INTRODUCTION
Sexual dimorphism and allometry are two widely known sources of phenotypic variation in the animal kingdom (Shine, Reference Shine1989; Weckerly, Reference Weckerly1998). Various hypotheses explain patterns of sexual dimorphism, such as natural selection on reproductive traits, niche divergence or other ecological causes (Abouheif & Fairbairn, Reference Abouheif and Fairbairn1997; Fairbairn, Reference Fairbairn1997). Hypotheses regarding selective processes have a common property that pattern (relative dimorphism of different traits), direction (which sex is larger) and magnitude of sexual dimorphism may predict components of reproductive success. On the other hand, the niche divergence or ecological sexual dimorphism hypothesis proposes that sexual dimorphism evolves to reduce intraspecific competition for food and is not associated directly with selection on reproductive traits (Fairbairn, Reference Fairbairn1997).
Morphological differences between sexes may arise either during development or at the adult stage only. In both cases, growth rate during development and level of allometry will determine sexual dimorphism. Allometry can be defined as differences in body proportions correlated with changes in the absolute magnitude of the total organism (Fairbairn, Reference Fairbairn1997; Klingenberg, Reference Klingenberg1998). There are three kinds of allometry: (1) Ontogenetic allometry (association between size and shape across different age stages), frequently used as an estimate of a population's ontogenetic trajectory; (2) Static allometry (association between size and shape within the same species and developmental stage), often used to explain coevolution of size and shape; and (3) Evolutionary allometry (covariation between size and shape among species), as a model for functional and behavioural adaptations (Cock, Reference Cock1966; Klingenberg, Reference Klingenberg, Marcus, Corti, Loy, Naylor and Slice1996).
Growth and reproduction are antagonistic processes and constitute a trade-off during development (Roff, Reference Roff2000; Koene & Ter Maat, Reference Koene and Ter Maat2004). In crustaceans the energetic demand for reproduction may interrupt somatic growth or culminate in the development of secondary sexual characters due to positive allometry of certain body structures. This occurs in males and females in a different way, resulting in distinct energetic expenditure in each sex (Hartnoll, Reference Hartnoll and Wenner1985). When there are modifications during ontogeny, it is possible to quantify how much one would contribute to a disparity between life stages (juveniles and adults) and how morphological changes vary between sexes (Sheets & Zelditch, Reference Sheets and Zelditch2013). Thus, information regarding sexual dimorphism and ontogenetic allometry can be useful to understand energy use during development, reproductive processes and sexual selection (Stearns & Koella, Reference Stearns and Koella1986; Dalabona & Pinheiro, Reference Dalabona and Pinheiro2005).
Studies on ontogenetic allometry and sexual dimorphism in brachyuran crabs are common, especially those using relative growth to determine the onset of morphological sexual maturity (Hartnoll, Reference Hartnoll1978, Reference Hartnoll2001; Marochi et al., Reference Marochi, Moreto, Lacerda, Trevisan and Masunari2013). However, these studies are related to classical morphometrics based on linear measurements. More recently, the geometric morphometrics (GM) approach has improved these analyses, as this technique is based on anatomical landmarks to describe shape-related variation and identify full shape changes (Rosenberg, Reference Rosenberg2002; Adams et al., Reference Adams, Rohlf and Slice2004). Furthermore, stepped growth through moulting combined with the crustacean calcified exoskeleton facilitate morphometric comparisons between sexes and life stages (juvenile and adult).
In order to analyse the period in which sexual dimorphism appears during ontogeny and increase the understanding of ontogenetic and sexual evolutionary trends in Sesarmidae crabs, a comparative approach was carried out in Aratus pisonii (H. Milne Edwards, 1873) and Armases rubripes (Rathbun, 1897). Our aims were to evaluate both: (1) sexual dimorphism in shape and size of the carapace and cheliped propodus of juveniles and adults, and (2) the ontogenetic trajectory, using geometric morphometrics (GM) techniques. We tested the hypothesis that sexual dimorphism of Sesarmidae shape and size is present before the puberty moult (adult phase), during juvenile development. These two sesarmid species were chosen because both are abundant semi-terrestrial crabs that inhabit estuarine regions and display sexual dimorphism known only during the adult phase (male body and chelipeds larger than female's) (Lima et al., Reference Lima, Soares and Oshiro2006; Pescinelli et al., Reference Pescinelli, Davanso and Costa2015). Such characteristics indicate these species are good models to evaluate sexual dimorphism in shape and size during ontogeny (from juvenile to adult phase) using a sensitive technique such as GM.
MATERIALS AND METHODS
Sampling
Aratus pisonii individuals were sampled from Paranaguá Bay (25°30′58″S 48°29′58″W), while Armases rubripes individuals were sampled from Guaratuba Bay (25°51′29.82″S 48°44′0.14″W), both in the Paraná state coast, Brazil. These were collected by hand and preserved in 75% ethanol. A total of 77 females (31 juveniles and 46 adults) and 70 males (33 juveniles and 37 adults) of Aratus pisonii and 253 females (25 juveniles and 228 adults) and 247 males (53 juveniles and 194 adults) of Armases rubripes were sampled. Only intact carapaces and cheliped propodus without regeneration signs were included in the analyses.
Size at onset of morphological sexual maturity
For Aratus pisonii, we considered as juveniles (both sexes) individuals smaller than 10 mm in carapace width (CW), based on a previous study by Pescinelli et al. (Reference Pescinelli, Davanso and Costa2015). For Armases rubripes, a relative growth analysis was performed to obtain the size at onset of morphological sexual maturity since this information was not available in the literature.
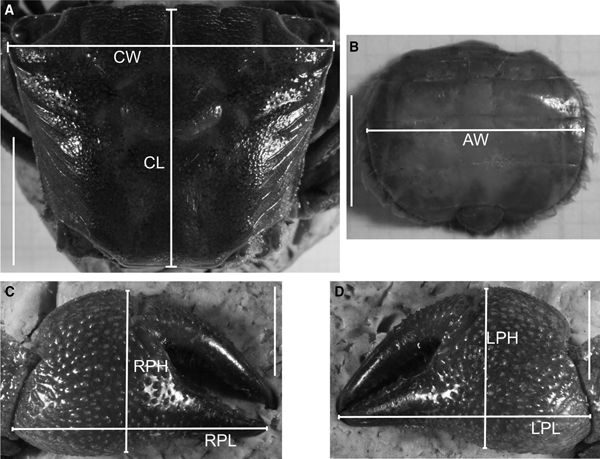
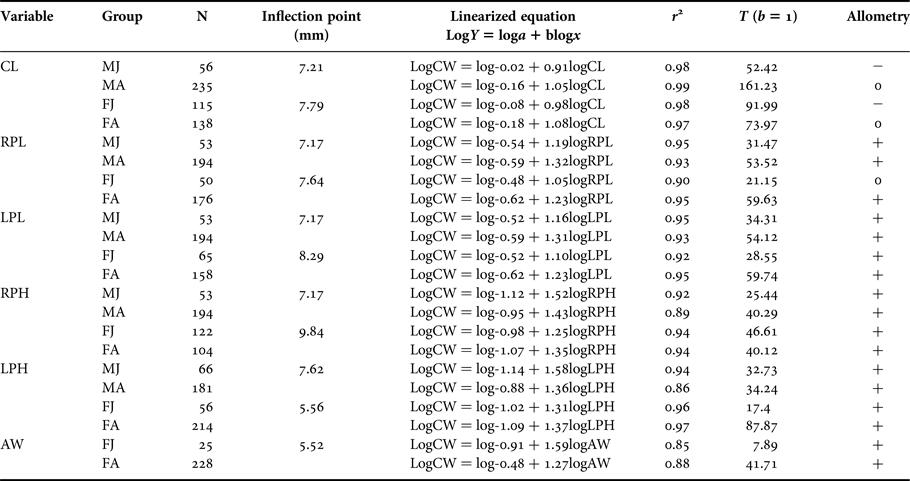
For the study of relative growth in Armases rubripes, the following linear body dimensions were measured for 247 males, 186 non-ovigerous females and 67 ovigerous females, using a digital calliper (0.01 mm precision): carapace width (CW) and length (CL), right cheliped propodus length (RPL) and height (RPH), left cheliped propodus length (LPL) and height (LPH) for both sexes, and abdomen width (AW) at the basis of the 4th somite in females only (Figure 1). CW was considered as an independent variable and the remaining, dependent. Due to visible homochely in these species (chelipeds with similar size and shape), chelipeds were designated as right and left, and not as major and minor. Body dimensions choice was based on Hartnoll (Reference Hartnoll1974).
