Significant outcomes
-
Galangin, a flavonoid compound, presented antipsychotic-like effects in experimental schizophrenia models in mice.
-
Galangin enhanced apomorphine-induced PPI disruption indicating a cognitive enhancement.
-
Galangin may be considered as a potential antipsychotic agent in the management of schizophrenia and also schizophrenia-associated cognitive dysfunctions.
Limitations
-
This study investigated the acute effects of galangin. It would be better if chronic effects of this agent were also investigated.
-
In this study, to investigate the involvement of nicotinic, muscarinic cholinergic, and serotonergic mechanisms in the effects of galangin, we administered the antagonists of these receptors (mecamylamine, scopolamine, serotonin-1A receptor antagonist WAY-100635, respectively) in combination with galangin. It would be better if the mechanisms of action of galangin were explored on a molecular basis.
-
We investigated the effects of galangin on brain acetylcholine levels. It would be better if we also investigated the effects of galangin on brain dopamine levels.
Introduction
Schizophrenia is a mental disorder characterised by delusions, perceptual disturbances, hallucinations, asociality, anhedonia, and cognitive deficits (Madireddy & Madireddy, Reference Madireddy and Madireddy2020). Dopaminergic hyperfunction and glutamatergic hypofunction are suggested hypotheses for the pathophysiology of schizophrenia. In addition, it has been suggested that subcortical dopamine neurons are modulated by cortical glutamatergic neuronal projections (Rodvelt et al., Reference Rodvelt, Kracke, Schachtman and Miller2008). Furthermore, the central cholinergic system has been associated with the pathophysiology of schizophrenia (Scarr et al., Reference Scarr, Gibbons, Neo, Udawela and Dean2013). This hypothesis originates from the consideration of the interactions between cholinergic, dopaminergic, and glutamatergic systems. In post-mortem studies, it was reported that some subunits of nicotinic and muscarinic receptors were disrupted (Uslu et al., Reference Uslu, Savci, Buyukuysal and Goktalay2014). Nicotinic and muscarinic acetylcholine receptors are suggested to modulate dopamine release and be involved in the pathophysiology of schizophrenia (Zhang et al., Reference Zhang, Yamada, Gomeza, Basile and Wess2002; Rodvelt et al., Reference Rodvelt, Kracke, Schachtman and Miller2008). It has been also suggested that a decrease in muscarinic M1 receptors led to glutamate hypofunction and a decline in M1 and/or M4 receptors enhanced dopamine release (Scarr et al., Reference Scarr, Gibbons, Neo, Udawela and Dean2013).
The cholinergic system is crucial for the normal maintenance of cognitive functions. Also, evidence shows that cholinergic dysfunction is associated with cognitive deficits seen in schizophrenia. It was reported that nicotine administration reduced cognitive deficits in patients with schizophrenia (D’Souza & Markou, Reference D’Souza and Markou2012). Sensorimotor gating is an essential mechanism for healthy cognitive functions, which filters sensory information and integrates with motor responses (Larrauri et al., Reference Larrauri, Burke, Hall and Levin2015; Khoja et al., Reference Khoja, Asatryan, Jakowec and Davies2019). The cholinergic system is also reported to be involved in the modulation of the sensorimotor gating mechanism (Jin et al., Reference Jin, Cheng, Lee, Amreen, McCabe, Pitcher, Liebmann, Greengard and Flajolet2019). Prepulse inhibition (PPI) is defined as the attenuation of the startle response to a sensory stimulus (pulse) when it is preceded by a weaker stimulus (prepulse) (Khoja et al., Reference Khoja, Asatryan, Jakowec and Davies2019). PPI has been reported to be an operational measure of the sensorimotor gating mechanism reflecting its functioning (Poje & Filion, Reference Poje and Filion2017), and disruption of PPI is considered as one of the typical features of schizophrenia (Uslu et al., Reference Uslu, Savci, Buyukuysal and Goktalay2014).
