Published online by Cambridge University Press: 21 January 2005
It is a signal honour to be invited to deliver the John Keith Memorial Lecture. Although I never had the good fortune to meet Dr Keith, I certainly know of him through his richly deserved reputation as a teacher and innovator in the field of paediatric cardiology. These aspects shone through during my frequent consultations of his monumental textbook.1 His influence has been huge, in part through the textbook, running to three editions thanks to the collaboration of his colleagues Dick Rowe and Peter Vlad, but also through the numerous students who learnt at his side, and took his clinical wisdom back for further dissemination in their countries of origin. His heritage as Chief of Paediatric Cardiology similarly been enhanced by the efforts of his successors at the Hospital for Sick Children, Dick Rowe, Bob Freedom, and Andrew Redington, all of whom I am pleased to be able to count as good friends and colleagues, albeit that Dick, sadly, is no longer with us.2
I have chosen as the topic for my lecture the field of cardiac development. This may come as a surprise to several of my friends, since it is not too long since Anton Becker and I roundly condemned cardiac embryology as being a hindrance rather than a help in understanding the morphology of congenital cardiac malformations.3 That, however, was in the “bad old days”, when concepts of cardiac development were usually based on limited study of serially sectioned normal embryos, with theories then being advanced on the basis of inferences made from the anatomic study of the cardiac malformations themselves. I had been guilty of such exercises myself4 and although some of the concepts advanced at that time have stood up well to the passage of time, and the emergence of evidence-based studies, I must confess that I cannot now fully understand some of my writings from 30 years ago! The advances made in the accumulation of evidence over the past 10 years, however, have been truly remarkable. Now, we are able to examine the structure of the developing normal heart using scanning electron microscopy, not only in animals such as the chick and mouse, but also in the human. The visually explicit preparations can now be supplemented by immunocytochemical studies that enable us to demonstrate the temporal appearance and morphological location of genes and their precursors, not only in the normal heart but in models of known congenital malformations reproduced consistently either by careful in-breeding or by knock-out technology.
All these new techniques have mandated a significant re-thinking of our concepts of cardiac development, not least in the mechanics of development and looping of the heart tube.5 I am more than grateful, therefore, that my close colleagues, Sandra Webb, Antoon Moorman, and Nigel Brown, permitted me to use so much of their material for the lecture, and now join me as co-authors in this written version.
Ideally, we would like to be able to illustrate how this newly available evidence has enabled us to clarify the details of morphology of the entire range of congenital cardiac malformations, since we firmly believe that it is now the case that knowledge of development is a major help rather than a stultifying hindrance. The available space, however, will not permit such a wide-ranging exploration. With this constraint in mind, therefore, we will concentrate our attention on just one of the cardiac segments, namely the atrial chambers and their venous connections. We will show how the evidence accrued concerning development has helped us to clarify such diverse areas as the number of definitive atrial components, the morphology of lesions, such as the various types of interatrial communication, the structure of hearts possessing a common atrioventricular junction, and the significance of isomeric atrial appendages. We will commence, nonetheless, by reviewing important new evidence concerning the formation of the heart tube, since this is the advance that has mandated the most radical of the revisions needed with regard to concepts of normal cardiac development.
Proteins encoding for messenger ribonucleic acid, such as light chain myosin, can first be demonstrated in the developing mouse embryo when it is no more than a flat plate, and with formation of no more than three or four somites. At this early stage, the cells containing the myosin are found in a crescentic area within the embryonic disc (Fig. 1). With growth of the embryo, the myocardial cells gather themselves round a newly developed endothelial plexus to form the initial part of the so-called primary heart tube, the myocardial cells attaching to the wall of the developing mediastinum throughout the length of the tube as the dorsal mesocardium. The heart tube is initially suspended by this mesocardium within the developing pericardial cavity. The endothelial components of the tube are themselves continuous caudally with the systemic venous plexuses, formed on both sides of the embryo at the same time as the heart tube itself. When first seen in a tube-like arrangement the heart takes the form of an inverted “Y” (Fig. 2). Marking experiments have now shown conclusively that the stem of the “Y”, representing the entirety of the heart tube at this early stage, is destined to form the primordium of only the left ventricle.6 Elegant experiments formed by several groups in recent years have shown that the right ventricle, along with the ventricular outflow tract, is recruited from a so-called secondary heart field.7–9 Significantly, however, this so-called secondary field is confluent with the area of the primary myocardial crescent, and the distinction between the two components is as much temporal as morphological.10 The earlier workers7–9 showed that recruitment from the secondary field at the cranial part of the developing heart built the outflow tract and right ventricle, but the more recent evidence has shown that the cells growing through the cranial pole also contribute, albeit in a minor fashion, to the developing left ventricle.10 At the cranial end of the growing tube, the outflow segment retains its continuity with the developing arteries passing through the pharyngeal pouches, these arteries arising from the aortic sac, with the margins of the newly formed pericardial cavity marking the boundary between outflow tract and the aortic sac.11 For the purposes of this review, we are concerned only with the development of the atrial component of the heart tube. In this respect, nonetheless, the most recent findings concerning the extent of the heart fields is of utmost importance, since the cells formerly expressing Islet 1, a transcription factor marking the secondary heart field, are found not only within the cranial pole, but also within both the right and left atriums.10

Figure 1. This early mouse embryo, with seven somites, has been processed to demonstrate the gene for myosin light chain. The myocardial gene demarcates the so-called cardiac crescent. The embryo is viewed from the front, with the developing head placed superiorly.