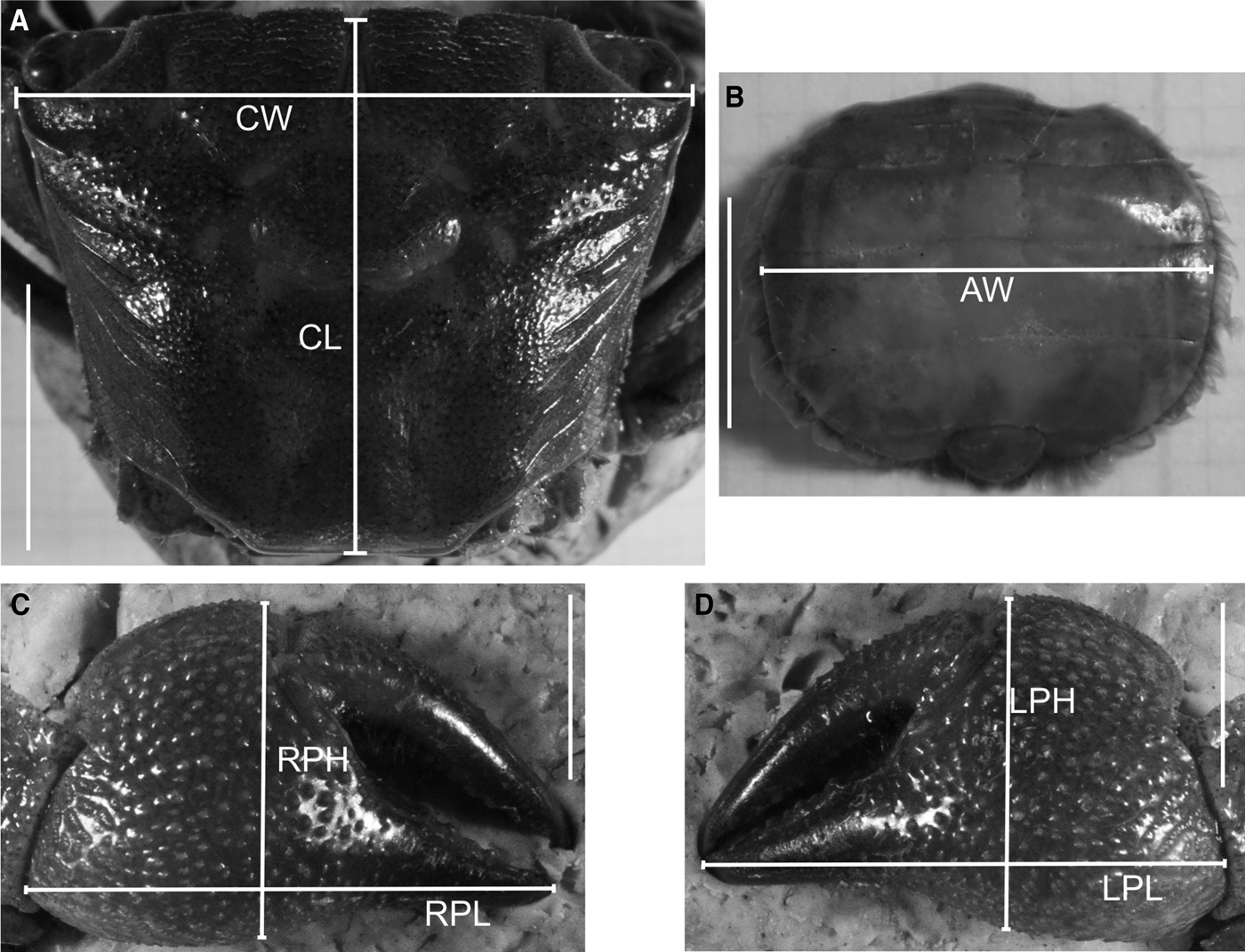
Fig. 1. Armases rubripes. Measured body dimensions for relative growth analysis. CW, carapace width; CL, carapace length; RPL, right cheliped propodus length; RPH, right cheliped propodus height; LPL, left cheliped propodus length; LPH, left cheliped propodus height; AW, abdomen width at the basis of the 4th somite. Scale: A = 10 mm; B, C and D = 5 mm.
Changes in the proportion of body dimensions in relation to CW were tested using the allometric equation y = ax b. This equation was linearized (logy = loga + blogx), where x represents the independent variable (CW) and y represents the dependent variables (all other body measurements) (Huxley, Reference Huxley1950).
The allometric coefficient (b) was assessed with a Student's t-test against the null hypothesis of isometry (b = 1). The slopes of lines and intersections between lines for juveniles and adults were tested through an analysis of covariance (ANCOVA), using a 95% confidence interval (Sokal & Rohlf, Reference Sokal and Rohlf1979). To adjust regression lines and calculate the onset of morphological sexual maturity the software REGRANS (Pezzuto, Reference Pezzuto1993) was used. All remaining analyses were performed in BioEstat 5.0 (Ayres et al., Reference Ayres, Ayres, Ayres and Santos2007).
Sexual dimorphism: geometric morphometrics procedures and statistical analysis
Pictures from the carapace in dorsal view for all individuals of both species were obtained with a Fujifilm Finepix HS10 camera with a resolution of 10 megapixels. Likewise, pictures of the right (Aratus pisonii, N = 35 females and 25 males; Armases rubripes, N = 62 females and 80 males) and left (Aratus pisonii, N = 32 females and 25 males; Armases rubripes, N = 62 females and 79 males) cheliped propodus were obtained for both species, only for adults. Cheliped propodus of juveniles were not analysed because it was not possible to establish the same landmarks as in the adults. Differences in the number of carapaces and chelipeds used in the morphometrics analyses were due to the fact that only intact right and left chelipeds were used.
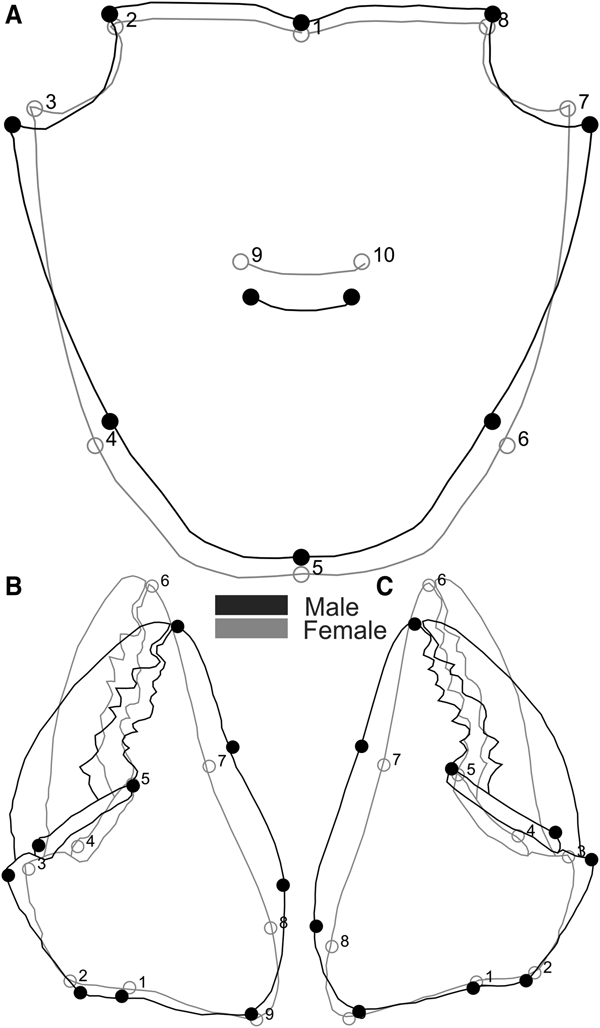
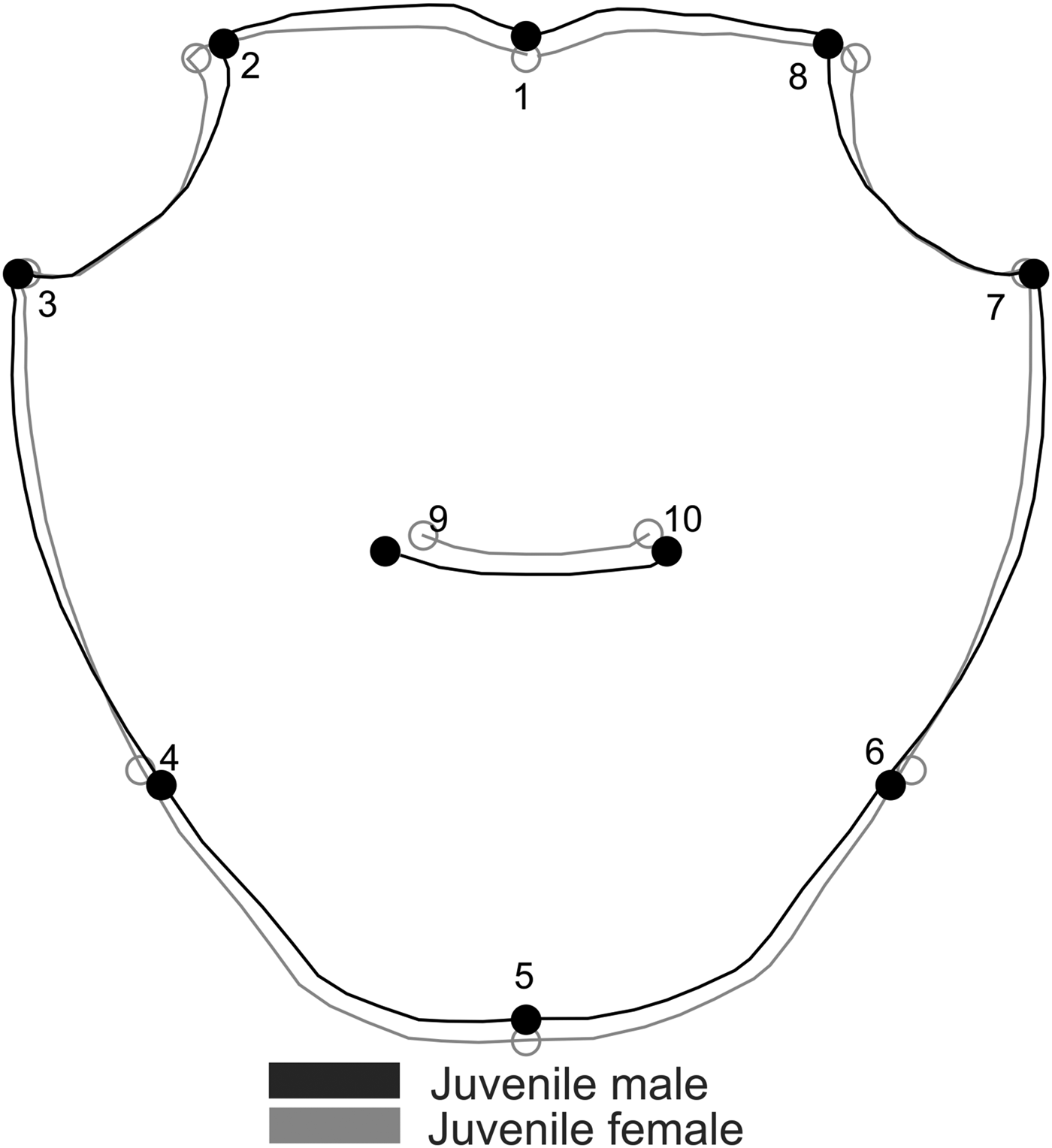