Inhibition of acetylcholinesterase, which catalyses the degradation of acetylcholine, enhances cholinergic transmission and improves cognitive functions (Ghezzi et al., Reference Ghezzi, Scarpini and Galimberti2013). The acetylcholine inhibitors donepezil, galantamine, and rivastigmine are commonly used in the symptomatic relief of cognitive dysfunctions in Alzheimer’s disease and other dementias (Pohanka, Reference Pohanka2014). Recently, these agents have been used for cognitive improvement in schizophrenia (Santos et al., Reference Santos, González-Fraile, Zabala, Guillén, Rueda and Ballesteros2018). The clinical data are limited and consist of both positive and negative reports on the use of acetylcholine inhibitors as adjunctive agents to antipsychotics for cognitive enhancement in schizophrenia (Bora et al., Reference Bora, Veznedaroğlu and Kayahan2005; Buchanan et al., Reference Buchanan, Conley, Dickinson, Ball, Feldman, Gold and McMahon2008; Dyer et al., Reference Dyer, Freudenreich, Culhane, Pachas, Deckersbach, Murphy, Goff and Evins2008; Kumar et al., Reference Kumar, Mohemmedali, Anish and Andrade2017; Santos et al., Reference Santos, González-Fraile, Zabala, Guillén, Rueda and Ballesteros2018). Another issue to consider is whether acetylcholinesterase inhibitors affect antipsychotic efficacy. Galantamine has been reported to increase the efficacy of atypical antipsychotics in schizophrenia models (Wadenberg et al., Reference Wadenberg, Manetti, Romanelli and Arias2017). Similarly, it was reported that galantamine reversed PPI disruptions induced by apomorphine, MK-801, or scopolamine (Hohnadel et al., Reference Hohnadel, Bouchard and Terry2007; Koda et al., Reference Koda, Ago, Kawasaki, Hashimoto, Baba and Matsuda2008). In addition to cholinergic receptors, serotonin-1A (5-HT1A) receptors have also been suggested to be involved in the aetiology of schizophrenia, depression, and anxiety (Ago et al., Reference Ago, Takuma and Matsuda2014). Furthermore, it was reported that 5-HT1A agonists alleviated PPI disruption (Matsuda, Reference Matsuda2013).
Flavonoids are phytochemicals derived from the secondary metabolisms of plants and are involved in various biologic processes. They are reported to be beneficial molecules for human health (Singh et al., Reference Singh, Kaur and Silakari2014; Zanoaga et al., Reference Zanoaga, Braicu, Jurj, Rusu, Buiga and Berindan-Neagoe2019). Galangin (3,5,7 trihydroxyflavone) is a flavonoid compound and is one of the main active constituents of Alpinia officinarum (Chen et al., Reference Chen, Tan, Li, Qin, Cai, Lai, Zhang, Li, Guan, Li and Zhang2015; Fang et al., Reference Fang, Xiong, Xu, Yin and Luo2019). Besides its antiproliferative, anticancer, and anti-inflammatory features, the acetylcholinesterase inhibiting action of galangin has also been exhibited (Guo et al., Reference Guo, Xie, Choi, Zheng, Bi, Xu, Dong and Tsim2010; Jung et al., Reference Jung, Kim, Yoon, Park, Youn, Lee and Lee2014; Chien et al., Reference Chien, Shi, Lee, Te and Shih2015).
In this study, we aimed to investigate the effects of galangin on apomorphine-induced PPI disruption and the involvement of cholinergic muscarinic, nicotinic, and 5-HT1A receptors in rats and experimental schizophrenia models in mice. The effects of galangin on brain acetylcholine levels in rats were also studied.
Material and methods
Experimental animals
Adult male Wistar rats (250–300 g) and adult male Swiss albino mice (25–35 g) were used in the study (n = 8 in each group for both rats and mice). The animals were sheltered in standard laboratory conditions (12-h light/12-h dark cycle, 22 ± 2°C, with 45–50% relative humidity), and food and water were available ad libitum. The study was approved by the Local Ethics Committee for Animal Experimentation at Eskisehir Osmangazi University (approval number: 277/2012). The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.