Figure 2. This embryo is slightly older than the one shown in Figure 1, with nine somites. The cardiac crescent has developed into the cardiac tube, which is continuous inferiorly with the systemic venous tributaries. The tubular part of the inverted “Y” will become the definitive left ventricle. Note the bulge at the junction with the left-sided venous tributary (arrow). This is the first sign of breaking of symmetry in the developing embryo, and this area will become the left side of the atrioventricular canal.
The real significance of the recent findings concerning the heart fields7–10 is that the atrial chambers of the developing heart, like the right ventricle and outflow tract, have no representation in the initial straight heart tube (Fig. 2). It was an oversimplification, therefore, when some of us initially prepared cartoons suggesting that all of the cardiac segments were represented in the initial primary heart tube,12 with the processes of looping and septation then being sufficient to account for eventual formation of the four-chambered definitive organ. The atrial component of the heart tube is formed at the junction between the caudal extent of the initially straight left ventricular part of the tube and the branching systemic venous tributaries. Significantly, the first sign of breaking of symmetry in the developing heart is seen in this region, with a bulge (Fig. 2) developing on the left side that eventually becomes incorporated within the atrioventricular canal.13 It is part of the limbs of the initially inverted “Y”, therefore, that are incorporated into developing heart as the basis of the atrial segment. Subsequent to its incorporation as part of the heart, this developing atrial cavity retains its initial continuity caudally with the systemic venous tributaries. This caudal incorporation of the primary atrium has occurred concomitant with the addition of the right ventricle and outflow tract cranially, with most of the cells in both regions being derived from the secondary heart field (Fig. 3). At the same time, there has been disruption of the dorsal mesocardium that initially anchored the initial left ventricular component of the tube to the body of the embryo. It is in this period that the heart tube bends rightwards in the process of looping, at the end of which the heart has begun, for the first time, to achieve some resemblance to the definitive organ (Fig. 4). At this stage in the mouse, the embryo has developed 40 somites, and the atrial appendages have begun to “balloon” from the lumen of the primary heart tube, protruding ventrally to each side of the outflow tract. At a comparable stage in the developing human embryo, the atrioventricular canal by now has achieved appreciable length (Fig. 5). At this stage, however, the trachea has only just begun to bud forward from the gut, and, as yet, there is only minimal development of the lungs. Even at a much earlier stage (Fig. 6), trans-section of the developing atrial component in its short axis reveals its continuity with the systemic venous tributaries, and shows that that the left-sided tributary, the left sinus horn, is beginning to diminish in size. Importantly, although the dorsal mesocardium breaks down throughout the length of the ventricular part of the heart tube, the cross-section shows that the atrial component of the tube has retained its mesocardial connection with the body of the embryo (Fig. 6).

Figure 3. This scanning electron micrograph shows a slightly older embryo, one with 13 somites. The heart tube is beginning to loop, and is incorporating the right ventricle cranially from the so-called secondary heart field. LV: left ventricle; RV: right ventricle; AV: atrioventricular.

Figure 4. This scanning electron micrograph shows a mouse embryo with 42 somites. The heart has begun to assume its definitive configuration. Note the “dog-leg” bend that separates the outflow tract into proximal and distal parts. Note also the atrial appendages (starred) that are ballooning from the primary atrial component of the tube.

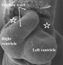
Figure 5. This sagittal section is from a human embryo at Carnegie stage 14. Note the length of the atrioventricular canal (brackets), with the cushions (starred) within its lumen. Note also the inferior position of the solitary pulmonary vein, and the discrete walls of the left sinus horn (LSH) within the left atrioventricular junction.

Figure 6. This cross-section from a mouse embryo with 13 somites, shown using the scanning electron microscope, reveals how the atrial segment of the heart tube retains its dorsal mesocardial connection with the body wall. The pulmonary vein will eventually enter the heart through this connection. Note that already the left sinus horn is marginally smaller than the right horn.
This feature is of crucial importance since, concomitant with growth of the lungs, there is a marked reorientation in the arrangement of the systemic venous tributaries. In humans, the left sinus horn continues to diminish in calibre compared to the right sinus horn, and becomes incorporated into the newly formed left atrioventricular junction, albeit retaining its own discrete walls as it courses through the junction (Fig. 7). As part of this reorientation, both systemic tributaries come to open into the right side of the developing atrial component, with the border between atrium and systemic venous sinus now marked anatomically by the venous valves (Fig. 8).