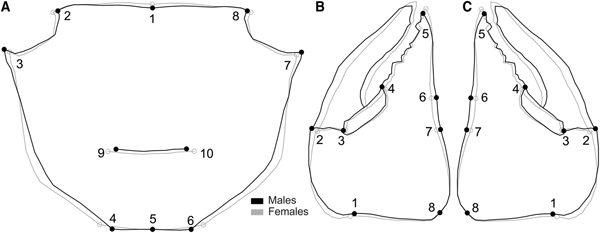
We defined 10 two-dimensional landmarks in the carapace of both species, nine in the propodus of the right and left cheliped for Aratus pisonii (Figure 2) and eight for Armases rubripes (Figure 3), using TPS Dig 2, version 2.16 (Rohlf, Reference Rohlf2010). In juveniles, the same carapace landmarks were used, except for the cardiac line (landmarks 9 and 10) in Armases rubripes due to difficulties in establishing said juveniles.
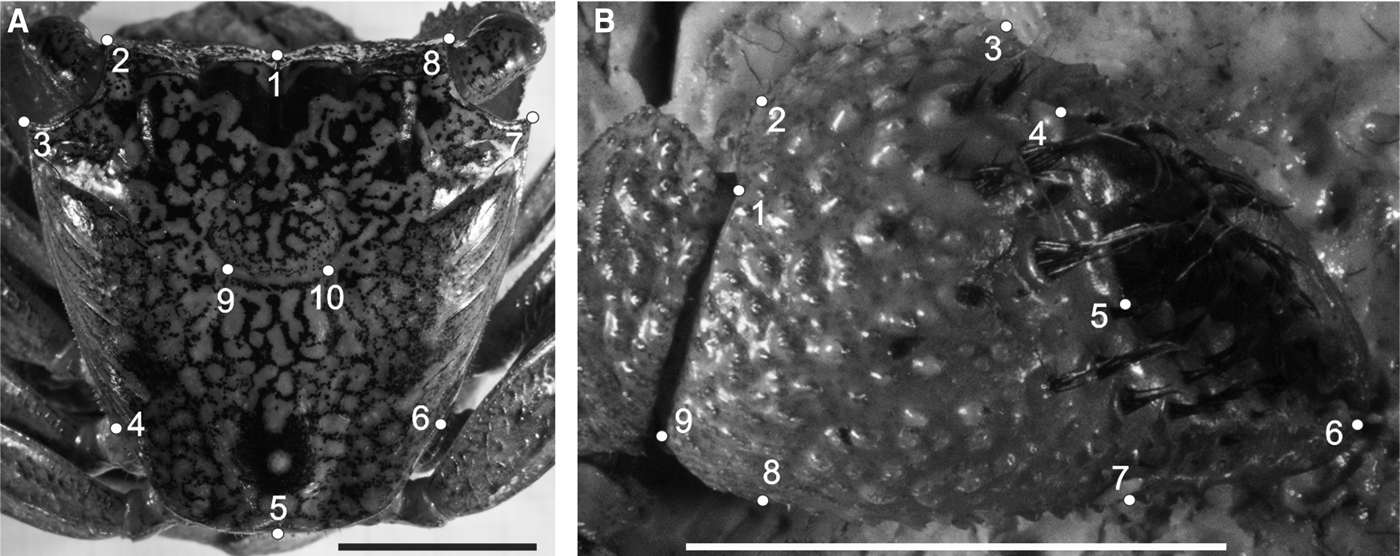
Fig. 2. Aratus pisonii. Position of anatomical landmarks in the carapace (A) and cheliped propodus (B). Scale 10 mm. (A) 1: Extreme of protogastric region; 2 and 8: End of antero-lateral line; 3 and 7: Tip of antero-lateral tooth; 4 and 6: Beginning of the lateral margin; 5: Posterior margin of intestinal region; 9 and 10: Extremes of cardiac line. (B) 1: Inner base of the carpo-propodus articulation; 2: Proximal tip of the cheliped propodus; 3: Distal tip of the cheliped propodus; 4: Suture in the intersection between ‘pre-dactilar’ lobe and the base of cheliped propodus; 5: Base of the fixed finger of the cheliped propodus; 6: Tip of the fixed finger; 7: Limit of fixed finger in the margin of cheliped propodus; 8: Vertical line through the tip of the cheliped propodus; 9: Outer base of the carpo-propodus articulation.

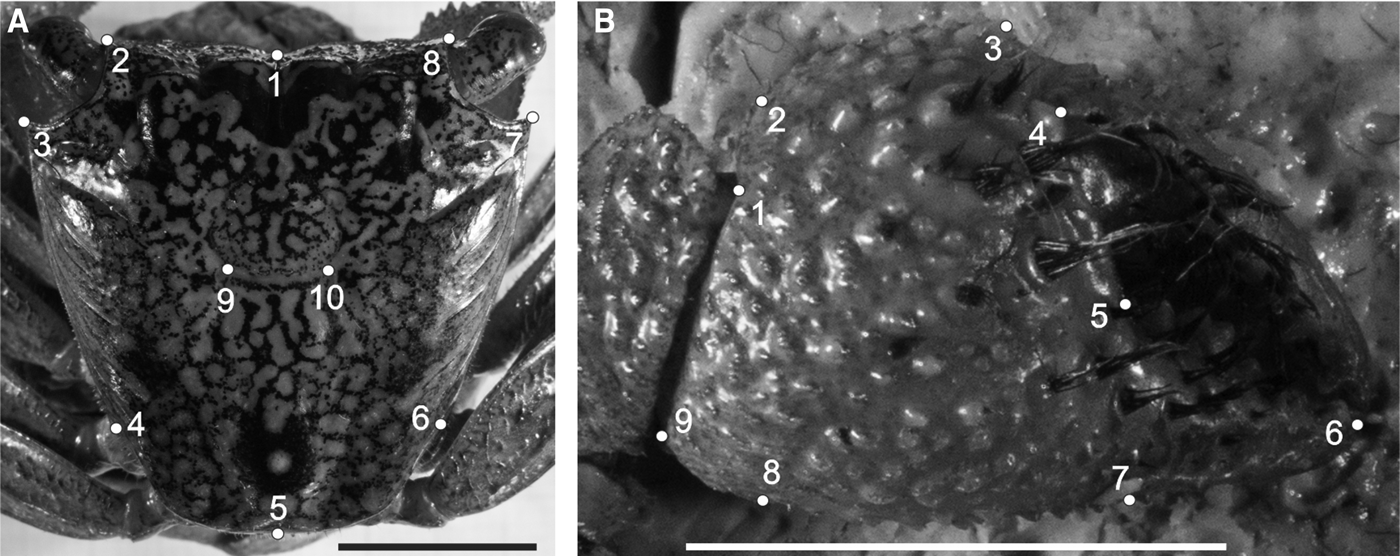
Fig. 3. Armases rubripes. Position of anatomical landmarks in the carapace (A) and right cheliped propodus (B) for sexual dimorphism and allometric ontogeny comparisons. (A) 1: Extreme of protogastric region; 2 and 8: End of antero-lateral line; 3 and 7: Tip of antero-lateral tooth; 4 and 6: End line of intestinal region; 5: Posterior margin of intestinal region; 9 and 10: Extremes of cardiac line. (B) 1: Inner base of the carpo-propodus articulation; 2: Distal tip of the cheliped propodus; 3: Suture in the intersection between ‘pre-dactilar’ lobe and the base of cheliped propodus; 4: Base of the fixed finger of the cheliped propodus; 5: Tip of the fixed finger; 6: Limit of fixed finger in the margin of cheliped propodus; 7: Vertical line through the distal tip of the cheliped propodus; 8: Outer base of the carpo-propodus articulation. 10 mm scale.
