis, Lu, Rock, Kutish, Ali, Doster and Osorio1999; Nelsen et al., Reference Nelsen, Murtaugh and Faaberg1999). In addition, nsp2 is also the key region for genomic differentiation between PRRSV genotypes NA and EU strains. Natural mutations, insertions, or, most notably, deletions are seen in the middle region or near the N-terminal region of the nsp2 protein in field strains, while the putative PL2 domain, predicted TM domain, as well as the predicted cleavage sites of nsp2 remain well conserved (Allende et al., Reference Allende, Lewis, Lu, Rock, Kutish, Ali, Doster and Osorio1999; Nelsen et al., Reference Nelsen, Murtaugh and Faaberg1999; Shen et al., Reference Shen, Kwang, Liu and Liu2000; Fang et al., Reference Fang, Kim, Ropp, Steen, Christopher-Hennings, Nelson and Rowland2004; Gao et al., Reference Gao, Guo and Yang2004; Ropp et al., Reference Ropp, Wees, Fang, Nelson, Rossow, Bien, Arndt, Preszler, Steen, Christopher-Hennings, Collins, Benfield and Faaberg2004; Han et al., Reference Han, Wang and Faaberg2006).
Areas in nsp2 with deletions and aa hypervariability are predicted to be immunologically important (Fang et al., Reference Fang, Schneider, Zhang, Faaberg, Nelson and Rowland2007). During the past 2 years, an atypical clinical outbreak, caused by a highly pathogenic PRRSV strain with a unique 30 aa deletion in its nsp2 coding region, was implicated in China (Li et al., Reference Li, Wang, Bo, Tang, Yang, Jiang and Jiang2007; Tian et al., Reference Tian, Yu, Zhao, Feng, Cao, Wang, Hu, Chen, Hu, Tian, Liu, Zhang, Deng, Ding, Yang, Zhang, Xiao, Qiao, Wang, Hou, Wang, Yang, Kang, Sun, Jin, Wang, Kitamura, Yan and Gao2007; Zhou et al., Reference Zhou, Hao, Tian, Tong, Yoo, An, Zhou, Li, Qiu, Wei and Yuan2008). In the field, the virus was associated with high mortality/morbidity in pigs of all ages, with mortality as high as 90% in some herds. The mechanism for enhanced virulence was not determined but seems to be associated with the nsp2 30 aa deletion (Li et al., Reference Li, Wang, Bo, Tang, Yang, Jiang and Jiang2007). Recently, few full-length infectious cDNA clones were generated, in which the nsp2 region containing the 30 aa deletion was replaced by the corresponding region of the low virulence PRRSV strain (Zhou et al., Reference Zhou, Zhang, Zeng, Yin, Li, Zheng, Guo, Ge and Yang2009). The researchers concluded that the 30 aa deletion is not related to the virulence of the highly pathogenic PRRSV that recently emerged in China (Zhou et al., Reference Zhou, Zhang, Zeng, Yin, Li, Zheng, Guo, Ge and Yang2009).
The nsp2 gene may be an ideal marker for monitoring genetic variation and for developing differential diagnostic tests. Since this region tolerates mutations, including insertions and deletions, nsp2 may represent the ideal site for the development of recombinant marker vaccines derived from infectious clones (Fang et al., Reference Fang, Kim, Ropp, Steen, Christopher-Hennings, Nelson and Rowland2004). In that regard a number of studies have indicated that PRRSV nsp2 is able to tolerate aa deletion and foreign gene insertion (Gao et al., Reference Gao, Guo and Yang2004; Fang et al., Reference Fang, Kim, Ropp, Steen, Christopher-Hennings, Nelson and Rowland2004, Reference Fang, Rowland, Roof, Lunney, Christopher-Hennings and Nelson2006; Han et al., Reference Han, Liu, Wang and Faaberg2007; Kim et al., Reference Kim, Calvert, Chang, Horlen, Kerrigan and Rowland2007; Tian et al., Reference Tian, Yu, Zhao, Feng, Cao, Wang, Hu, Chen, Hu, Tian, Liu, Zhang, Deng, Ding, Yang, Zhang, Xiao, Qiao, Wang, Hou, Wang, Yang, Kang, Sun, Jin, Wang, Kitamura, Yan and Gao2007; Ran et al., Reference Ran, Chen, Guo, Ge, Yoon and Yang2008) and is then an excellent gene candidate for modifications (Fang et al., Reference Fang, Rowland, Roof, Lunney, Christopher-Hennings and Nelson2006; Kim et al., Reference Kim, Calvert, Chang, Horlen, Kerrigan and Rowland2007; de Lima et al., Reference de Lima, Kwon, Ansari, Pattnaik, Flores and Osorio2008).
