Introduction
Laryngotracheal stenosis in the paediatric population can have multiple causes, most commonly occurring as a result of prolonged intubation. Endoscopic techniques have been used to dilate laryngotracheal stenosis since 1870.Reference Barwell and Lond 1 By the 1970s, open procedures such as laryngotracheal reconstruction were introduced to manage severe subglottic stenosis.Reference Holinger, Kutnick, Schild and Holinger 2 , Reference Cotton 3
Reported modalities for endoscopic expansion of the airway include rigid dilatation using a bronchoscope or bougie, cold knife techniques, and laser.Reference Ang, Modi, Raithatha, April and Ward 4 With the advent of balloon angioplasty catheters for cardiac procedures, the use of balloon dilatation in the airway has become increasingly popular.Reference Guarisco and Yang 5 The advantages include less post-operative pain, reduced in-patient stay, fewer complications and better voice results when compared to open procedures.Reference Lando, April and Ward 6 Rigid dilatation results in longitudinal shearing forces in the mucosal and submucosal layers, which can cause subepithelial fibrosis that can lead to recurrent stenosis. In contrast, balloon dilatation results in dilatation in a radial direction, thus reducing trauma to the mucosal and subepithelial layers.Reference Brown, Hedlund and Glasier 7 There are also theories that balloon dilatation causes full- or partial-thickness fractures of the cricoid cartilage, decompressing the subglottis.Reference Ang, Modi, Raithatha, April and Ward 4
Although patients often experience symptomatic improvement after dilatation, the recurrence rates have been reported as between 40 and 70 per cent over months to years.Reference Hseu, Benninger, Haffey and Lorenz 8 The use of mitomycin C has also been extensively investigated, with the theory that it can improve wound healing in the airway.Reference Smith and Elstad 9
There is very little information available in the literature to guide balloon sizing, and currently surgeons often use their own breadth of experience to guide balloon sizing, inflation pressures and length of inflation time. Excessively high inflation pressures can damage or rupture the airway, and inadequately high pressures can reduce the effectiveness of procedures and result in the requirement for even more procedures. Although two systematic reviews have concluded that airway balloon dilatation is very effective in treating subglottic stenosis, there is no consensus regarding balloon diameters or inflation pressures.Reference Wentzel, Ahmad, Discolo, Gillespie, Dobbie and White 10 , Reference Lang and Brietzke 11 One animal study that used rabbit models to determine the optimum balloon sizes found that the minimum balloon size required to create a fracture in the cricoid cartilage in an airway diameter of 5.4 mm was 7.0 mm, at a pressure of 6.0 atm.Reference Ang, Modi, Raithatha, April and Ward 4 A similar study on a live rabbit model showed that airway balloon dilatation with balloon diameters that exceeded the airway diameter by 2.6 mm was associated with cricoid fractures.Reference Modi, Visaya and Ward 12
This study aimed to describe our experience regarding the use of balloon dilatation and to provide guidelines for maximum safe balloon sizes according to age in children undergoing this procedure.
Materials and methods
This study was approved by the Great Ormond Street Hospital for Children NHS Foundation Trust Clinical Governance Department.
A retrospective review of case notes and the otolaryngology department operative database was performed for all children who underwent balloon dilatation of their airway for subglottic stenosis in a paediatric tertiary referral unit (Great Ormond Street Hospital for Children) over a 10-year period, between May 2006 and February 2016. Patient demographics, co-morbidities, number of procedures and procedure details (including balloon size (in millimetres), inflation pressure (in atmospheres) and length of inflation time (in seconds)) were all recorded and analysed. Patients for whom there were insufficient data recorded were excluded.