From the initial configurations, we performed a generalized Procrustes analysis (GPA) with raw landmark coordinates, which consisted of configurations superimposition based on the centroid (i.e. mass centre of the configuration), scaling the centroid size of each configuration to a value of 1 and rotating them (Monteiro & Reis, Reference Monteiro and Reis1999). GPA overlapping removed the effect of position, orientation, and size of landmark configuration, in such a way that the aligned configurations corresponded exclusively to structure shape (Adams et al., Reference Adams, Rohlf and Slice2004).
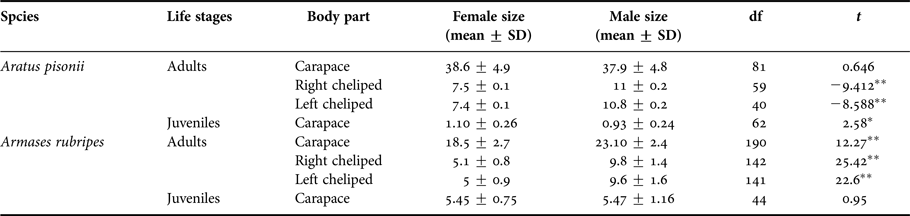
As the carapace right and left sides are separated by a central axis (symmetrical object), shape components (GPA aligned coordinates) can be separated into symmetrical and asymmetrical (Klingenberg et al., Reference Klingenberg, Barluenga and Meyer2002). Symmetrical components represent shape variation among individuals and were used to analyse carapace shape. Size of each structure was estimated through centroid size, which is calculated as the root sum of square distances of a group of points to that centroid (Monteiro & Reis, Reference Monteiro and Reis1999). Sexual size dimorphism (SSD) of the carapace and cheliped propodus in adults, and of the carapace in juveniles was analysed through a t-test using centroid size (log standardized). Analyses were performed in MorphoJ, version 1.06d (Klingenberg, Reference Klingenberg2011) and via the R environment (R Development Core Team, 2016).
When sexual size dimorphism was detected, a multivariate regression of symmetric components on log-transformed centroid size was done and regression residuals were used in sexual dimorphism shape analyses (Monteiro & Reis, Reference Monteiro and Reis1999; Klingenberg, Reference Klingenberg2016). This procedure performed a size correction of shape data. Shape of sexual dimorphism (SShD) was analysed using Procrustes shape coordinates. Correct percentage of classification was determined by cross-validation and the difference between mean Procrustes distance of groups was tested by permutation (Klingenberg, Reference Klingenberg2011).
Ontogenetic allometry: laboratory procedures and statistical analysis
Carapace measurements from all individuals of both species were used. After the GPA, carapace shape variation during development (ontogenetic allometry) was described through a multivariate regression of symmetric Procrustes coordinates components with the log-transformed centroid size separated by sex. The significant level of this multivariate regression was evaluated through a permutation test considering the null hypothesis of independency between variables. To avoid interference of static allometry, a multivariate regression of symmetric components of shape variables on log-transformed centroid size was done separately for juveniles and adults of both sexes (Monteiro & Reis, Reference Monteiro and Reis1999; Klingenberg, Reference Klingenberg2016). To test allometric trajectories of males and females, the angle between regression vectors was compared between sexes. Allometry was tested in both life stages (juvenile and adult) within each sex and between sexes. Analyses were performed in MorphoJ, version 1.06d (Klingenberg, Reference Klingenberg2011).
RESULTS
Relative growth of Armases rubripes
Carapace width (CW) ranged from 2.80 to 20.39 mm in males, from 3.62 to 18.23 mm in non-ovigerous females, and from 6.1 to 16.34 mm in ovigerous females. The regression between CL and CW had a negative coefficient for juveniles, indicating negative allometric growth and isometric growth in adults of both sexes (Table 1). This indicates that CW grows at a higher rate than CL in juveniles and at the same rate during adulthood.
Table 1. Armases rubripes. Regression analyses of morphometrics data.

CW, Carapace width; CL, Carapace length; RPL, Right cheliped propodus length; RPH, Right cheliped propodus height; LPL, Left cheliped propodus length; LPH, left cheliped propodus height; AW, Abdomen width at the basis of the 4th somite. MJ, Juvenile males; MA, Adult males; FJ, Juvenile females; FA, Adult females; N, Number of individuals; r 2 = Determination coefficient.
Cheliped propodus had a positive allometric growth in the majority of the demographic categories (for both sexes). However, allometric growth of RPL in juvenile females was isometric, meaning most dimensions grew at a faster rate than CW.
There were significant differences in straight line intercepts (a) and slopes (b) between juveniles and adults in all dimension comparisons (P < 0.05), except for slopes of CW × LPH (α = 0.13) and CW × AW (α = 0.59) in females. These exceptions showed distinct intercepts (P < 0.05), but a similar slope (Table 2). The inflection point for three (RPL, RPH and LPL) out of four dimensions of male cheliped propodus tested was 7.17 mm (Figure 4A–C). These can be an indicative of a good estimation of size at onset of morphological sexual maturity in A. rubripes males.

Fig. 4. Armases rubripes. Regression between CW and cheliped propodus dimensions (males), and CW and AW (females). The circles represent juveniles and squares represent adults. A: CW × LPL, B: CW × RPL, C: CW × RPH and D: CW × AW.
Table 2. Armases rubripes. Comparisons of the straight line slopes (b) and intercepts (a) for juveniles and adults of both sexes based on a covariance analysis (ANCOVA).
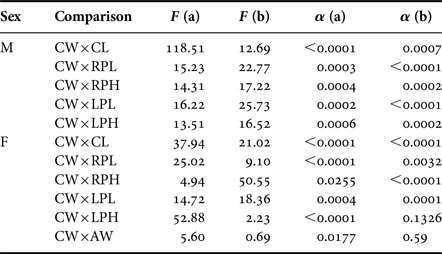
CW, Carapace width; CL, Carapace length; RPL, Right cheliped propodus length; RPH, Right cheliped propodus height; LPL, left cheliped propodus length; LPH, left cheliped propodus height; AW, Abdomen width at the basis of the 4th somite; M, Males; F, Females.
On the other hand, inflection point values obtained for female cheliped propodus dimensions were not related to sexual maturity. The dimension that showed a point of inflection separating juvenile females from adults was AW, with a positive allometric growth in both phases of development (Table 1) and an estimated value of 5.52 mm CW (Figure 4D). Size of the smallest female (6.1 mm CW) was closer to our estimated value for the onset of sexual maturity which may indicate a good estimate for the present purpose.
Sexual size dimorphism (SSD)
Aratus pisonii juveniles showed sexual dimorphism in carapace size (centroid size) during the juvenile phase, but not into adulthood, while in Armases rubripes SSD only manifested in adults (Table 3). Conversely, there was a pattern of sexual size dimorphism in the cheliped propodus: males had larger propodus than females in both species (Table 3).
Table 3. t-test results comparing body parts size (centroid size) of females and males of Aratus pisonii and Armases rubripes.

*P < 0.05, **P < 0.001.
Sexual shape dimorphism (SShD)
Carapace shape showed sexual dimorphism in adults of both species: for Aratus pisonii, Procrustes distance of 0.036, P < 0.001, with a correct classification of 96.3% (for both sexes), and for Armases rubripes, Procrustes distance of 0.012, P = 0.04, with a correct classification of 60% for females and 62.5% for males. In Aratus pisonii variation occurred mainly in landmarks 4 and 6, and 5, in the posterior margin of the carapace. Females showed a wider posterior margin than males, and variation in the hepatic region (landmarks 9 and 10). Males showed broader antero-lateral margins (landmarks 3 and 7) than females (Figure 5A). For Armases rubripes shape variation occurred mainly in landmarks 3–4 and 6–7, in the posterior margin of the carapace. Females showed a wider posterior margin than males, which in turn exhibited an anterior margin wider than females (landmarks 3 and 7) (Figure 7A).

Fig. 5. Aratus pisonii. Sexual dimorphism in adults, regarding shape of carapace (A), right (B) and left (C) cheliped propodus. Magnification: A – 3 times, B and C – 2 times.
