The population of grown-up congenital heart disease patients is growing due to advancements in surgical techniques, medical therapies, and instruments. Reference Giamberti, Pluchinotta and Chessa1 The increase in the population brings long-term problems. Arrhythmia is one cause for mortality and morbidity in long-term follow-ups. Reference Somerville2 The incidence of arrhythmia increases with increasing age in patients with congenital heart disease. Reference Khairy and Balaji3 The most common arrhythmias are atrial arrhythmias and most of them are intra-atrial macro-reentrant tachycardias originating from the right atrium. Reference Labombarda, Hamilton and Shohoudi4 Right atrial dilatation due to overpressure overload, surgical scars, and prosthetic materials in the right atrium may be the cause of the arrhythmia focus originating in the right atrium. Reference Giamberti, Pluchinotta and Chessa1,Reference Walsh and Cecchin5,Reference Walsh6 In the presence of left atrial dilation and obstructive lesions of the left heart, atrial fibrillation (AF) which is a micro-reentrant tachycardia originating from left atrium can be seen. Reference Kirsh, Walsh and Triedman7 The transcatheter ablation procedure has a very important role in the treatment of arrhythmia, but due to complex anatomy and multiple mechanisms of tachycardia in congenital cardiac diseases, the efficacy of ablation procedure can be limited. Reference Triedman, Bergau, Saul, Epstein and Walsh8 Surgical ablation can be performed for prophylaxis or treatment purposes in congenital cardiac surgery as an isolated procedure or concomitant to a cardiac procedure. Reference Mavroudis, Stulak and Ad9
The aim of the study was to evaluate the early outcomes of patients who underwent a concomitant therapeutic maze procedure for congenital heart surgery in our centre.
Materials and methods
This retrospective study was approved by the hospital review board. All patients were informed about the planned surgical procedure and consent forms were obtained from all patients detailing that the hospital data could be used for any scientific purpose.
Patient selection
The hospital database was reviewed retrospectively to identify congenital heart disease patients who underwent a maze procedure using a cryoablation probe. According to the hospital policy, cardiac surgeries of patients diagnosed with congenital heart disease, including reoperation, are performed by the paediatric cardiac surgery team regardless of age. Between 2019 and 2020, the data of eight patients who underwent open-heart surgery and concomitant cryoablation were collected and examined. There were four male patients and four female patients. Patient characteristics and demographics are shown in Table 1.
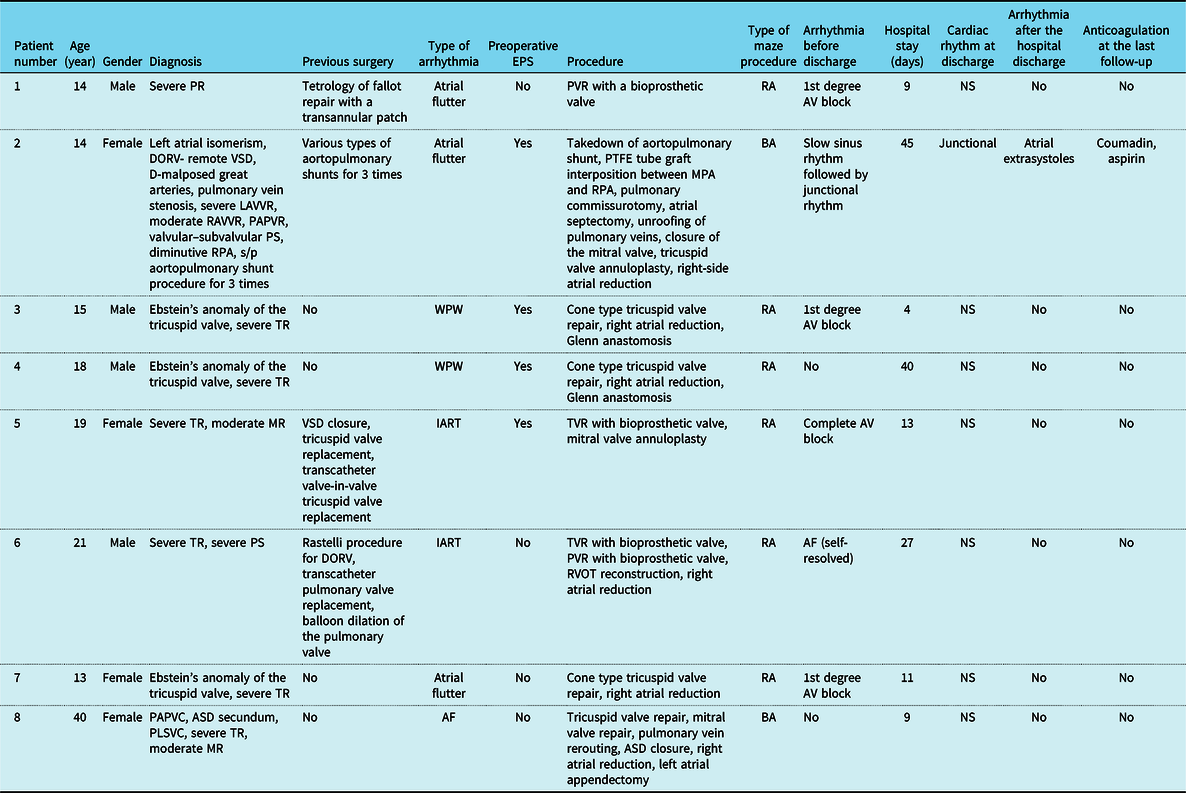
Table 1. Patients’ demographics, preoperative, intraoperative, and post-operative data.