Drugs and chemicals
Galangin (Alfa Aesar), R-(-) apomorphine hydrochloride hemihydrate (Sigma), (+)-MK-801 hydrogen maleate (Santa Cruz Biotechnology), donepezil hydrochloride monohydrate (Sigma), mecamylamine hydrochloride (Santa Cruz Biotechnology), (−)-scopolamine hydrobromide trihydrate (Acros Organics), haloperidol (Sigma), olanzapine (Sigma), and WAY-100635 (Sigma) were used in the study. All the drugs and chemicals were dissolved in saline except for galangin, which was dissolved in distilled water: DMSO with 1:1 ratio, and R-(-) apomorphine hydrochloride hemihydrate was dissolved in saline containing 0.1% ascorbic acid. Galangin, (+)-MK-801 hydrogen maleate, (−)-scopolamine hydrobromide trihydrate, haloperidol, olanzapine were administered intraperitoneally (i.p.), and R-(-) apomorphine hydrochloride hemihydrate, donepezil, mecamylamine hydrochloride, and WAY-100635 were administered subcutaneously (s.c.).
Experimental design
The effects of acute galangin administration on schizophrenia-associated cognitive deficits and the involvement of nicotinic, muscarinic, and 5-HT1A receptors were investigated through apomorphine-induced PPI disruption in rats. The PPI of the acoustic startle reflex test was used for this purpose and is described in detail below. In this test, galangin was administered alone at doses of 50 and 100 mg/kg. Then, for the investigation of the involvement of nicotinic, muscarinic, and 5-HT1A receptors, galangin 50 mg/kg was combined with the nicotinic receptor antagonist mecamylamine (1 mg/kg), the muscarinic receptor antagonist scopolamine (0.05 mg/kg), and the 5-HT1A receptor antagonist WAY-100635 (1 mg/kg). In the combination groups, galangin was administered at a dose of 50 mg/kg because both 50 and 100 mg/kg doses of galangin similarly enhanced apomorphine-induced PPI disruption. Immediately after the PPI test, the rats were sacrificed and their brains were removed and stored at −80°C until required for the determination of acetylcholine levels. A low dose of scopolamine that does not disrupt PPI was selected because it was reported that scopolamine disrupted PPI (Jones & Shannon, Reference Jones and Shannon2000). The effects of galangin on the PPI of the acoustic startle reflex test were compared with the reference drug donepezil (1 mg/kg), a commonly used agent in Alzheimer’s disease with acetylcholinesterase inhibiting action, and reference typical antipsychotic haloperidol (1 mg/kg) and atypical antipsychotic olanzapine (3 mg/kg).
The effects of acute galangin administration on animal models of schizophrenia were examined through apomorphine-induced climbing, MK-801-induced hyperlocomotion, and catalepsy tests in mice, as described in detail below. In these tests, galangin was administered at a dose of 50 mg/kg considering the similar effects of 50 and 100 mg/kg galangin in the PPI test. The effects of galangin on animal models of schizophrenia were compared with the reference typical antipsychotic haloperidol (1 mg/kg) and atypical antipsychotic olanzapine (3 mg/kg).