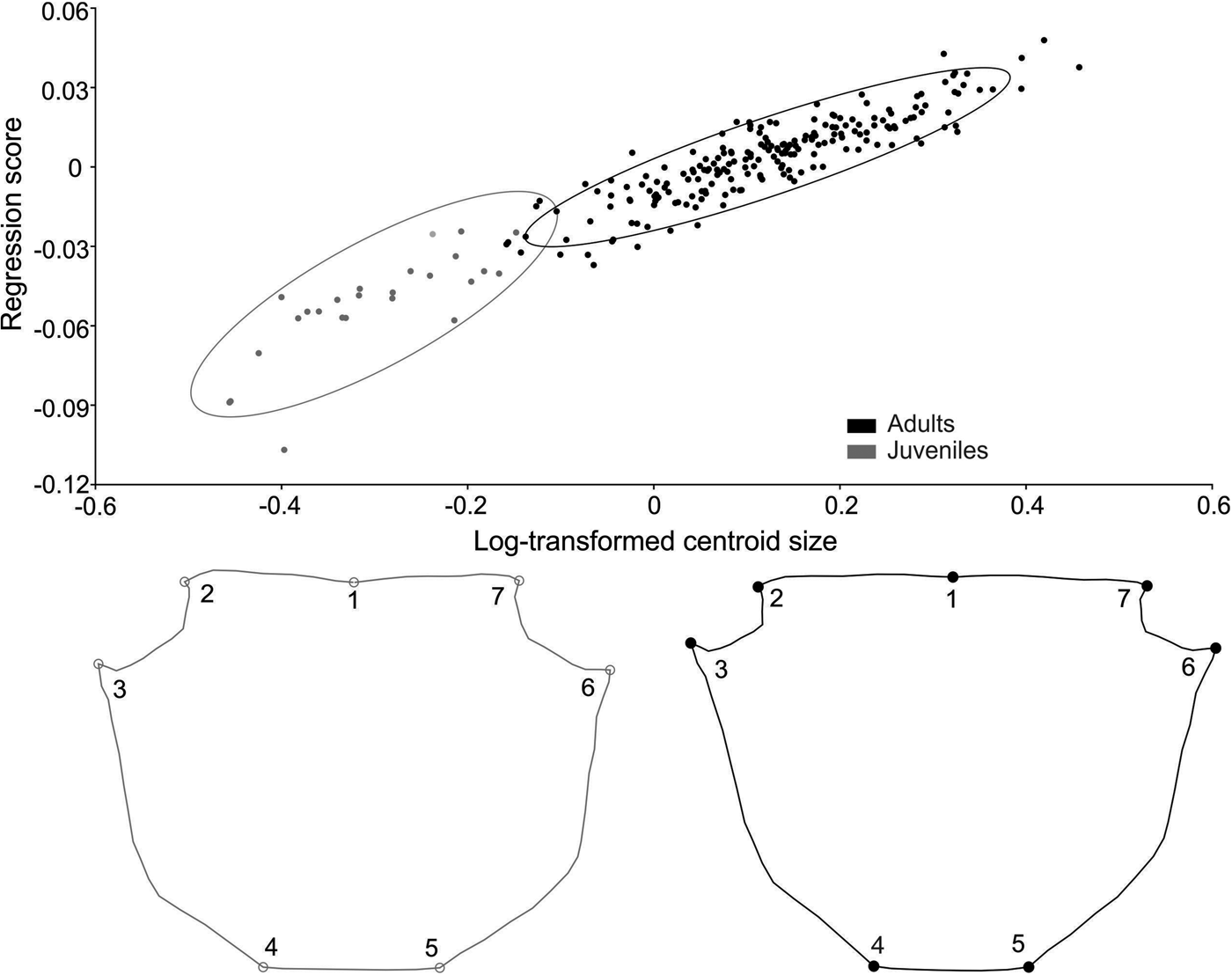
Carapace shape of juvenile A. pisonii also revealed sexual dimorphism (Procrustes distance of 0.018, P = 0.03), with a correct classification of 80% (for both sexes). This variation was subtle and occurred in the posterior margin of the carapace (landmarks 4, 5 and 6), with females having wider and longer posterior carapaces (Figure 6). Even after size correction, carapace SShD of juveniles showed the same dimorphism pattern (Procrustes distance of 0.018, P = 0.01). In contrast, no sexual dimorphism was recorded in the shape of juvenile carapace in A. rubripes (Procrustes distance of 0.012, P = 0.067), with a correct classification of 53% for females and 62% for males.
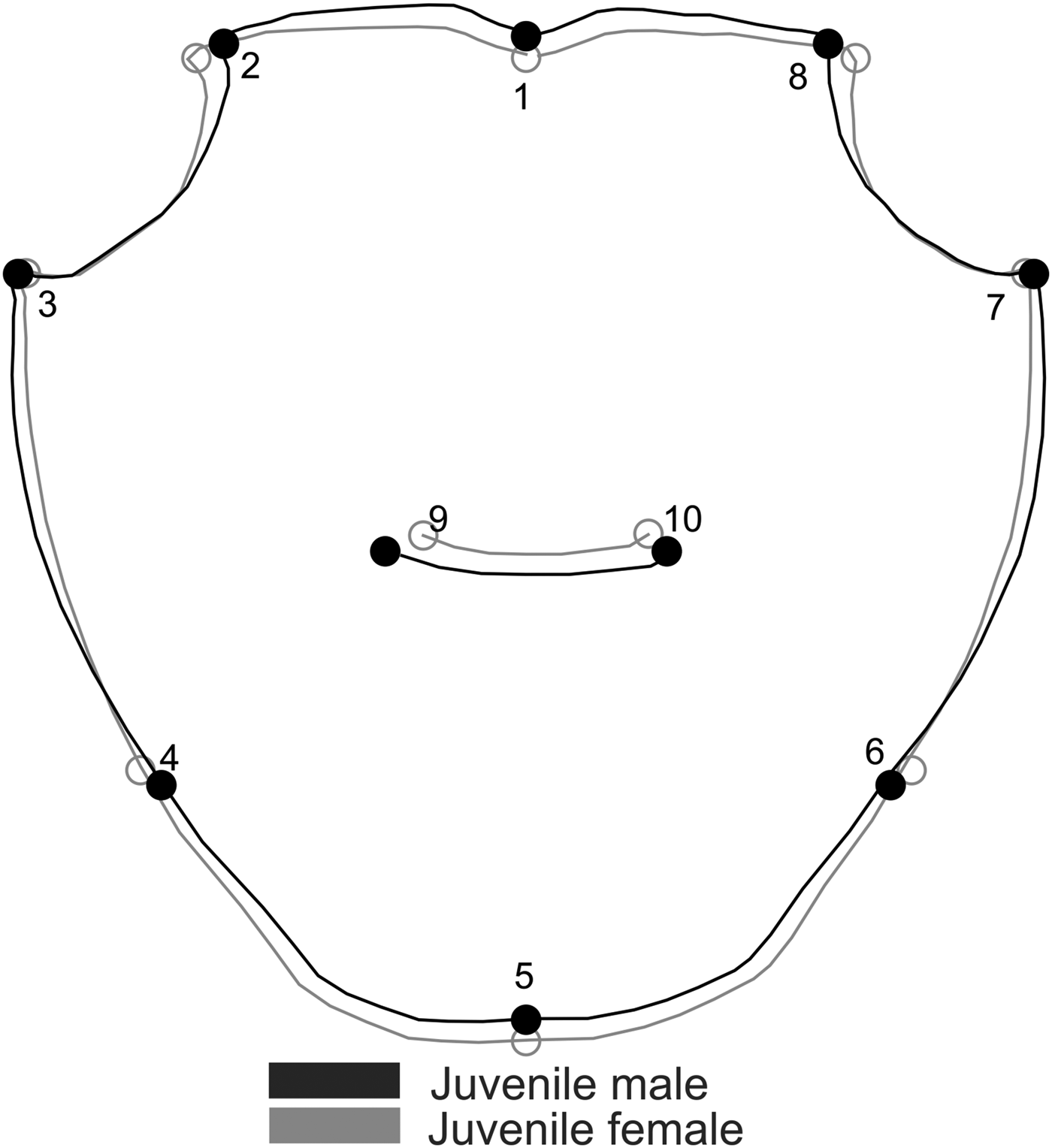
Fig. 6. Aratus pisonii. Sexual dimorphism in the shape of juvenile carapace. Magnification: 3 times.

Fig. 7. Armases rubripes. Sexual dimorphism of carapace (A), right (B) and left cheliped propodus in adults. Magnification: A = 5 times, B and C = 4 times.
Shape of both cheliped propodus also differed between sexes in Aratus pisonii: right propodus (Procrustes distance of 0.026, P < 0.01), with a correct classification of 92.3%; and left propodus (Procrustes distance of 0.044, P < 0.001), with a correct classification of 76.9%. Cheliped propodus of both sides showed to be more robust in males (wider in landmarks 8, 7, 3 and 4) and longer/thinner in females (narrow in landmarks 8, 7, 3 and 4). On the other hand, shape of both cheliped propodus did not differ between sexes after size correction (Figure 5B, C) (right cheliped propodus: Procrustes distance of 0.018, P = 0.21; left cheliped propodus: Procrustes distance of 0.024, P = 0.22). This showed that the differences between male and female cheliped propodus shape are derived from distinct allometric growth.