AF = atrial fibrillation; ASD = atrial septal defect; A.V = atrioventricular; BA = biatrial; DORV = double outlet right ventricle; IART = intra-atrial reentrant tachycardia; LAVVR = left atrioventricular valve regurgitation; MPA = main pulmonary artery; MR = mitral valve regurgitation; NS = normal sinus; PAPVC = partial anomalous pulmonary vein connection; PLSVC = persistent left superior vena cava; PR = pulmonary regurgitation; PS = pulmonary stenosis; PTFE = polytetrafluoroethylene; PVR = pulmonary valve replacement; RA = pight atrium; RAVVR = right atrioventricular valve regurgitation; RPA = right pulmonary artery; s/p = status post; TR = tricuspid valve regurgitation; TVR = tricuspid valve replacement; VSD = ventricular septal defect; WPW = Wolf–Parkinson–White.
All patients were symptomatic and indicated a need for cardiac surgery. The implementation of a maze procedure was decided according to the consensus of the electrophysiology team. All patients had documented recurrent atrial arrhythmias. Cryoablation was performed for therapeutic purposes.
The hospital database was reviewed for patient demographics, preoperative diagnostic studies, preoperative antiarrhythmic therapies, operative data, post-operative antiarrhythmic therapies, post-operative anticoagulation usage, post-discharge follow-up data, morbidity, and mortality.
Surgical technique
Cryoablation was performed as endocardial in all patients. The cryoFORM® probe (AtriCure Inc., Cincinnati, OH, United States of America) was used as the cryoablation probe. The probe utilises nitrous oxide (N2O), has an active defrost mode, and is made from stainless steel. Reference Schroeter and Misfeld10 The ablation procedure was performed at −60 °C for 140 seconds. According to the surgeon’s judgment of the thickness of the tissue and the width of the frozen place, this time was reduced to 100–120 s for very thin tissues. The ablation time was also reduced for the lesions around the coronary sinus (CS), where the conduction system could be compromised.
The ablation procedure in the right atrium was made from the incision of the right atrium free wall. The atrial reduction was performed on both sides of the atriotomy towards the inferior vena cava by removing tissue in the shape of a fish mouth. Ablation was performed according to the planned surgical procedure, conduction system, and the location of the arrhythmogenic focus indicated in the preoperative electrophysiological study (EPS). Lesion sets were as follows: (1) superior vena cava (SVC) to inferior vena cava (IVC), (2) atriotomy to atrial septal defect (ASD) or patent foramen ovale (PFO), (3) around IVC, (4) IVC to the tricuspid annulus (isthmus), (5) IVC to CS, (6) atriotomy to anterior tricuspid annulus, (7) round lesion in appendage to the tricuspid annulus, (8) CS to tricuspid annulus, and (9) ASD patch or PFO to CS. We do not perform right atrial appendectomies.
Left atrial ablation was done via a left atriotomy or transseptal depending on the surgeon’s preference. Lesion sets were as follows: (1) around the four pulmonary veins, (2) between the pulmonary veins and the mitral valve annulus, and (3) between the base of the left atrial appendage to the pulmonary veins. The left atrial appendage was either amputated or ablated by a circular lesion.
A biatrial or isolated left atrial ablation was performed when a preoperatively documented atrial arrhythmia accompanied the AF or enlarged left atrium.