Statistical analyses and comparisons (conducted using GraphPad Software, La Jolla, California, USA) were carried out using a linear regression model, and significance was set at p < 0.05. 13
Results
A total of 166 patients underwent balloon dilatation of the airway during the study period. There were 75 male patients and 91 female patients. Mean (± standard deviation (SD)) patient age was 4.5 ± 3.99 years. Of the 166 patients, 94 (57 per cent) also had concurrent airway pathologies (e.g. laryngomalacia, laryngeal cleft, tracheomalacia, trachea-oesophageal fistula). The most common co-morbidities included cardiac conditions (e.g. patent ductus arteriosus, tetralogy of Fallot) (48 out of 166, 29 per cent) and trisomy 21 (19 out of 166, 11 per cent).
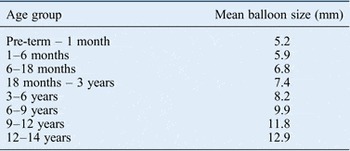
The reason for balloon dilatation in all cases was subglottic stenosis. Grade of subglottic stenosis was measured using the Myer–Cotton classification.Reference Cotton 3 The median grading of stenosis was grade 2. The median balloon size used was 8 mm, the median balloon inflation pressure was 10 atm and the mean (± SD) balloon inflation time was 65.1 ± 18.6 seconds. We also calculated the mean balloon diameters (in millimetres) for different age groups (Table I). The majority of patients (148 out of 166 patients, 89 per cent) underwent multiple balloon dilatation procedures. Multiple balloon dilatations were generally not performed during the same operation unless it was felt that the initial balloon dilatation was not effective. No significant unexpected events occurred.
Table I Mean balloon size for different age groups

We looked at the correlation between patient age and balloon size used. The Pearson correlation co-efficient (R value) was 0.85 (p = 0.001), suggesting a strongly positive correlation between these variables (Figure 1). We also examined the correlation between subglottic stenosis grade and balloon size used. The Pearson correlation co-efficient (R value) was 0.15 (p = 0.09), suggesting no correlation between these variables. The Pearson correlation co-efficient (R value) between subglottic stenosis grade and balloon inflation pressure was 0.68 (p = 0.001), suggesting a moderately positive correlation between these variables (Figure 2). The Pearson correlation co-efficient (R value) between subglottic stenosis grade and balloon inflation time was 0.22 (p = 0.06), suggesting little correlation between these variables.

Fig. 1 Correlation between patient age and balloon size.

Fig. 2 Correlation between subglottic stenosis grade and balloon inflation pressure.
Discussion
Airway balloon dilatation has become increasingly popular for the management of subglottic stenosis. It has the advantages of relative ease and low morbidity when compared to open reconstruction techniques, such as laryngotracheal reconstruction and cricotracheal resection, which are usually reserved for more severe cases. Success rates of balloon dilatation used for the treatment of subglottic stenosis are reported as up to 100 per cent, although many patients require repeat airway dilatations.Reference Avelino, Maunsell and Wastowski 14 The success of airway balloon dilatation is defined by the long-term resolution of stenosis and the avoidance of open surgery such as laryngotracheal reconstruction. Modern balloon catheters have low profiles, which allow them to be passed atraumatically through small stenotic segments, to be subsequently inflated. They are also oblong in shape, creating greater stability and resistance to motion when inflated.Reference Visaya, Ward and Modi 15 In addition, they apply force in a radial direction, thus theoretically allowing the force to be applied to a more focused area and limiting mucosal trauma.Reference Modi, Visaya and Ward 12
There is little in the literature that provides guidance for balloon diameter size and inflation pressures. There are National Institute for Health and Clinical Excellence Interventional Procedure guidelines in the UK regarding the use of endoscopic balloon dilatation for subglottic or tracheal stenosis, which were published in December 2011. 16 The guidance concludes that the procedure is relatively safe, but there is a poor evidence base, and no clear recommendations regarding balloon size, inflation pressures or inflation time. The introduction of the Airway Intervention Registry in the UK (in March 2015), designed to collect information on paediatric balloon dilatation procedures for airway stenosis on a national scale, will be useful to improve the evidence base for such procedures in children, and may help create guidelines. 17