Figure 7. This dissection shows the atrial chambers of a mouse embryo with 36 somites, again revealed using scanning electron microscopy. Note the location of the left sinus horn, with its own discrete walls, within the left atrioventricular groove.

Figure 8. This mouse embryo, with 42 somites, has been transected in the four-chamber plane, and is again photographed using scanning electron microscopy. The systemic venous tributaries, entering the systemic venous sinus, are now draining exclusively to the right side of the primary atrium, and their boundaries are delimited by the so-called venous valves (star). The pulmonary vein opens to the left side through the so-called pulmonary ridges, formed at the initial site of the dorsal mesocardium (see Fig. 6).
Subsequent to these changes, much of the initial atrial component remains within the developing left atrium. The ballooned appendage, along with the extensive systemic venous sinus, now form the larger parts ofthe developing right atrium. Only subsequent to formation of the lungs is the pulmonary venous plexus formed within their substance. The intraparenchymal pulmonary veins are then connected to the developing left atrium by the primary pulmonary vein, which develops from further venous plexuses within the mediastinal tissues, the canalising vein gaining access to the left side of the atrial component through the persisting remnant of the dorsal mesocardium. When first seen, therefore, the pulmonary vein enters the atrial cavity between the ridges marking the original attachments of the dorsal mesocardium to the body wall (Fig. 8), structures that, when viewed internally, appear as the so-called “pulmonary ridges”.14, 15 If we take the appearance of the venous valves (Fig. 8) as the first sign of an anatomic boundary between the primary atrium and the systemic venous sinus, we are unaware of any evidence that supports the claim that the pulmonary vein is derived from the systemic venous sinus (sinus venosus).16
With the appearance of the pulmonary vein, all of the putative atrial components are in place, albeit that significant remoulding, along with septation, is needed to produce the definitive anatomic arrangements. It is subsequent to the reorientation of the systemic venous tributaries that the scene is set for atrial septation (see below). At this stage, therefore, we will review the contributions of the various developmental components to the definitive atrial chambers. Much of the initial atrial component of the heart tube remains as the body of the left atrium. Initially, it had been thought that the atriums possessed no more than a venous component, an appendage, and a vestibule.17 We had appreciated, of course, that they were separated by the septum, which is shared by the two atriums. It is a careful examination of hearts with totally anomalous pulmonary venous connection that reveals the flaw in this initial concept since, if composed only of appendage, vestibule, and septum, the left atrium would lack any volume in the absence of its pulmonary venous component. In reality, the atrium in this setting retains a significant body (Fig. 9). Re-examination of our embryonic material then shows how the larger part of the left atrium is produced within the initial atrial component of the heart tube (Fig. 10). Elegant immunocytochemical studies have shown that the atrial body is distinct from the appendages, which balloon from each side of the initially common atrial chamber, and stain positively for atrial natriuretic factor.18 The appendages, therefore, are the most characteristic of components with which to distinguish the right and left sides of the developing atriums, not only in morphological terms,19 but also in the developmental sequence. This is because they develop in bilateral fashion from the same initial cardiac segment, namely the atrium. It is the right appendage, along with the systemic venous component, that then forms the bulk of the newly developed morphologically right atrium. The smooth vestibule is the right side of the initial atrioventricular canal (Fig. 5), this part becoming sequestrated as an atrial structure by formation of the fibrous plane of atrioventricular insulation.20 The part of the embryonic right atrium derived from the initial heart tube is no more than the small strip found between the attachment of the left venous valve and the newly formed atrial septum (Fig. 10).

Figure 9. This specimen has totally anomalous pulmonary venous connection, with the pulmonary venous confluence draining to an infradiaphragmatic site (yellow and red arrow). Note that, in addition to the appendage and vestibule, the left atrium retains a significant body. See text for discussion.