Follow-up
All patients’ follow-ups were done 1 week, 1 month, 6 months, and 1 year after the hospital discharge. All patients were evaluated using electrocardiography (ECG) and echocardiography. Holter monitoring was performed in the case that arrhythmia was detected at the ECG or in the event of a clinical complaint. Holter monitoring was performed routinely 1 year after hospital discharge. For patients living a distance, the hospitals where they were followed up were contacted. Arrhythmia recurrence was defined by an ECG +/− a Holter monitoring or as a result of a decline of complaints after antiarrhythmic drug treatment.
Statistics
Continuous variables were presented as median values and within the interquartile range. Categorical variables were presented as n (%).
Results
There were eight patients in the study group. Of these eight patients, four of them were female and four were male. Patients demographics, underlying cardiac diagnosis, type of arrhythmia, and surgical procedure are summarised in Table 1. The age range was 13–40 years.
Three of these eight patients had atrial flutter, two patients had Wolf–Parkinson–White (WPW) syndrome, two had intra-atrial reentrant tachycardia (IART), and one of them had AF. Four patients underwent preoperative EPS. During EPS, one patient underwent cardioversion, one patient was defibrillated due to VF, and one patient underwent radiofrequency ablation for atrioventricular (AV) nodal reentrant tachycardia. Mapping was performed in three patients during EPS. Preoperatively, one patient was on 3, two patients were on 2, five patients were on 1 antiarrhythmic drug of any type of class I–II–III. Preoperative rhythm status and antiarrhythmic therapies are shown in Table 1.
Four of the patients had previous heart surgery. All of the study patients underwent therapeutic maze procedure. Of the eight study patients, six underwent right atrial maze and two underwent bilateral atrial maze. Five out of six right atrial maze patients underwent right atrial reduction. The atrial reduction was done before ablation. In Ebstein’s anomaly patients, the lesion from CS to displaced tricuspid annulus was delicately performed. Lesion between ASD and CS was skipped if the round lesion around IVC were wide enough to include that lesion. During the left atrial ablation, all three lesions mentioned above were created. A left atrial appendectomy was done in one patient and a lesion was created between the appendage base and the pulmonary box after inserting the ablation probe through the appendectomy.
The extubation time was 7 hours–20 days. The length of the post-operative hospital stay was 9–45 days. One patient went to the OR for bleeding control, one patient for additional mitral valve repair, and two of the Ebstein’s anomaly patients for Glenn anastomosis. There was no hospital mortality. Post-operative data are shown in Table 1.
Post-operative arrhythmias before discharge
One patient had an AV block that turned to normal sinus rhythm on post-operative day 2. Two patients had a 1st degree AV block. One patient had AF that was converted to sinus rhythm spontaneously. The single ventricle patient who underwent bilateral maze had a junctional rhythm following a slow sinus rhythm. Post-operative rhythm status is shown in Table 1.
Post-operative rhythm status and arrhythmias after the hospital discharge
One patient experienced atrial extrasystoles 2 months after surgery. This patient had a junctional rhythm. Seven of the eight patients were on sinus rhythm. No patient needed permanent pacemaker placement.
Post-operative antiarrhythmic therapies and anticoagulation
All patients received aspirin after the surgery. All of the valve replacement patients were receiving warfarin on discharge. Biological prosthetic valve-replaced patients received warfarin for 3–6 months. Aspirin was discontinued 6 months after the surgery. One patient continued to receive warfarin and aspirin due to the interposed graft in the pulmonary artery.
Four out of the eight patients were receiving antiarrhythmic drugs at their last follow-up. Post-operative anticoagulation and antiarrhythmic usage are shown in Table 1.
Discussion
Maze procedure is the gold standard surgical treatment of AF. Reference Gillinov11 The original Cox-Maze I procedure was developed and modified to maze II, III by Dr Cox. Reference Cox, Schuessler and Lappas12 With the introduction of new energy sources that decreases the procedure time and lower the bleeding complications, it has started to be called the Maze IV procedure. Although there are definitions such as right atrial, left atrial, biatrial, and mini-maze, they are all considered as a maze procedure. Reference Mavroudis and Deal13 Surgical maze procedures began to be performed and popularised for the treatment of atrial arrhythmias in congenital heart surgery over time. Reference Theodoro, Danielson, Porter and Warnes14-Reference Giamberti, Chessa and Abella17 Apart from therapeutic use, it is also used in prophylactic purposes for the prevention of late arrhythmias in experienced centres. Reference Mavroudis, Stulak and Siegel18 We performed a maze procedure for treatment purposes in this study.