Conversely, the shape of both cheliped propodus showed no sexual dimorphism in A. rubripes after size correction (Figure 7B and C) (right cheliped propodus: Procrustes distances of 0.016, P = 0.83; left cheliped propodus: Procrustes distances of 0.015, P = 0.85), with a correct classification of the right cheliped propodus of 38% for males and 43% for females, and for the left cheliped propodus, 41% for males and 46% for females. Before size correction, males were shown to have both propodus more robust (longer and wider) than females.
Ontogenetic allometry
Males and females showed ontogenetic allometry in carapace shape in both species (P < 0.001). The pattern of shape variation was estimated from the regression of shape on centroid size in ontogenetic groups (juvenile or adult).
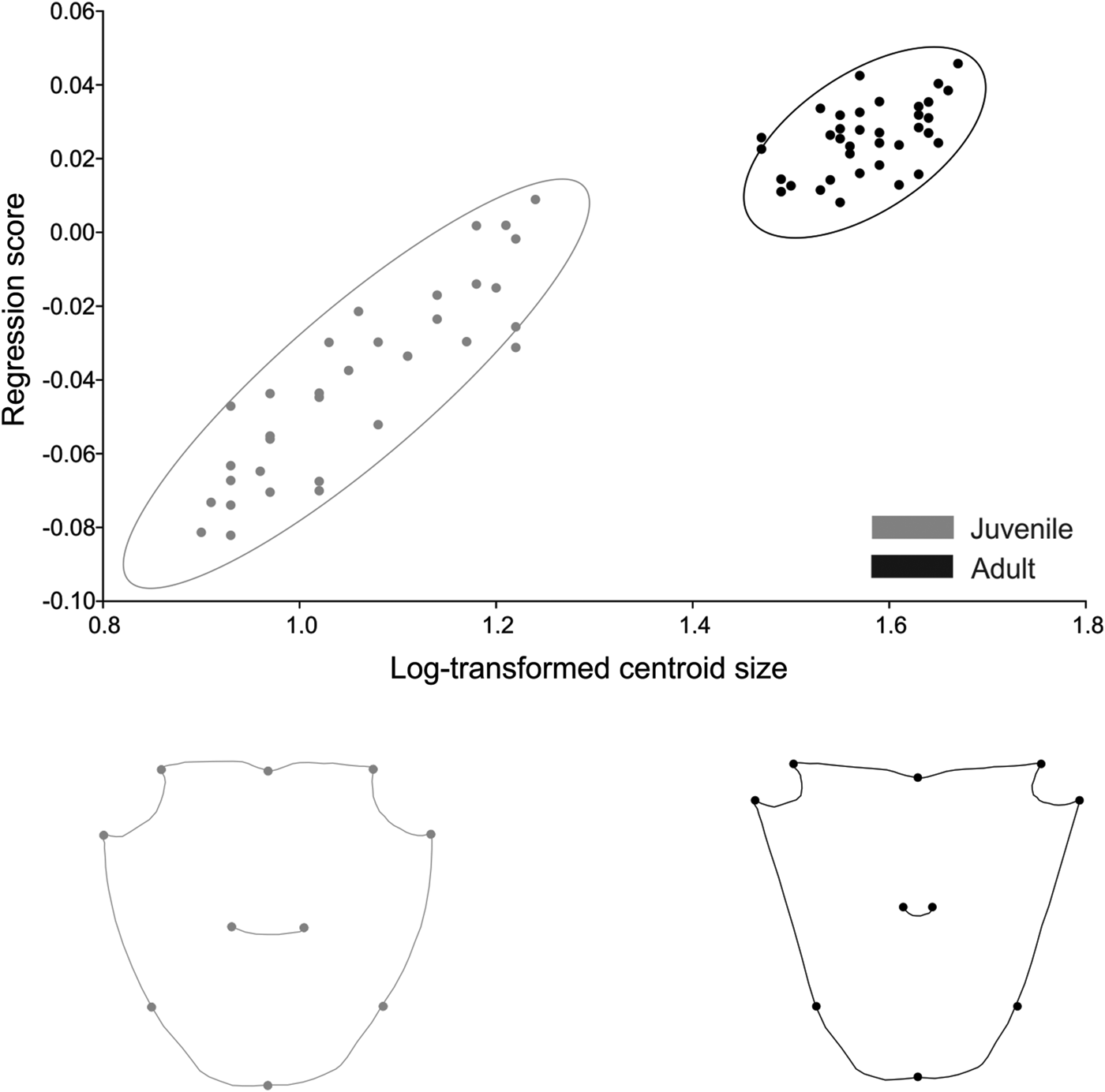
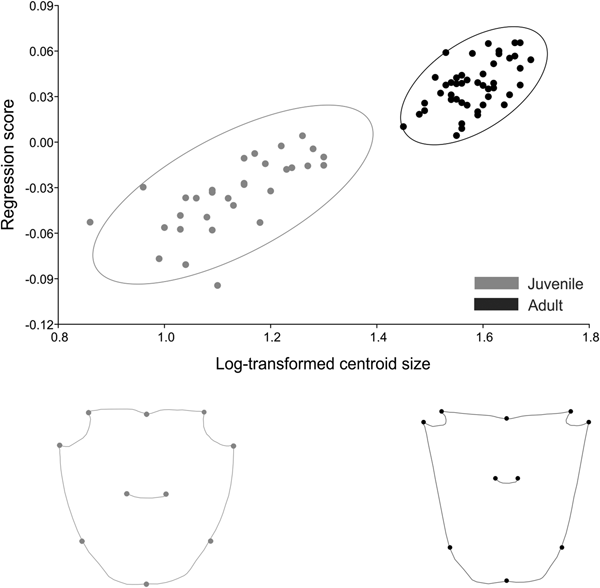
For A. pisonii, size was responsible for 61% of shape variation in females and 62% in males. In both sexes the increase in size produced a compression of the anterior region and elongation of the posterior region (Figures 8 and 9). The angle between regression vectors of the ontogenetic trajectory between males and females was 23° and smaller than expected for the pairs of random vectors (P < 0.001).

Fig. 8. Aratus pisonii. Ontogenetic allometry of male carapace shape, based on multivariate regression of symmetrical components on log-transformed centroid size. The two drawings show shapes expected for changes by 0.8 and 1.8 units of log-transformed centroid size from the mean shape (the extremes at the left and right of the plot).

Fig. 9. Aratus pisonii. Ontogenetic allometry of female carapace shape, based on multivariate regression of symmetrical components on log-transformed centroid size. The two drawings show shapes expected for changes by 0.8 and 1.8 units of log-transformed centroid size from the mean shape (the extremes at the left and right of the plot).
In A. pisonii males, static allometry (estimated from the regression of shape on centroid size) occurred in both life stages, juvenile (P < 0.001) and adult (P < 0.001). Size was responsible for 42% and 17% of shape variation in each life stage, respectively. The angle between regression vectors of male juveniles and adults was 29° and differed significantly from the expected pairs of random vectors (P < 0.001) (Figure 8). In females, allometry also occurred in both juvenile (P < 0.001) and adult (P < 0.001) stages, and size was responsible for 21% and 11% of shape variation, respectively. The angle between regression vectors of juvenile and adult females was 33° and differed significantly from the expected pairs of random vectors (P < 0.001) (Figure 9).
Carapace shape in A. pisonii differed between life stages in both sexes (female: Procrustes distance of 0.072, P < 0.001; male: Procrustes distance of 0.066, P < 0.001). The difference in shape between juveniles and adults was related to the allometric effect, with size correction eliminating this effect of shape variation between life stages in females (Procrustes distance of 0.0024, P = 0.98) and males (Procrustes distance of 0.0068, P = 0.36). The angle between regression vectors of juvenile males and juvenile females was 19° (P < 0.001), while between adult females and adult males it was 36° (P < 0.001), indicating that males and females have a similar static allometry during the juvenile phase and it is intensified during adulthood.
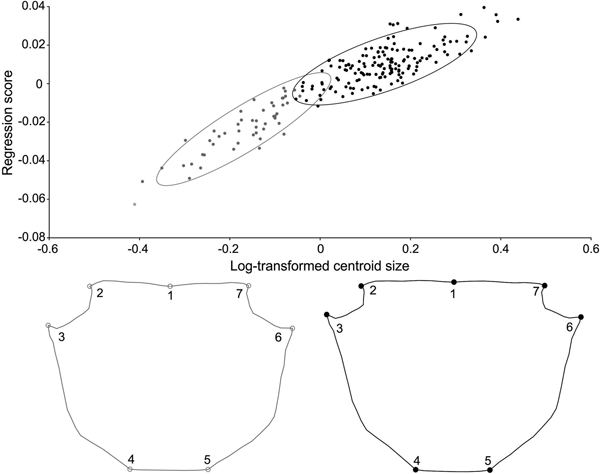
In Armases rubripes size was responsible for 34% of shape variation in males and 46% in females. In both sexes, the increase in size generated an elongation of the frontal region of the carapace and shortening of the orbital region in adults (Figures 10 and 11). The angle between regression vectors of ontogenetic trajectories between males and females was 8° and differed significantly from the expected for pairs of random vectors (P = 0.001). In males, static allometry occurred in both life stages, juvenile (P < 0.001) and adult (P < 0.001), and size was responsible for 21% and 9% of shape variation in each life stage, respectively. The angle between regression vectors of male juveniles and adults was 31° and differed significantly from the expected for the pairs of random vectors (P = 0.015). For females, static allometry also occurred in both juvenile (P < 0.001) and adult (P < 0.001) stages and size was responsible for 33% and 32% of shape variation, respectively. The angle between regression vectors of juvenile and adult females was 61° and did not differ significantly from the expected for the pairs of random vectors (P < 0.17). These results indicate that the allometric trajectory is similar in males but distinct in juvenile and adult females.