Figure 10. This cartoon shows how the various embryonic building blocks are incorporated into the definitive atrial chambers.
It is the body of the morphologically left atrium that represents the greater part of the initial atrial component of the heart tube, with the tube-like appendage budding from its superior and leftward margin. The vestibule of the mitral valve, as with that of the tricuspid valve, is derived from initial atrioventricular canal, this time the left side (Fig. 5). This muscle becomes sequestered as an atrial structure subsequent to formation of the fibrous atrioventricular junction.20 Significantly, the pulmonary vein, when first seen in the human embryo is located dorsally and inferiorly, directly adjacent to the left atrioventricular junction, and possesses a solitary opening (Fig. 5). It takes quite some time beyond the completion of atrial septation (see below) for the developing pulmonary venous component to achieve its definitive position within the atrial roof (Fig. 11), with one of four pulmonary veins draining to each of its corners.21 It also takes time for the musculature of the newly formed atrial dome to develop the non-uniform anisotropic arrangement seen in then postnatal heart.22 The new myocardium of the atrial dome also extends along the veins themselves as myocardial sleeves. It is now established that the cells within these myocardial sleeves are important in the genesis of focal atrial fibrillation.23 It has also been suggested that the cells are “morphologically specialised”.24 In our opinion, there is neither histological nor developmental evidence to support this claim. The cells fail to satisfy the histological criterions for recognition as being “specialised”.25 In developmental terms, the cells are newly formed working myocardium, expressing connexion40, and are not derived from the primary myocardium that forms the cardiac nodes.26

Figure 11. This four-chamber section is from a human embryo of 9 weeks gestation, a stage at which septation has been completed. Only at this late stage are the pulmonary veins being incorporated to form the dome of the left atrium – compare with Figure 5.
Our study of the development of the atrial chambers, therefore, permits us to provide a rational explanation for the definitive atrial components, with each of the atriums possessing a body, albeit of different proportions on the two sides, a venous component, an appendage, and a vestibule. The two atriums, thus formed, are separated by the septum (Fig. 12). The new evidence accruing from development, combined with re-examination of older studies, has now permitted us also to clarify several features not only of the structure of the normal septum, but also the morphology of interatrial communications.

Figure 12. This adult heart has been sectioned in four-chamber plane to show that the so-called “septum secundum” is, in reality, a deep fold between the connections of the superior caval veins (SCV) to the right atrium and the pulmonary veins (PV) to the left atrium. The true septal structures are the flap valve, derived from the primary atrial septum, and the antero-inferior rim, formed by muscularisation of the vestibular spine and the mesenchymal cap carried on the primary septum.
It has long been recognised that the essence of division of the atrial chambers is the growth of the primary muscular septum (septum primum) from the roof of the initially common atrial component of the heart tube, with the newly formed muscular septum then interposing between the systemic and pulmonary venous components. As the septum grows in this fashion, it approaches the atrioventricular endocardial cushions, which are fusing to divide the atrioventricular canal into its mitral and tricuspid components (Fig. 13). It is less well recognised, however, that as the muscular primary atrial septum grows towards the atrioventricular canal, it carries a mesenchymal cap on its leading edge (Fig. 13). Thus, it is the mesenchymal structures that fuse to obliterate the primary atrial foramen. The upper part of the muscular primary septum fenestrates as part of normal development, thus producing the secondary interatrial foramen, which permits the continued shunting of the richly oxygenated placental blood into the left atrium (Fig. 14). Formation of the rims of the foramen occurs much later in development, and is dependent on folding of the atrial roof between the site of connection of the caval veins to the right atrium, and the pulmonary veins to the left atrium. This process is not completed until well after the completion of ventricular septation within the heart (Fig. 15). The rims of the fossa formed superiorly and posteriorly are particularly deep infoldings, rather than true septal structures. Antero-inferiorly, there is formation of a secondary atrial septal component. At the time of fusion of the mesenchymal components to close the primary atrial foramen, an additional septal component grows into the region of the developing atrioventricular junctions from the posterior wall of the embryo. This structure was originally described by Wilhelm His the Elder as long ago as 1865,27 and named by him the “spina vestibuli” (Fig. 16). This particular septal component became somewhat neglected during the latter half of the twentieth century, but its importance was then re-emphasised by several groups just prior to the time of the millennium.28–30 We now know that the material growing into the heart through the vestibular spine muscularises to form the antero-inferior rim of the oval fossa.31 The true role of this structure as a septal component was confirmed by discovery of a congenitally malformed heart with a hole within this rim (Fig. 17), a hole that can be explained only on the basis of failure of fusion of the various muscularised mesenchymal components making up the antero-inferior rim of the oval fossa.32 Our account of formation of the atrial septum (Fig. 12), therefore, now needs to take cognisance of growth of the vestibular spine to complete the formation of the rims of the oval fossa. This knowledge of septal development then permits us to clarify the structure of the various interatrial communications, and also to make inferences concerning the timing of maldevelopment responsible for their appearance.

Figure 13. This four-chamber section is from a human embryo at Carnegie stage 16. The primary atrial septum is growing towards the atrioventricular (AV) cushions, and carries a distinct mesenchymal cap on its leading edge. The upper part of the septum has broken down (bracket).

Figure 14. This four-chamber section is from another human embryo at Carnegie stage 16, but in this embryo, the mesenchymal cap has fused with the atrioventricular endocardial cushions to close the primary atrial foramen. Note that the superior margin of the secondary atrial foramen is flat, since as yet, the pulmonary veins have not been incorporated into the dome of the left atrium. SCV: superior caval vein.