When applying the right atrial maze, we used the nine lesion sets as described above, and sometimes combined some of them, as one. One of the advantages of the malleable probe was that we were able to apply many lesions at the same time by shaping the probe. In particular, we were able to make lines 3 and 4 at once by keeping the IVC round lesion wide and extending the ablation time. Likewise, lines 2, 8, 9 could be done at once. Combining the lesions might be an approach that shortens the ablation time, even if we can’t statistically show this in our study because of the low number of patients. In addition to combining lesions, some of the lesion sets can be extended. In WPW patients, lesion from CS to the tricuspid annulus (line 8) was extended towards the right ventricle due to the downwardly displaced septal leaflet.
There are differences in the pathways of congenital heart disease patient’s conduction system that require special attention. The sinus node in left atrial isomerism is dislocated and the AV conduction may be damaged during the procedure. In right atrial isomerism, AV conduction can be damaged due to dual sinus node and AV node. Reference Ho, Seo, Brown, Cook, Fagg and Anderson19,Reference Ho, Fagg, Anderson, Cook and Allan20 Due to the chronotropic incompetent known in Ebstein’s anomaly and the repaired Fallot tetralogy Reference Chen, Dimopoulos, Sheehan, Gatzoulis and Kilner21,Reference Rathore, Agrawal and Kapoor22 , a pacemaker may be needed after the maze procedure. While the need for post-mace pacemaker placement in congenital heart patients was 12% in the Boston group series, Giamberti stated that 3 out of 80 patients needed pacemaker in the early period. Reference Giamberti, Pluchinotta and Chessa1,Reference Corcia, Walsh and Emani23 No pacemaker placement was required in our study group. Only the patient with left atrial isomerism who underwent biatrial maze was in junctional rhythm.
Apart from intracardiac damage that requires a pacemaker after the maze procedure, some complications may also develop due to surrounding tissue damage. Radiofrequency (RF) ablation may cause thermal injury to the surrounding tissue. Reference Prasad, Maniar and Camillo24 Cryoablation is a more preferable option than RF ablation because of its low risk of endothelial damage, damage to surrounding tissue organs such as the esophagus and phrenic nerve, and lower risk of thrombosis. Reference Yaghoubi, Rostamzadeh, Pezeshkian, Parvizi and Imani25-Reference Gillinov, Pettersson and Rice29 There was no thrombosis, surrounding tissue, or organ damage in our study population. In order not to cause any surrounding tissue damage, especially when creating appendage and free wall lesions, care was taken to free the atrium from surrounding tissues. For the possibility of thrombosis, heparin, or low-molecular-weight heparin (LMWH) was started within 4–6 hours after surgery according to the bleeding status. LMWH at a dose of 0.1 mg/kg was given for patients weighing more than 50 kg. In patients under 50 kg, heparin infusion was started at a dose of 5–10 U/kg/h and titrated to keep the activated partial thromboplastin time at 50–60 s.
The depth of the lesions is more important than the location of the lesions and the type of energy used. The thickness of their atriums varies according to the underlying cardiac pathology, such as a thick atrial wall in tricuspid atresia and thin atrial wall in double inlet left ventricle. Reference Mavroudis and Deal13 In cases where the atrium is thick, ablation time can be extended to affect full thickness. In our study population, ablation was mostly performed for 140 seconds. According to the surgeon’s assessment, ablation time for the lesions on the atrial walls were sometimes reduced to 100–120 s in thin atrial walls.
Maze procedure can be performed using energy sources such as RF, microwave, ultrasound, cryothermia, and laser, other than using a scalpel. Reference Gillinov11 In the literature, there are publications presenting early and late-term outcomes of application of either different types of energy sources and “cut and sew technique” for the maze procedure. In the study of Giamberti et al., an RF ablation catheter was used for 80 congenital heart diseased adult patients, freedom from arrhythmic events at 1 and 5 years of follow-up was, respectively, 88 and 75% for those who underwent right-sided maze procedure, and 82 and 77% for those who received a Cox-Maze III. No statistical difference was shown between the two procedures. Reference Giamberti, Pluchinotta and Chessa1 In the Boston group’s study in which a scalpel or cryoablation probe was used, 1- and 5-year arrhythmia-free rates were 79 and 67%, respectively. These rates were calculated ignoring the 3-month period after surgery that was shown to have the highest and temporary arrhythmia rate. Reference Corcia, Walsh and Emani23 In a multicentre study consist of 372 patients, the authors emphasised the high recurrence rate (AF in 32% of the study group) in the long-term follow-ups of patients who underwent percutaneous RF ablation due to right atrial tachycardia following surgery for congenital and acquired heart disease. Reference Anguera, Dallaglio and Macías30 We preferred to use cryothermia as the energy source, not because of its success rate but also its low risk of surrounding tissue damage.