Many of the previously published case series of patients with airway stenosis undergoing airway balloon dilatation do not explain the rationale for the selection of particular balloon diameters.Reference Axon, Hartley and Rothera 18 – Reference Schweiger, Smith, Kuhl, Manica and Marostica 20 One study suggests using diameters based on the age-appropriate airway size.Reference Whigham, Howell, Choi, Pena, Zalzal and Preciado 21 Another study suggests the use of a balloon 1 mm larger than the age-appropriate endotracheal tube, whilst a slightly larger study suggests sizing the balloon 2–4 mm larger than the normal airway diameter, or 100–125 per cent of the normal airway diameter.Reference Maturo and Hartnick 22 – Reference Bent, Shah, Nord and Parikh 24 A balloon diameter column has recently been added to the Myer–Cotton subglottic stenosis table, which suggests that the balloon diameter should be no larger than 1 mm above the age-appropriate subglottic diameter.Reference Myer, O'Connor and Cotton 25
With regard to inflation pressure, there is a wide range quoted in the literature.Reference Modi, Visaya and Ward 12 Some papers do not report the inflation pressures used. Some quote 2 atm,Reference Schweiger, Smith, Kuhl, Manica and Marostica 20 , Reference Durden and Sobol 26 others report between 4 and 7 atm,Reference Hebra, Powell, Smith and Othersen 27 and one paper quotes up to 16 atm.Reference Schweiger, Smith, Kuhl, Manica and Marostica 20 One paper suggests reading the package information leaflet and adopting the manufacturers’ recommended settings.Reference Maturo and Hartnick 22 This is because the pressure applied depends on the balloon type and diameter with compliant balloons.
Animal studies, such as those using New Zealand white rabbits, suggest that airway balloon dilatation with balloon diameters that exceed the airway diameter by 2.6 mm is associated with cricoid fractures.Reference Modi, Visaya and Ward 12 In that particular study, when balloon diameter was kept constant, inflation pressure had no correlation with mucosal injury or probability of cricoid fracture. An older pilot study by the same research group suggested that the cricoid cartilage remained intact when using a balloon 0.6 mm or smaller than the measured subglottis diameter, and a fracture occurred when using a balloon 1.6 mm or larger than the measured subglottis diameter with a pressure as low as 6 atm.Reference Ang, Modi, Raithatha, April and Ward 4 They also found that when a gross fracture did occur, it was always at the anterior cricoid ring. Another animal study reported that intra-operative mortality from cardiopulmonary arrest reached 50 per cent when the balloon diameter exceeded the airway diameter by 4.6 mm.Reference Visaya, Ward and Modi 15 In addition, they found that post-operative feeding difficulties could occur with any balloon diameter or inflation pressure.
Appropriate balloon catheter sizing is important given the risk of potential cricoid rupture with excessive expansion. This has been reported in animal studies, as described previously, but has never been reported in humans. Standard practice involves the use of an endotracheal tube to determine the airway diameter. This information, together with expected airway diameter for age in a normal airway, is used to identify the balloon size to be selected.Reference Myer, O'Connor and Cotton 25
Our results demonstrate our experience with balloon dilatation. They show that there are currently no clear patterns when deciding on appropriate balloon diameters, inflation pressures and inflation times, although increasing balloon size should be used with increasing age. However, given that we had no adverse complications, such as cricoid rupture, we can infer that it is safe to keep within the limits of balloon dilatation described in our results. Therefore, the results suggest that using a balloon diameter which is equal to the outer diameter of an age-appropriate endotracheal tube +2 mm is safe for treating airway stenosis, without causing complications. Our data do not, however, give any idea as to the maximum balloon diameter size that can be used before complications, such as cricoid fracture, ensue. Previous animal studies have demonstrated in vivo maximum balloon diameter sizes for varying tracheal diameters. However, there are no reports regarding maximum balloon dilatation diameters in the airways of children.