Figure 15. It is only with incorporation of the pulmonary veins into the dome of the left atrium, as shown in this human embryo of 9 weeks, after the completion of septation, that the upper rim of the secondary foramen becomes infolded (dotted line) between the right pulmonary (star) and the caval veins – compare with Figure 12.

Figure 16. This drawing is a copy of the original work of Wilhelm His the Elder, and shows the location of the “spina vestibili” or vestibular spine.

Figure 17. This picture shows the so-called vestibular defect (arrowed), first described by Sharratt et al.32 It is the consequence of improper muscularisation of the vestibular spine and the mesenchymal cap of the primary atrial septum. SCV, ICV: superior and inferior caval veins. The red dotted line shows the rims of the intact oval fossa.
We have appreciated for some time that not all the lesions producing the potential for shunting between the two atriums are true septal defects.33 The commonest defects of the atrial septum represent either fenestration or exuberant absorption of the flap valve, this structure being the persisting lower margin of the primary atrial septum. Recognition that the upper rim of the foramen is no more than an infolding also permits explanation of how the volume-overloaded atrial chambers can produce the potential for transient interatrial shunting, concomitant with temporary effacement of the interatrial fold. And we have already discussed how incomplete fusion of the mesenchymal elements at the base of the septum can produce a vestibular defect (Fig. 17).32 The other lesions that produce interatrial shunting, however, share the feature that all are outside the confines of the oval fossa. The most frequent defect, the “ostium primum” defect, has as its phenotypic feature a common atrioventricular junction. We will discuss the potential mechanisms for this lesion below. The other two lesions are found not only with normal folding of the antero-superior rim of the fossa, but also with normal formation of the separate right and left atrioventricular junctions, and normal muscularisation of the antero-inferior rim of the oval fossa. On the basis of the morphology (see below), it is possible to infer that these sinus venosus and coronary sinus defects must develop subsequent to the incorporation of the pulmonary veins into the left atrium, and subsequent to the growth and muscularisation of the vestibular spine.
It seems to be abnormal incorporation of the pulmonary veins that are responsible for the genesis of this defect. In most examples of the malformation, the mouth of the superior caval vein is connected within the left as well as the right atrium, so that its orifice overrides the intact superior rim of the oval fossa. The rim of the fossa itself contains a core of extra-cardiac tissue through which it is possible to pass a probe (Fig. 18). Most examples also have anomalous connection of one or more of the right pulmonary veins (Fig. 18), albeit that the left pulmonary veins are incorporated normally into the dome of the left atrium. This morphology initially suggested to us that the phenotypic feature of the lesion was biatrial connection of the mouth of one or other of the caval vein.34 We have now seen a specimen (Fig. 19) in which the superior caval vein is connected exclusively within the right atrium, yet anomalous connection of the right upper pulmonary vein still produces an interatrial communication outside the confines of the oval fossa. This finding, to us, confirms the paramount role of atrial incorporation of the right pulmonary veins in the genesis of the lesion, albeit that, as yet, we are unable to offer an explanation for the abnormal mechanics.

Figure 18. This picture shows the essence of the superior sinus venosus defect. The mouth of the superior caval vein (SCV) overrides the superior rim of the intact oval fossa. A probe has been passed through the core of fibro-adipose tissue contained within this rim. Note the connections of the right pulmonary veins (RPV) to the superior caval vein.

Figure 19. In this superior sinus venosus defect (arrow), there is no overriding of the superior caval vein, which is exclusively connected within the right atrium. It is the anomalously connected right upper pulmonary veins that produce an extra-cardiac conduit between the cavities of the right and left atriums. Note the normal connection of the right lower pulmonary vein to the left atrium. The oval fossa is intact.
This fascinating lesion is no more than a hole at the anticipated site of the mouth of the coronary sinus that permits interatrial shunting due to absence of the walls that normally separate the sinus from the left atrium.35 When it was thought that a “party wall” separated the sinus from the left atrium, and it was argued that the wall was formed by active growth of a purported left sinuatrial fold,36 it was an easy matter to explain the lesion (Fig. 20) simply on the basis of incomplete or absent formation of the fold. We now know, however, that the coronary sinus always possesses its own discrete myocardial wall as it courses through the left atrioventricular junction.37 Examination of our embryonic material also shows that this wall exists from the earliest formation of the systemic venous tributaries, representing the wall of the left sinus horn that becomes the coronary sinus (Figs 5 and 7). The walls of the left atrium and coronary sinus are closely apposed, albeit that it is always possible, with careful study, to distinguish between the two components. The spectrum of malformation seen in the coronary sinus defect, therefore, ranging from a simple fenestration between the sinus and the left atrium38 to complete absence of the walls of the coronary sinus, with drainage of a persistent left superior caval vein to the left atrial roof (Fig. 20), can only be explained on the basis of dissolution of both of the walls that initially separated the developing coronary sinus from the body of the left atrium.35 Again, although we can offer an explanation to account for the existence of the coronary sinus defect, we are still at a loss to account for the abnormal mechanism causing the dual walls to disappear.