The probability of early and late period arrhythmias may be different after the maze procedure. In Boston Children’s Hospital’s study about the long-term results of atrial maze surgery in patients with congenital heart disease, the rate of early and late atrial arrhythmias free in patients in the therapeutic group was 65% in the first year after maze, 61% in the third year, and 56% in the fifth year. In the same study, only 35% of patients in the therapeutic group who had arrhythmia in the first 3 months after maze was shown to have atrial arrhythmias in the long-term follow-up. In case the first 3-month period in which temporary arrhythmias are frequently observed after maze is ignored, the freedom from atrial arrhythmias was 79% at 1 year, 77% at 3 years, and 67% at 5 years post-maze for the entire study group. Reference Corcia, Walsh and Emani23 In that study, the majority of patients underwent cryoablation. In our study group, temporary tachycardia or bradycardia attacks were seen in 3 patients before discharge. Only the patient with left atrial isomerism had atrial tachycardia attack 2 months after the hospital discharge. Other than that patient, no patient experienced any rhythm disturbances 3 months after the hospital discharge.
We prefer to use antiarrhythmic drugs after a maze procedure since the first 3 months of arrhythmia recurrence is common. Reference Corcia, Walsh and Emani23 The recurrences in this period are not counted as a true recurrence. Reference Andrade, Khairy and Verma31 Our clinical approach is reducing the dose of the antiarrhythmic drug as appropriate and stopping it according to the Holter monitoring result done in the first year after surgery. Since most of the patients in the study have been operated recently, 3 patients are still on beta-blockers.
The underlying rhythm should be evaluated in detail preoperatively for deciding on the lesion sets. There are muscle bridges between the left and right atrium. Reference Ho and Sánchez-Quintana32,Reference Platonov, Mitrofanova, Ivanov and Ho33 Due to these muscle bridges, arrhythmias originating from the right atrium can make similar changes in left atrium tissues and cause arrhythmogenic changes in the left atrium. Therefore, paroxysmal atrial tachycardia or IART originating from the right atrium may turn to AF over time. Reference Koh, Uemura, Kada, Kagisaki, Hagino and Yagihara34,Reference Uemura35 For this reason, in the patients with right atrium originated arrhythmia, the left atrium can be included in the ablation procedure in patients when they start experiencing paroxysmal AF. Especially in such patients, EPS and mapping may be beneficial before the maze procedure. There was no patient with paroxysmal AF in our study group. In three of the EPS patients, there was atrial flutter or IART causing hemodynamic instability. Since the catheter ablation procedure of these patients failed, the right atrial maze was applied during the operation. The patient who had EPS before the biatrial maze was the patient with atrial flutter with left atrial isomerism.
According to the expert consensus and opinion statement, the prophylactic application of the maze procedure is an approach accepted in many centres. Reference Cox, Schuessler and Lappas12,Reference Khairy, Van Hare and Balaji36,Reference Hernandez-Madrid, Paul and Abrams37 But in a patient without a complaint, the potential risks and benefits should be considered before applying a prophylactic maze procedure. Because incomplete lines might be proarrhythmic. Reference Corcia, Walsh and Emani23 Also, the sinus node dysfunction might happen, which can be a complication of the maze procedure. Due to these possible complications of the maze procedure, the conduction system should be avoided and lesion sets should only be created close to potential arrhythmia foci.
Limitations
The limited number of patients, retrospective nature of the study, short follow-up period limited the strength of conclusions and the analysis of the results statistically.
Conclusion
Cryomaze procedure can be applied in the treatment of atrial tachycardias in congenital heart diseases with low complication rates and acceptable arrhythmia-free rates by selecting the appropriate materials and suitable lesion sets. The depth of the lesion set is as crucial as its location. Application of cryomaze in heterotaxy patients can be challenging due to differences in the conduction system and complex anatomy. Consensus with the electrophysiology team about the choice of the right, left, or biatrial maze procedure is mandatory for operational success.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.