PRRSV nsps play a key role in processing and maturation of the repertoire of structural and nsp proteins of the virion, but little is known about the anti-nsp immune response. In one recent study (Johnson et al., Reference Johnson, Yu and Murtaugh2007), it was hypothesized that pronounced Ab responses are generated against PRRSV nsp1 and nsp2. Accordingly, nsp1 and nsp2 were cloned and expressed, and Ab responses in the sera of infected and vaccinated pigs were determined (Johnson et al., Reference Johnson, Yu and Murtaugh2007). Pigs mounted significant cross-reactive Ab responses against nsp1 and nsp2 that appeared equivalent to or greater than the response to N protein. Ab reactivity to nsp1 and N was highly dependent on refolding of denatured proteins, suggesting that the porcine Ab response is directed primarily to conformational epitopes. The proteins were recognized with sera from pigs infected with PRRSV heterologous strains, indicating that multiple epitopes are conserved. Ab responses against nsp1 and nsp2 were much higher than those directed against nsp4.
PRRSV is a poor inducer of IFN in vitro and in vivo. Recently, several nsps (nsp1, nsp2, nsp4 and nsp11) were shown to down-regulate the immune response (Beura et al., Reference Beura, Sarkar, Kwon, Subramaniam, Jones, Pattnaik and Osorio2010). They possess an inhibitory effect on IFN-beta (IFN-β) promoter activation for which the strongest inhibitory effect was exhibited by nsp1 followed by, nsp2, nsp11 and nsp4 (Beura et al., Reference Beura, Sarkar, Kwon, Subramaniam, Jones, Pattnaik and Osorio2010). In addition, nsp1α, nsp1β and nsp11 strongly inhibit dsRNA signaling pathways. The nsp1β inhibits both IFN regulatory factor 3 (IRF3) and NF-κB-dependent gene induction by dsRNA and Sendai virus. Investigation of IRF3 phosphorylation status after dsRNA stimulation showed that nsp1β inhibits IRF3 (Ser396) phosphorylation and subsequently its nuclear translocation (Beura et al., Reference Beura, Sarkar, Kwon, Subramaniam, Jones, Pattnaik and Osorio2010). Another recent report revealed that nsp1α and nsp1β dramatically inhibit IFN-β expression (Chen et al., Reference Chen, Sun, Zhou, Guan, Christopher-Hennings, Nelson and Fang2010). This study indicates that nsp1β possesses the ability to inhibit both IFN synthesis and its signaling pathway, while nsp1α alone strongly inhibits IFN synthesis.
The four predicted ORF1b non-structural polypeptides comprise a putative RNA-dependent RNA polymerase (POL or nsp9), an nsp10 protein (nsp10) which contains a putative metal-binding domain (MBD) and nucleoside triphosphate binding or helicase motif (HEL), and two proteins of unknown functions (nsp11 and nsp12) (Gorbalenya et al., Reference Gorbalenya, Koonin, Donchenko and Blinov1989; den Boon et al., Reference den Boon, Snijder, Chirnside, de Vries, Horzinek and Spaan1991; Godeny et al., Reference Godeny, Chen, Kumar, Methven, Koonin and Brinton1993; Meulenberg et al., Reference Meulenberg, de Meijer and Moormann1993a; Herold et al., Reference Herold, Siddell and Ziebuhr1996; van Dinten et al., Reference van Dinten, Wassenaar, Gorbalenya, Spaan and Snijder1996, Reference van Dinten, Rensen, Gorbalenya and Snijder1999; Snijder and Meulenberg, Reference Snijder and Meulenberg1998; Nelsen et al., Reference Nelsen, Murtaugh and Faaberg1999; Allende et al., Reference Allende, Lewis, Lu, Rock, Kutish, Ali, Doster and Osorio1999, Reference Allende, Kutish, Laegreid, Lu, Lewis, Rock, Friesen, Galeota, Doster and Osorio2000; Shen et al., Reference Shen, Kwang, Liu and Liu2000; Wootton et al., Reference Wootton, Yoo and Rogan2000; Bautista et al., Reference Bautista, Faaberg, Mickelson and McGruder2002). PRRSV nsp10 possess a thermolabile and pH-sensitive NTPase activity that is modulated by polynucleotides and is able to unwind dsRNA in a 5′ to 3′ polarity (Bautista et al., Reference Bautista, Faaberg, Mickelson and McGruder2002). These results provide the first evidence of the functional properties of PRRSV helicase and further support the finding that nidovirus helicases possess properties that distinguish them from other viral helicases. Based on significant homology in the catalytic domains and in predicted cleavage sites of PRRSV strains it was suggested that PRRSV polymerase may undergo similar proteolytic processing as that of EAV (Wassenaar et al., Reference Wassenaar, Spaan, Gorbalenya and Snijder1997; Snijder and Meulenberg, Reference Snijder and Meulenberg1998; van Dinten et al., Reference van Dinten, Rensen, Gorbalenya and Snijder1999; Ziebuhr et al., Reference Ziebuhr, Snijder and Gorbalenya2000).