Figure 20. In this heart, shown through the left atrium, the mouth of the coronary sinus functions as an interatrial communication because the walls of the left atrium and persistent left superior caval vein (LSCV) have disappeared. The putative course of the left-sided caval vein is shown by the dotted lines. The caval vein now drains to the left atrial roof between the origin of the left pulmonary veins and the mouth of the appendage. The oval fossa is intact.
Perhaps the most interesting of the lesions permitting interatrial shunting outside the confines of the oval fossa is the so-called “primum” defect. We have long recognised that the defect represents persistence of the primary interatrial foramen, and is part of the family of defects known as “atrioventricular canal malformations”39 or “endocardial cushion defects”.40 The focus in the past for understanding the defect, however, had been on the structure of the atrial septum. In more recent years, we have come to appreciate that the phenotype of the defect, rather than involving the septal structures, is the presence of a common atrioventricular junction.41 The fact that, very rarely, hearts can be found with an “atrioventricular septal defect”, but with intact, albeit abnormal, atrial and ventricular septal structures,42, 43 emphasises the importance of the common junction (Fig. 21) as the phenotypic feature rather than the septal deficiency. The key to understanding abnormal development, therefore, is found when we are able to unravel the mechanisms involved in normal separation of the mitral and tricuspid valvar orifices.

Figure 21. This photograph shows the common atrioventricular junction, which is the phenotypic hallmark of the so-called “ostium primum” defect. The aorta is positioned antero-superior relative to the common junction. A tongue of leaflet tissue (blue line) joins together the superior (red line) and inferior (green line) bridging leaflets, which are also attached to the crest of the ventricular septum, confining shunting through the associated atrioventricular septal defect at atrial level. Because the bridging leaflets are joined together by the tongue, there are separate right and left valvar orifices (RAVO, LAVO) within the common junction.
We now know that one of the major components separating the two normal atrioventricular junctions is the vestibular spine first described by His in 1864 (Fig. 16).27 We have already described the formation and muscularisation of this important structure. It is no coincidence that, in examples of the “ostium primum” defect with well-formed atrial septal structures, one feature lacking from the morphological make-up is the bulbous muscular antero-inferior rim of the oval fossa. Furthermore, we now have two important animal models that have a common atrioventricular junction as part of their phenotype. These are the mouse with Trisomy 1644 and the Pitx2 knock-out mouse.45 As we examine the developing embryos from those models, we can see that a feature shared by both is incomplete formation of the vestibular spine (Fig. 22). Our previous studies of the mouse with Trisomy 16 had shown that, even though the endocardial cushions were abnormal,44 it was possible for the superior and inferior cushions to be fused one to the other, and yet still the animal could develop with a common atrioventricular junction.46 We have now noted similar features in a minority of the Pitx2 knock-out mice (Fig. 23). Thus, it is no longer adequate to offer explanations for formation of the “atrioventricular canal malformations” on the basis of failure of fusion of the atrioventricular endocardial cushions. Rather, in our opinion, attention needs to be focussed on the atrioventricular junctions, and in particular the mechanics of normal and abnormal formation of the vestibular spine.

Figure 22. This dissection, illustrated using scanning electron microscopy, shows the dorsal aspect of the atrial chambers in a mouse embryo of 35 somites, on day 10, with Pitx2 knock-out. Note the rudimentary formation of the vestibular spine. There is also symmetrical arrangement of the sinus horns (see Fig. 24).