Fig. 10. Armases rubripes. Ontogenetic allometry of male carapace shape, based on multivariate regression of symmetrical components on log-transformed centroid size. The two drawings show shapes expected for changes by −0.4 and 0.4 units of log-transformed centroid size from the mean shape (the extremes at the left and right of the plot).
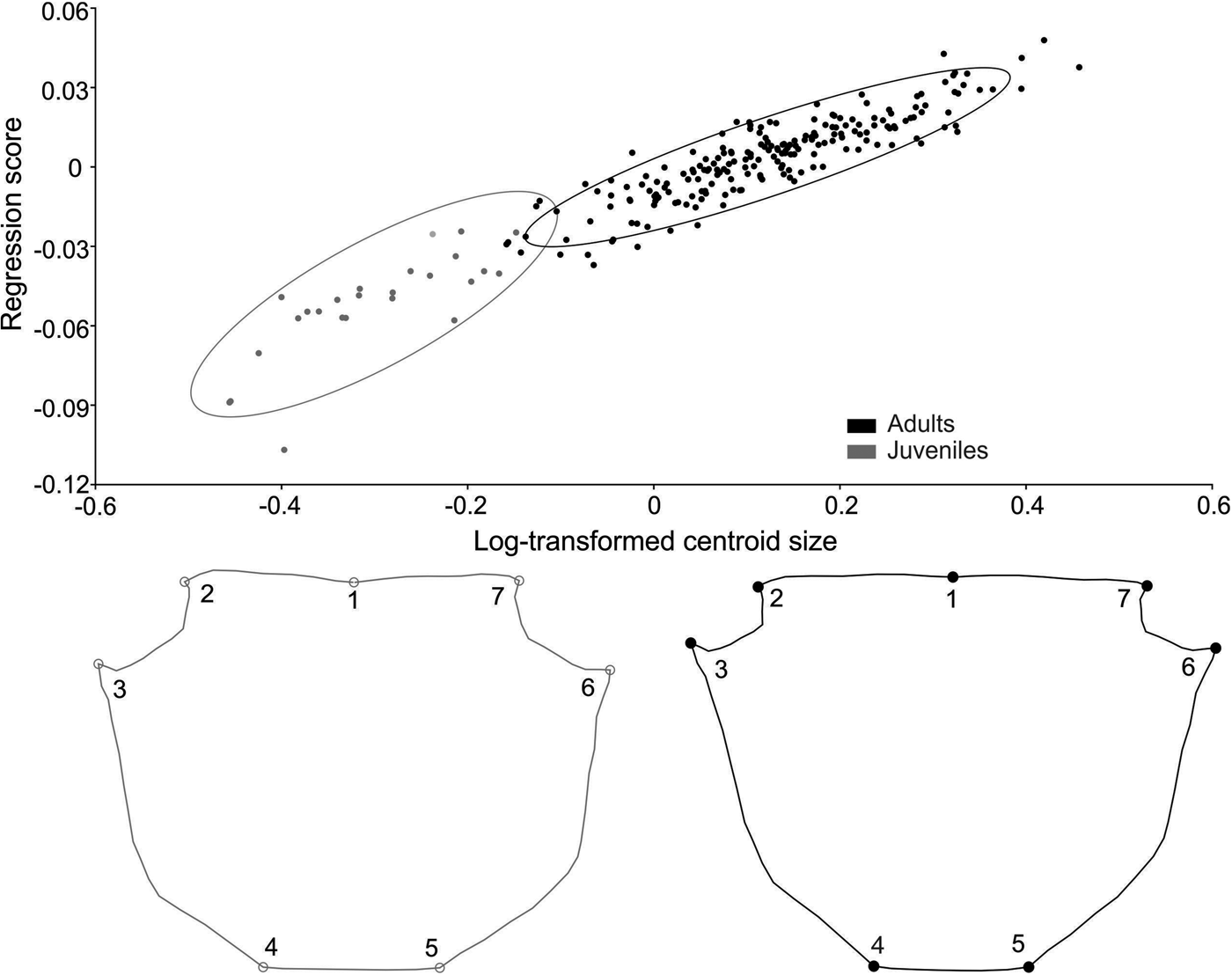
Fig. 11. Armases rubripes. Ontogenetic allometry of female carapace shape, based on multivariate regression of symmetrical components on log-transformed centroid size. The two drawings show shapes expected for changes by −0.4 and 0.4 units of log-transformed centroid size from the mean shape (the extremes at the left and right of the plot).
Carapace shape differed between life stages in both sexes (male: Procrustes distance of 0.033, P < 0.001; female: Procrustes distance of 0.056, P < 0.001). However, after size correction males and females showed distinct patterns. The difference in shape between juvenile and adult males was related to the allometric effect and size correction eliminated this effect of shape variation between life stages (Procrustes distance of 0.002, P = 0.17). While in females, the difference in shape between life stages reflected distinct trajectories (Procrustes distance of 0.011, P < 0.001). The angle between regression vectors of juvenile males and juvenile females was 25° (P = 0.006) while between adult females and males it was 23° (P = 0.001), indicating that males and females have a similar static allometry during juvenile and adult phases.
DISCUSSION
Although shape variance used in geometric morphometrics studies has been found to be fairly accurate even with small samples, mean shapes and angles within static allometric trajectories are affected by sampling size (error) (Cardini & Elton, Reference Cardini and Elton2007). Thus, it is important to note that the results found here for Aratus pisonii may show a bias due to reduced number of individuals when compared with Armases rubripes.
Our results indicated that sexual dimorphism shows a different pattern in the two species analysed. In Aratus pisonii there is shape sexual dimorphism of the carapace during juvenile stage (before puberty moult) and the variation between juveniles and adults is a size-associated variation especially in the carapace frontal region. For Armases rubripes the shape sexual dimorphism was detected after puberty moult only (adult phase). Carapace variation between male juveniles and adults is also a size-associated variation especially in the carapace frontal region, but for females there is a shape change with distinct trajectories. We partially rejected our initial hypothesis since sexual dimorphism before puberty moult was detected only in one species.
Relative growth in Armases rubripes
The positive allometric growth in most body dimensions found in the present study, especially in cheliped propodus of males and abdomen width of females, indicates a common pattern related to sexual selection. For taxa such as lizards, insects and crabs, it is hypothesized that exaggerated male traits have evolved under sexual selection as ornaments to attract mates and to be utilized as weapons to face rivals, with directional sexual selection leading to the evolution of positive allometry (Green, Reference Green1992; Petrie, Reference Petrie1992; Kodric-Brown et al., Reference Kodric-Brown, Sibly and Brown2006). The same pattern occurs across distinct animal taxa for females. Positive allometric growth in the female abdomen is commonly associated with directional sexual selection to an increase of fat reserve or to area enlargement in order to accommodate offspring or eggs (Hartnoll, Reference Hartnoll1974; Anholt et al., Reference Anholt, Marden and Jenkins1991; Braña, Reference Braña1996). In both sexes, directional selection is likely related to an increase in reproductive success (Kodric-Brown et al., Reference Kodric-Brown, Sibly and Brown2006).
Since males showed mean CW larger than females (10.36 and 9.56 mm, respectively), it was expected that onset sizes of morphological sexual maturity in males and females would also be close. However, there is a 1.65 mm difference. This difference was exactly the same as that found in a population from São Paulo (Castiglioni et al., Reference Castiglioni, Santos, Reigada and Negreiros-Fransozo2004). Furthermore, although males attained sexual maturity before females in a population from Rio de Janeiro (Lima & Oshiro, Reference Lima and Oshiro2006), the difference above is still close to both populations (1.8 mm). This variation may be partially explained by the distinct reproductive strategies of each sex. Since males reach sexual maturity after females they can increase in body and weapon size (cheliped propodus) to fight for a mate, thus getting advantages in intraspecific competition and copulation. Conversely, females reaching sexual maturity earlier can invest energy in egg production as well as in abdomen development (Hartnoll, Reference Hartnoll and Abele1982, Reference Hartnoll and Wenner1985). The pattern of females becoming sexually mature at a smaller size than males was similar to that found for the São Paulo population but distinct from the Rio de Janeiro population (males became sexually mature while smaller than females) (Castiglioni et al., Reference Castiglioni, Santos, Reigada and Negreiros-Fransozo2004; Lima et al., Reference Lima, Soares and Oshiro2006; Lima & Oshiro, Reference Lima and Oshiro2006). This indicates that this parameter is a population characteristic and not a species pattern.