Prepulse inhibition of the acoustic startle reflex test
The test was performed according to the method introduced by Chang et al. (Reference Chang, Weber, Breier, Saint Marie, Hines and Swerdlow2012) by using four acoustic startle response chambers (SR-LAB Startle Response System, San Diego Instruments, San Diego, CA, USA). Each chamber consisted of a cylindrical plexiglas animal enclosure. Acoustic stimuli were supplied using a speaker mounted 24 cm above the animals in the chambers. Startle responses were transduced by a piezoelectric accelerometer positioned below the cylinder, rectified, digitised, and recorded as data on the computer. Rats were handled daily for a week, and they were placed into chambers for 15 min, 24 h before performing the test sessions for habituation to the test system without any acoustic stimuli. The background noise level was 70 decibels (dB) [A] soundpressure level (SPL) throughout the test sessions. Apomorphine was administered at a dose of 0.5 mg/kg s.c. 30 min after the administration of the drugs (galangin 50 or 100 mg/kg, haloperidol 1 mg/kg, olanzapine 3 mg/kg, donepezil 1 mg/kg), and rats were immediately placed in the chambers and the test sessions were started. Apomorphine dose was determined based on the study of Geyer et al. (Reference Geyer, Swerdlow, Lehmann-Masten, Teschendorf, Traut and Gross1999). In the combination groups, mecamylamine (1 mg/kg, s.c.) was administered 15 min before, scopolamine (0.05 mg/kg, i.p.) was administered 30 min before, and WAY-100635 (1 mg/kg, s.c.) was administered just before the administration of galangin (50 mg/kg, i.p.) or the reference drugs (haloperidol 1 mg/kg, olanzapine 3 mg/kg, donepezil 1 mg/kg). In all the combination groups, apomorphine (0.5 mg/kg, s.c.) was administered 30 min before galangin or the reference drug administrations and immediately the test sessions were started. Mecamylamine, scopolamine, and WAY-100635 doses and administration time schedules were performed according to the related literature (Jones & Shannon, Reference Jones and Shannon2000; Krebs-Thomson et al., Reference Krebs-Thomson, Ruiz, Masten, Buell and Geyer2006; Kohnomi et al., Reference Kohnomi, Suemaru, Goda, Choshi, Hibino, Kawasaki and Araki2010; Koda et al., Reference Koda, Ago, Yano, Nishimura, Kobayashi, Fukada, Takuma and Matsuda2011).
At, the beginning of the test sessions, a 5-min acclimation period with only 70 dB background noise was started. The prepulse intensities were +4, +8, +16 dB [A] SPL above background noise (74, 78, 86 dB, respectively). After the acclimation period, five consecutive startle pulse-alone trials (120 dB) were applied, which were followed by 10 blocks of trials. Each block consisted of five types of trials in a random order as below:
-
1. Pulse-alone stimulus (120 dB)
-
2. Prepulse (74 dB) + pulse stimulus (120 dB)
-
3. Prepulse (78 dB) + pulse stimulus (120 dB)
-
4. Prepulse (86 dB) + pulse stimulus (120 dB)
-
5. No stimulus (70 dB background noise only)
The test session concluded with five consecutive startle pulse-alone trials (120 dB). PPI was described as a reduction in the amplitude of the startle reflex in the presence of the prepulse stimulus, which was calculated for each prepulse intensity with the formula: PPI (%) = 100 − (average startle reflex in presence of the prepulse × 100/average startle reflex without a prepulse).
Apomorphine-induced climbing test
The test was performed according to the related literature (Akhtar et al., Reference Akhtar, Uma Devi, Ali, Pillai and Vohora2006; Mahmood et al., Reference Mahmood, Khanam, Pillai and Akhtar2012). Apomorphine was administered (1.5 mg/kg, s.c.) 30 min after galangin (50 mg/kg, i.p.) or the reference drug administration (haloperidol 1 mg/kg, olanzapine 3 mg/kg). Then, the mice were immediately placed in a cylindrical wire cage (height 13 cm, diameter 14 cm, and pores 3 mm). The total climbing time on the inside of the wire cage was recorded for 30 min.
MK-801-induced hyperlocomotion test
The test was performed according to the related literature (Bardgett et al., Reference Bardgett, Boeckman, Krochmal, Fernando, Ahrens and Csernansky2003; Sławińska et al., Reference Sławińska, Wierońska, Stachowicz, Marciniak, Lasoń-Tyburkiewicz, Gruca, Papp, Kusek, Tokarski, Doller and Pilc2013; Canal et al., Reference Canal, Morgan, Felsing, Kondabolu, Rowland, Robertson, Sakhuja and Booth2014). MK-801 was administered (0.3 mg/kg, s.c.) 30 min after galangin (50 mg/kg, i.p.) or the reference drug administration (haloperidol 1 mg/kg, olanzapine 3 mg/kg). Then, the mice were immediately placed in activitymeter apparatus (MAY-AMS 02 Animal Activity Monitoring System, Commat Ltd. Ankara/TURKEY), which automatically recorded the total number of movements for 1 h.