Figure 23. This scanning electron micrograph, again from a mouse with Pitx2 knock-out, but this time on the 17th day of gestation, shows a common atrioventricular junction (yellow dotted line), albeit with fusion between the superior and inferior bridging leaflets (solid line). The superior and inferior bridging leaflets (1, 2) are derived from the atrioventricular cushions (white dotted lines), and, with the mural leaflet of the left valve (3), form a trifoliate left atrioventricular valvar orifice.
The final lesion to receive our attention is again one that has attracted much previous attention. These are the hearts found in the setting of visceral heterotaxy. There has been heated debate in the past as to whether these hearts showed evidence of isomerism,47, 48 a feature known to exist in the lungs and bronchial trees in this setting.49 Indeed, it was consideration of these hearts some years ago that persuaded one of us, at the time, that embryology was a hindrance rather than help.3 The wheel has now turned full circle, and it is embryological studies that now provide the convincing evidence for the existence of isomerism.
As we have already explained, the atrial appendages balloon out in parallel from the primary atrial segment of the heart tube. Developing in this way, from the same part of the heart, the morphological differences between the appendages are under the control of a cascade of genes that produce leftness as opposed to rightness. Included in this cascade are the genes nodal, lefty, and Pitx2.50 We have already referred to the animal model with knock out of the Pitx2 gene, and explained that the mice posses a common atrioventricular junction, known to be a common finding in patients with visceral heterotaxy. Examination of the mice with Pitx2, however, shows that the common atrioventricular junction is one of the anatomical features in a phenotype dominated by right isomerism. These animals have right pulmonary and bronchial isomerism, along with abdominal heterotaxy. Examination of the heart now provides unequivocal evidence of isomeric formation of morphologically right atrial appendages, with additional bilateral formation of the systemic venous sinus, with isomeric venous valves, and formation of no more than an atrial septal strand (Fig. 24). The embryological evidence, therefore, is overwhelming, with knock out of one of the genes responsible for morphological leftness producing right isomerism. And, within the heart, this produces isomerism of the morphologically right atrial appendages, just as seen in humans with so-called “asplenia syndrome”. The embryology, therefore, has proved of the utmost help in establishing isomerism of the atrial appendages as a morphological reality.

Figure 24. This dissection is again from a mouse with Pitx2 knock-out, and is again illustrated with scanning electron microscopy. The mouse was in the 17th day of gestation. There is symmetry of the atrial appendages and the systemic venous sinuses (starred) – in other words right isomerism. Note again the poorly formed vestibular spine.
We hope that we have demonstrated, in the setting of normal and abnormal formation of the atrial chambers, the crucial importance of knowledge of development in clarifying the morphological structure of the heart. Now, with the proper evidence in abundance, there is no justification in even hinting that embryology might be a hindrance rather than a help. Since John Keith retired as Chief of Paediatric Cardiology in Toronto, the revolution in technology has underscored huge advances in the diagnosis and treatment of children with congenital cardiac disease, many of these advances being made in the unit he directed so capably in the Hospital for Sick Children. It is now not unrealistic to hope that, in the very near future, the advances being made in molecular biology and genetics will permit us fully to unravel the mechanisms leading to congenital malformations of the heart. Within our lifetimes, therefore, we may look forward to the huge goal of modern medicine – prevention rather than cure.
The research on which this review is based was supported by grants from the British Heart Foundation together with the Joseph Levy Foundation. Research at the Institute of Child Health and Great Ormond Street Hospital for Children NHS Trust benefits from R&D funding received from the NHS Executive.

This early mouse embryo, with seven somites, has been processed to demonstrate the gene for myosin light chain. The myocardial gene demarcates the so-called cardiac crescent. The embryo is viewed from the front, with the developing head placed superiorly.

This embryo is slightly older than the one shown in Figure 1, with nine somites. The cardiac crescent has developed into the cardiac tube, which is continuous inferiorly with the systemic venous tributaries. The tubular part of the inverted “Y” will become the definitive left ventricle. Note the bulge at the junction with the left-sided venous tributary (arrow). This is the first sign of breaking of symmetry in the developing embryo, and this area will become the left side of the atrioventricular canal.
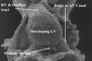
This scanning electron micrograph shows a slightly older embryo, one with 13 somites. The heart tube is beginning to loop, and is incorporating the right ventricle cranially from the so-called secondary heart field. LV: left ventricle; RV: right ventricle; AV: atrioventricular.
This scanning electron micrograph shows a mouse embryo with 42 somites. The heart has begun to assume its definitive configuration. Note the “dog-leg” bend that separates the outflow tract into proximal and distal parts. Note also the atrial appendages (starred) that are ballooning from the primary atrial component of the tube.

This sagittal section is from a human embryo at Carnegie stage 14. Note the length of the atrioventricular canal (brackets), with the cushions (starred) within its lumen. Note also the inferior position of the solitary pulmonary vein, and the discrete walls of the left sinus horn (LSH) within the left atrioventricular junction.
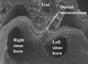
This cross-section from a mouse embryo with 13 somites, shown using the scanning electron microscope, reveals how the atrial segment of the heart tube retains its dorsal mesocardial connection with the body wall. The pulmonary vein will eventually enter the heart through this connection. Note that already the left sinus horn is marginally smaller than the right horn.

This dissection shows the atrial chambers of a mouse embryo with 36 somites, again revealed using scanning electron microscopy. Note the location of the left sinus horn, with its own discrete walls, within the left atrioventricular groove.

This mouse embryo, with 42 somites, has been transected in the four-chamber plane, and is again photographed using scanning electron microscopy. The systemic venous tributaries, entering the systemic venous sinus, are now draining exclusively to the right side of the primary atrium, and their boundaries are delimited by the so-called venous valves (star). The pulmonary vein opens to the left side through the so-called pulmonary ridges, formed at the initial site of the dorsal mesocardium (see Fig. 6).