The main size at onset of morphological sexual maturity in A. rubripes in the present study was distinct from that of the São Paulo (SP) and Rio de Janeiro (RJ) populations. In SP there was a variation in the onset of sexual maturity from 9.1 to 11.6 mm CW in males and from 7.6 to 9.8 mm CW in females. In RJ it varied from 6.6 to 7 mm CW in males and from 8.2 to 9 mm CW in females (Castiglioni et al., Reference Castiglioni, Santos, Reigada and Negreiros-Fransozo2004; Lima & Oshiro, Reference Lima and Oshiro2006). In Guaratuba Bay (present study), individuals became sexually mature earlier (7.17 and 5.52 mm CW, respectively) than the populations mentioned. Distinct biological and physical variables may be responsible for this population difference in the onset of maturity, such as: predator pressure, temperature range, photoperiod, food availability, substrate and population density, as observed for other brachyurans (Kuris, Reference Kuris1971; Wenner et al., Reference Wenner, Fusaro and Oaten1974; Hines, Reference Hines1989). All these factors can drive the onset size of morphological sexual maturity through the modulation of growth rates and longevity (Hines, Reference Hines1989). Temperature range and photoperiod may act in larger scales (latitudinal) reducing growth rate at low temperatures (higher latitudes), for example, as observed for the crab Panopeus herbstii H. Milne Edwards, 1834 (Hines, Reference Hines1989). This could be the case for the Guaratuba and Rio de Janeiro populations (500 km apart), but does not explain variation found between Guaratuba and São Paulo populations (200 km apart). It is thus more likely that factors occurring at a smaller scale (predator pressure, food availability, substrate and population density) would locally influence growth rate and longevity.
Sexual dimorphism
Size sexual dimorphism (SSD) of the carapace (in juveniles and adults) showed no common pattern between the species evaluated. SSD was only recorded in juveniles of Aratus pisonii and adults of Armases rubripes. SSD absence or presence can be explained in terms of several factors, including differential migration and/or mortality rate between sexes. These events may act together with natural and sexual selection to produce the observed size similarity or dissimilarity between sexes (Fairbairn & Preziosi, Reference Fairbairn and Preziosi1994, Reference Fairbairn and Preziosi1996). Nutritional status, food availability and genetic factors have also been suggested as a cause of population variability in Decapoda (Anastasiadou et al., Reference Anastasiadou, Liasko and Leonardos2009). Since none of those parameters was experimentally tested we cannot assert which parameter or group thereof are effectively acting on size sexual dimorphism.
Sexual shape dimorphism (SShD) of the carapace was marked by a wider posterior margin in females than in males of both species. As discussed previously, females of brachyuran crabs generally have positive allometric abdomen growth (ventral side), showing wider abdomens than males (Hartnoll, Reference Hartnoll1974). This allometric growth seems to affect the posterior carapace margin as well (dorsal side). Since the abdomen is close to the posterior carapace margin, abdomen growth may also affect growth of structures in the surrounding area, modifying female shape. A similar pattern is also observed in males of fiddler crabs that exhibit carapace asymmetry due to growth of the asymmetrically sized claw, which has been interpreted as a biomechanical response during development (Crane, Reference Crane1975). An increase in abdomen size can generate a biomechanical response to support weight increase during development and to accommodate the egg mass. This response would lead to an increase in musculature and consequently of the posterior carapace area. But it may also be a consequence of abdomen growth simply because the posterior carapace margin is located closer to the abdomen and would be influenced by a similar growth rate. Since the posterior region of the carapace is not characterized by developed musculature, the most likely reason for this pattern seems to be proximity between carapace margin and abdomen. The same pattern was also observed for the anomuran Aegla marginata Bond-Buckup and Buckup, 1994 (Trevisan et al., Reference Trevisan, Marochi, Costa, Santos and Masunari2012).
Cheliped propodus of adults, in both species, showed SSD (males larger than females) and males exhibited more robust chelipeds (taller and wider) than females. But after size correction this variation was not significant. Thus, cheliped sexual dimorphism is a consequence of size increase, and shape variation a consequence of the distinct effect of size on shape in males and females. The differences of cheliped size are related to growth rate (higher in males), which is influenced by sexual hormones, as observed for the shrimp Macrobrachium rosenbergii de Man, 1879 (Nagamine & Knight, Reference Nagamine and Knight1980; Nagamine et al., Reference Nagamine, Knight, Maggenti and Paxman1980.
A number of authors have suggested that size increase and shape variation may provide advantages during direct male-male competition and that sexual selection acts on male traits to increase their efficacy as functional weapons (e.g. the closing force of claws; Lailvaux et al., Reference Lailvaux, Reaney and Backwell2009). In distinct taxa, larger weapon-like male traits are favoured during male fighting, as well as female mate choices (Judge & Bonanno, Reference Judge and Bonanno2008; Milner et al., Reference Milner, Detto, Jennions and Backwell2010; Callander et al., Reference Callander, Kahn, Maricic, Jennions and Backwell2013). The probability of success during fighting is a strong sexual selection indicator because, usually, winning a fight provides direct benefits in the form of shelter, feeding territory, mating sites and quantity of mates (Smith & Miller, Reference Smith and Miller1973; Backwell & Passmore, Reference Backwell and Passmore1996; Koga et al., Reference Koga, Backwell, Christy, Murai and Kasuya2001).
Sexual dimorphism traits (wider posterior region in females and larger cheliped propodus in males) represent specialization of reproductive roles for breeding, hence the evolutionary success of sexual selection. These traits may also be the result of ecological sexual dimorphism (intersexual niche divergence) (Punzalan & Hosken, Reference Punzalan and Hosken2010). This hypothesis assumes that sexual dimorphism evolved to reduce intraspecific competition for food and it is not associated with reproductive selection. But empirical tests of this hypothesis with different taxa showed that even when conclusions are positively related, it is difficult to exclude the hypothesis of trophic dimorphism evolving as a consequence of pre-existing sexual dimorphism (for more details see Fairbairn, Reference Fairbairn1997).
Ontogenetic allometry
Growth generates compression of the anterior region (especially the orbital area) and elongation of the posterior carapace region. Since this occurred in both species, it may indicate a general pattern at family level. In early juvenile stages of brachyuran crabs, the eyestalk and eye are proportionally larger than in adults, and this proportion decreases progressively during development even with the increase in eye size (Meyer-Rochow et al., Reference Meyer-Rochow, Towers and Ziedins1990; Guerao et al., Reference Guerao, Anger, Nettelmann and Schubart2004, Reference Guerao, Anger and Schubart2007). Thus, the ontogenetic shape variation is likely a consequence of allometric modularity traits for the lineage since many crustacean species show the same pattern.
One of the possible hypotheses for this ontogenetic shape variation may be associated with biotype distribution during juvenile and adult phases. Adults of Aratus pisonii occur in abundance in upper arboreal strata (branches and upper trunks), while juveniles are frequently observed in lower trunks and aerial roots (Pahlas, Reference Pahlas2013). A similar pattern of biotype distribution is also observed for Armases rubripes, where adults live in supratidal and juveniles inhabit mainly intertidal regions (personal observation). In this scenario, ontogenetic plasticity may be a cause or a consequence of habitat preference. During growth the eyes of decapods show an increase in volume of retinal and dioptric structures, rhabdom width, crystalline cone length and focal length. These eye and eye-stalk ontogenetic modifications increase absolute sensitivity of single ommatidia, sensitivity to light sources and also increase the angular visual field of the whole eye (Meyer-Rochow et al., Reference Meyer-Rochow, Towers and Ziedins1990). Thus, this visual accuracy increase may allow adults to use resources that are unreachable during the juvenile stage.
Our data also indicate that the influence of size on shape and the ontogenetic trajectories are different in the two species. For Aratus pisonii, size influence over shape is similar in both sexes (62% in males and 61% in females) while for Armases rubripes, female shape (46%) is more influenced by size than male's (34%). Both sexes of A. pisonii and males of A. rubripes have similar trajectories from juvenile to adult phase and shape differences are mainly related to the influence of size over shape. On the other hand, females of A. rubripes have a distinct trajectory from juvenile to adult phase. This distinct trajectory reflects significant shape change during carapace growth and is likely a consequence of the development of secondary sexual characters (abdomen) over the posterior carapace area, as discussed previously.
Our results revealed that development of secondary sexual characters on the ventral body side also affects carapace dorsal surface in females. Although male cheliped propodus are larger than female's, our study revealed no differences in shape (after size effect was removed). We partially rejected our initial hypothesis since there is no pattern for the period of life in which morphological sexual dimorphism is present on the carapace of Sesarmidae. Our results also indicated that shape variation is a common pattern during growth in Sesarmidae. This ontogenetic shape variation may be associated with spatial partitioning between juveniles and adults. Further studies evaluating habitat use of juveniles and adults will be useful in order to understand the allometric evolution of this lineage.
ACKNOWLEDGEMENTS
We are grateful to Salise Brandt Martins for helping with collection. All biological samples collected for the present study complied with the current laws of the Brazilian Federal Government, and were conducted with the permission of the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA) (Authorization # 42931-1-DIFAP/IBAMA, 11 May 2015).
FUNDING
We acknowledge the National Council for Scientific and Technological Development (CNPq) for providing a scholarship to MZM (process no. 141212/2013-6).