Catalepsy test
The test was performed according to the related literature (Kinsey & Cole, Reference Kinsey and Cole2013; Neves et al., Reference Neves, Antonio, Betti, Pranke, Fraga, Barreiro, Noël and Rates2013). The mice were positioned with their forepaws on a glass bar 5 cm high from the floor, 30, 60, and 90 min after galangin 50 mg/kg or the reference drug administration (haloperidol 1 mg/kg, olanzapine 3 mg/kg). At each time point, the time that the mice remained in this position, which was defined as the cataleptic posture, was observed for a maximum of 60 s. The test was ended when mice corrupted this position by touching the floor with their forepaws or by climbing on the bar.
Determination of brain acetylcholine levels
Brain acetylcholine concentrations were determined in rats. Immediately after performing the PPI of the acoustic startle reflex test, the rats were sacrificed and their brains were removed. One cerebral hemisphere was weighed and homogenised in phosphate-buffered saline. Acetylcholine concentrations were determined by following the instructions of a commercial kit (Bioassay Systems Enzychrom TM Acetylcholine Assay Kit-EACL-100, CA, USA), and the results were analysed colorimetrically.
Statistical analysis
Data were statistically analysed using the SPSS 21.0 and Sigma Stat 3.5 programmes. The Kruskal−Wallis test was used to analyse the results of the PPI of the acoustic startle reflex, apomorphine-induced climbing, MK-801-induced hyperlocomotion, and catalepsy tests, and multiple comparisons were performed using the Student−Newman−Keuls or Tukey tests. One-way analysis of variance was used to analyse the outcomes of the brain acetylcholine concentrations, and multiple comparisons were made using the Tamhane test. A value of p < 0.05 was accepted as statistically significant.
Results
The effects of galangin on apomorphine-induced prepulse inhibition
The results of a single administration of galangin and comparison with haloperidol, olanzapine, and donepezil
Apomorphine significantly decreased PPI% at the three prepulse intensity levels (74, 78, and 86 dB) compared with the control, which meant there was a disruption in PPI (p < 0.05) (Fig. 1). Galangin 50 and 100 mg/kg significantly increased PPI% at the three prepulse intensity levels (74, 78, and 86 dB) compared with the apomorphine group, which reflected an enhancement in apomorphine-induced PPI disruption (p < 0.05) (Fig. 1). There was no significant difference between galangin 50 and 100 mg/kg doses in PPI% at the three prepulse intensity levels (74, 78, and 86 dB) (p > 0.05) (Fig. 1).