Following the first identification of PRRSV in the early 1990s, most of the research was oriented toward the viral structural proteins. During the last 5 years, an increasingly significant number of reports were oriented toward acquisition of new knowledge of the non-structural viral proteins and it has become evident that the nsps play a tremendous role in viral pathogenesis such as the immune response down regulation. The ability of the nsps to inhibit IFN indicates that they are important virulence determinants. Thus, we are now entering a new exciting PRRSV research era that will focus on a better understanding of how PRRSV takes control of the host immune response to its advantage.
Conclusion
Little is known about the biological functions of the individual nsps of PRRSV, except that they exhibit replicase, protease and polymerase activities. With the continuing emergence of viral strains, the nsp2 replicase protein with its noted mutations, insertions and deletions has become a vital region for monitoring the rapid viral genomic evolution and for molecular epidemiology research on PRRSV (Allende et al., Reference Allende, Lewis, Lu, Rock, Kutish, Ali, Doster and Osorio1999; Gao et al., Reference Gao, Guo and Yang2004; Ropp et al., Reference Ropp, Wees, Fang, Nelson, Rossow, Bien, Arndt, Preszler, Steen, Christopher-Hennings, Collins, Benfield and Faaberg2004; Fang et al., Reference Fang, Kim, Ropp, Steen, Christopher-Hennings, Nelson and Rowland2004, Reference Fang, Rowland, Roof, Lunney, Christopher-Hennings and Nelson2006, Reference Fang, Schneider, Zhang, Faaberg, Nelson and Rowland2007; Han et al., Reference Han, Wang and Faaberg2006, Reference Han, Liu, Wang and Faaberg2007; Kim et al., Reference Kim, Calvert, Chang, Horlen, Kerrigan and Rowland2007; Yoshii et al., Reference Yoshii, Okinaga, Miyazaki, Kato, Ikeda and Tsunemitsu2008). The PRRSV nsp anti-IFN activity is certainly involved in the paradoxical immune response observed in PRRSV infected swine and would explain the inability of the host to clear PRRSV. The capability of the PRRSV nsps to interfere with the establishment of the innate and cellular antiviral states suggests that nsps are critical virulence determinants. In the near future, research programs involving nsp immune regulation of the infected host will likely provide important insights into the mechanism of PRRSV pathogenesis which could lead to new and innovative approaches for the control of PRRSV. The N, M and GP5 viral structural proteins are essential and sufficient for particle formation, but the minor structural proteins are also required for virus infectivity (Wieringa et al., Reference Wieringa, de Vries and Rottier2003, Reference Wieringa, de Vries, van der Meulen, Godeke, Onderwater, van Tol, Koerten, Mommaas, Snijder and Rottier2004; Wissink et al., Reference Wissink, Kroese, van Wijk, Rijsewijk, Meulenberg and Rottier2005). The N protein seems to be the most immunogenic viral protein and an ideal target for the serological detection of infected pigs (Denac et al., Reference Denac, Moser, Tratschin and Hofmann1997; Dea et al., Reference Dea, Wilson, Therrien and Cornaglia2000b; Seuberlich et al., Reference Seuberlich, Tratschin, Thur and Hofmann2002; Ferrin et al., Reference Ferrin, Fang, Johnson, Murtaugh, Polson, Torremorell, Gramer and Nelson2004). Nonetheless, some non-structural viral proteins, such as nsp2, are also highly immunogenic (Oleksiewicz et al., Reference Oleksiewicz, Botner and Normann2001a, Reference Oleksiewicz, Botner and Normannb, Reference Oleksiewicz, Botner and Normann2002; Fang et al., Reference Fang, Kim, Ropp, Steen, Christopher-Hennings, Nelson and Rowland2004; Johnson et al., Reference Johnson, Yu and Murtaugh2007). In addition, nuclear and/or nucleolar localization of N protein could have an important role in cell gene expression regulation (Hiscox, Reference Hiscox2002, Reference Hiscox2003, Reference Hiscox2007; Rowland and Yoo, Reference Rowland and Yoo2003; Weidman et al., Reference Weidman, Sharma, Raychaudhuri, Kundu, Tsai and Dasgupta2003; Uchil et al., Reference Uchil, Kumar and Satchidanandam2006).