Based on expert opinion, and using our data and the previous literature, we recommend using a balloon with the outer diameter of the age-appropriate endotracheal tube for the child +1 mm for the larynx or subglottis and +2 mm for the trachea, leaving a safe margin for error. We have designed a table to show recommended safe maximum balloon catheter sizes for endoscopic dilatation of stenosis based on patient age and stenosis site (Table II). The table also includes existing data on recommended endotracheal tube size, and reference values for cricoid and tracheal transverse diameters for the same age groups.Reference Tweedie, Skilbeck, Cochrane, Cooke and Wyatt 28
Table II Our recommended safe maximum balloon catheter sizes for endoscopic dilatation of stenosis*

We include existing data on recommended endotracheal tube size, and reference values for cricoid and tracheal transverse diameters for the same age groups.Reference Tweedie, Skilbeck, Cochrane, Cooke and Wyatt 28 *Based on patient age and site of stenosis.
Balloon catheters may be compliant (i.e. continue to expand with increasing pressure) or non-compliant (i.e. expand to a fixed diameter with increasing pressure). Compliant balloon catheters should be used with care to avoid overstretching the cricoid with increasing inflation pressures. A recent study using a three-dimensional computer model identified that compliant balloons were at least as likely as non-compliant balloons to fracture the cricoid.Reference Johnson, Howell, Mettenburg, Rueggeberg, Howell and Postma 29 With compliant balloons, there is an element of unpredictability, as the external diameter of the balloon increases with increasing pressure, and there is a theoretical risk of damage to surrounding tissue. Non-compliant or semi-compliant balloons provide more focused pressure in a more predictable fashion, without altering balloon diameter with increasing inflation pressure, and these can be more effective in treating rigid stenosis.Reference Mauro, Murphy, Thomson, Venbrux and Morgan 30 Thus, we recommend the use of non-compliant or semi-compliant balloons.
-
• Appropriate balloon catheter sizing is important given the risk of potential cricoid rupture with excessive expansion
-
• Standard practice involves the use of an endotracheal tube to determine the airway diameter
-
• This information, together with expected airway diameter for the child's age in a normal airway, is used to select balloon size
-
• The results suggest an outer diameter of the age-appropriate endotracheal tube +1 mm for the larynx or subglottis and +2 mm for the trachea
-
• Balloon inflation pressure should be 10 atm for 1 minute
-
• Compliant or semi-compliant balloon catheters should be used with care to avoid overstretching the cricoid with increasing inflation pressures
With regard to balloon inflation pressures and inflation time, there do not appear to be clear patterns based on our data. However, we would recommend using a range of inflation pressures, between 6 and 14 atm, depending on the size of the airway and degree of stenosis, and guided by manufacturer-recommended pressure specifications for the size and type of balloon used. Our preferred balloon inflation time is 1 minute. In cases of acquired soft-tissue stenosis, radial cuts through the stenosis using a sickle knife, prior to balloon dilatation, can help with management.
This study was limited by the relatively small sample size. Furthermore, it was retrospective, relying on accurate database and operative note recording; thus, there may be an element of selection and reporting bias. Patients for whom there were insufficient data were excluded.
Further prospective studies are warranted to investigate the use of balloon dilatation in other paediatric tertiary centres. In addition, further in vitro studies demonstrating safe levels of balloon inflation would be useful, particularly those investigating the maximum levels of balloon dilatation in humans.
Conclusion
Appropriate balloon catheter sizing is important given the risk of potential cricoid rupture with excessive expansion described in animal studies. Standard practice involves the use of an endotracheal tube to determine the airway diameter. This information, together with expected airway diameter for age in a normal airway, is used to identify the balloon size to be selected. Based on expert opinion, animal studies and our data, we would suggest using the outer diameter of the age-appropriate endotracheal tube for the child +1 mm for the larynx or subglottis and +2 mm for the trachea. Compliant or semi-compliant balloon catheters should be used with care to avoid overstretching the cricoid with increasing inflation pressures.
Acknowledgement
Grateful thanks to the administrative staff in the ENT department in Great Ormond Street Hospital for Children.