This specimen has totally anomalous pulmonary venous connection, with the pulmonary venous confluence draining to an infradiaphragmatic site (yellow and red arrow). Note that, in addition to the appendage and vestibule, the left atrium retains a significant body. See text for discussion.

This cartoon shows how the various embryonic building blocks are incorporated into the definitive atrial chambers.

This four-chamber section is from a human embryo of 9 weeks gestation, a stage at which septation has been completed. Only at this late stage are the pulmonary veins being incorporated to form the dome of the left atrium – compare with Figure 5.

This adult heart has been sectioned in four-chamber plane to show that the so-called “septum secundum” is, in reality, a deep fold between the connections of the superior caval veins (SCV) to the right atrium and the pulmonary veins (PV) to the left atrium. The true septal structures are the flap valve, derived from the primary atrial septum, and the antero-inferior rim, formed by muscularisation of the vestibular spine and the mesenchymal cap carried on the primary septum.

This four-chamber section is from a human embryo at Carnegie stage 16. The primary atrial septum is growing towards the atrioventricular (AV) cushions, and carries a distinct mesenchymal cap on its leading edge. The upper part of the septum has broken down (bracket).

This four-chamber section is from another human embryo at Carnegie stage 16, but in this embryo, the mesenchymal cap has fused with the atrioventricular endocardial cushions to close the primary atrial foramen. Note that the superior margin of the secondary atrial foramen is flat, since as yet, the pulmonary veins have not been incorporated into the dome of the left atrium. SCV: superior caval vein.

It is only with incorporation of the pulmonary veins into the dome of the left atrium, as shown in this human embryo of 9 weeks, after the completion of septation, that the upper rim of the secondary foramen becomes infolded (dotted line) between the right pulmonary (star) and the caval veins – compare with Figure 12.

This drawing is a copy of the original work of Wilhelm His the Elder, and shows the location of the “spina vestibili” or vestibular spine.

This picture shows the so-called vestibular defect (arrowed), first described by Sharratt et al.32 It is the consequence of improper muscularisation of the vestibular spine and the mesenchymal cap of the primary atrial septum. SCV, ICV: superior and inferior caval veins. The red dotted line shows the rims of the intact oval fossa.

This picture shows the essence of the superior sinus venosus defect. The mouth of the superior caval vein (SCV) overrides the superior rim of the intact oval fossa. A probe has been passed through the core of fibro-adipose tissue contained within this rim. Note the connections of the right pulmonary veins (RPV) to the superior caval vein.

In this superior sinus venosus defect (arrow), there is no overriding of the superior caval vein, which is exclusively connected within the right atrium. It is the anomalously connected right upper pulmonary veins that produce an extra-cardiac conduit between the cavities of the right and left atriums. Note the normal connection of the right lower pulmonary vein to the left atrium. The oval fossa is intact.

In this heart, shown through the left atrium, the mouth of the coronary sinus functions as an interatrial communication because the walls of the left atrium and persistent left superior caval vein (LSCV) have disappeared. The putative course of the left-sided caval vein is shown by the dotted lines. The caval vein now drains to the left atrial roof between the origin of the left pulmonary veins and the mouth of the appendage. The oval fossa is intact.

This photograph shows the common atrioventricular junction, which is the phenotypic hallmark of the so-called “ostium primum” defect. The aorta is positioned antero-superior relative to the common junction. A tongue of leaflet tissue (blue line) joins together the superior (red line) and inferior (green line) bridging leaflets, which are also attached to the crest of the ventricular septum, confining shunting through the associated atrioventricular septal defect at atrial level. Because the bridging leaflets are joined together by the tongue, there are separate right and left valvar orifices (RAVO, LAVO) within the common junction.

This dissection, illustrated using scanning electron microscopy, shows the dorsal aspect of the atrial chambers in a mouse embryo of 35 somites, on day 10, with Pitx2 knock-out. Note the rudimentary formation of the vestibular spine. There is also symmetrical arrangement of the sinus horns (see Fig. 24).

This scanning electron micrograph, again from a mouse with Pitx2 knock-out, but this time on the 17th day of gestation, shows a common atrioventricular junction (yellow dotted line), albeit with fusion between the superior and inferior bridging leaflets (solid line). The superior and inferior bridging leaflets (1, 2) are derived from the atrioventricular cushions (white dotted lines), and, with the mural leaflet of the left valve (3), form a trifoliate left atrioventricular valvar orifice.

This dissection is again from a mouse with Pitx2 knock-out, and is again illustrated with scanning electron microscopy. The mouse was in the 17th day of gestation. There is symmetry of the atrial appendages and the systemic venous sinuses (starred) – in other words right isomerism. Note again the poorly formed vestibular spine.