It is further evident that the current vaccines based on a single PRRSV strain and on expression of GP5 alone are not or only partially effective (Kimman et al., Reference Kimman, Cornelissen, Moormann, Rebel and Stockhofe-Zurwieden2009). Viral epitopes that are capable of inducing neutralizing Abs appear to reside on the M, GP2a, GP3, GP4 and GP5 proteins (Yang et al., Reference Yang, Frey, Yoon, Zimmerman and Platt2000; Ansari et al., Reference Ansari, Kwon, Osorio and Pattnaik2006; Plagemann, Reference Plagemann2006; Kim and Yoon, Reference Kim and Yoon2008). Of these, neutralizing Abs to GP5 appear to be most relevant for protection since most of the neutralizing Abs found in infected animals are directed against the GP5. Unfortunately, it is also one of the most variable proteins among viral strains. Several recent studies using expression of the fusion proteins M–GP5 and GP3–GP5, GP4–GP5, or GP3–GP4–GP5 showed that specific immune response against GP5 was significantly increased by fusion of the proteins (Jiang et al., Reference Jiang, Jiang, Li, Tang, Wang and Ma2006a, Reference Jiang, Jiang, Wang, Li and Du2008). Glycosylation of GP5 of PRRSV plays an important role in escaping or minimizing virus-neutralizing Ab response by a N-glycan-shielding mechanism (Kimman et al., Reference Kimman, Cornelissen, Moormann, Rebel and Stockhofe-Zurwieden2009). The glycosylation of GP5 diminishes the immunogenicity of the nearby enclosed neutralizing epitope, and is thus critical for the induction or evasion of a neutralizing Ab response. These indicate that, for vaccine development, GP5 should be optimized in its ability to induce neutralizing Abs by allowing its fusion with other viral proteins or by reducing its glycosylation state.
The virulence of PRRSV is associated with multiple factors such as, host genetic, viral genetic, environment, concomitant infections, and consequently it is not easy to identify the determinant regions in the genome of PRRSV involved in the viral virulence (Wang et al., Reference Wang, Liang, Han, Burkhart, Vaughn, Roof and Faaberg2008). However, searching viral genomic regions or viral genes related to PRRSV virulence is an essential step toward making a contribution to the understanding of the pathogenicity of PRRSV. In order to determine virulence associated genes in PRRSV, reverse genetics has been abundantly used (Kwon et al., Reference Kwon, Ansari, Pattnaik and Osorio2008; Wang et al., Reference Wang, Liang, Han, Burkhart, Vaughn, Roof and Faaberg2008). Using this experimental approach, it was found that PRRSV virulence is multigenic and resides in both the non-structural and structural viral proteins (Kwon et al., Reference Kwon, Ansari, Pattnaik and Osorio2008; Wang et al., Reference Wang, Liang, Han, Burkhart, Vaughn, Roof and Faaberg2008).
Acknowledgment
The authors are grateful to Cynthia M. Guilbert for critically reviewing the manuscript. Dr C.A. Gagnon was supported financially by Natural Sciences and Engineering Research Council of Canada (NSERC) discovery grants, the Fédération des producteurs de porcs du Québec (FPPQ), the Centre d'insémination porcine du Québec Inc. (CIPQ), and the Conseil pour le développement de l'agriculture du Québec (CDAQ).