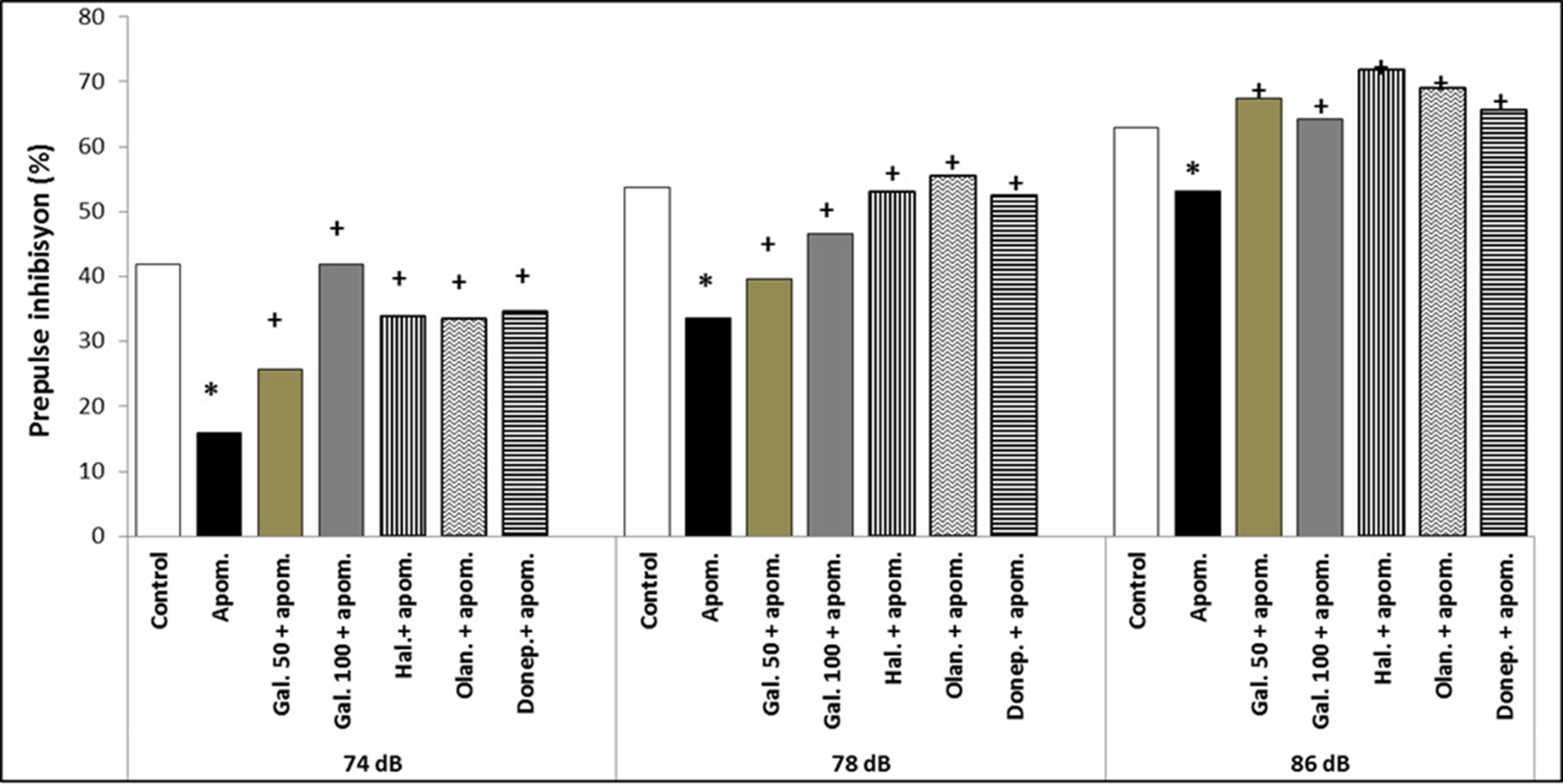
Fig. 1. The effects of a single galangin administration on apomorphine-induced PPI disruption at three prepulse intensity levels (74, 78, and 86 dB) and a comparison with haloperidol, olanzapine, and donepezil in rats. The results are expressed as median. Apom., apomorphine; Gal. 50, galangin 50 mg/kg; Gal. 100, galangin 100 mg/kg; Hal., haloperidol, Olan., olanzapine, Donep., Donepezil. * p < 0.05 refers to a statistically significant difference compared with the control group. + p < 0.05 refers to a statistically significant difference compared with the apomorphine group.
Haloperidol, olanzapine, and donepezil also significantly increased PPI% at the three prepulse intensity levels (74, 78, and 86 dB) compared with the apomorphine group (p < 0.05) (Fig. 1). There was no significant difference between galangin 50 and 100 mg/kg doses and haloperidol, olanzapine, donepezil in PPI% at the three prepulse intensity levels (74, 78, and 86 dB) (p > 0.05) (Fig. 1).
The results of combined administration of galangin with nicotinic, muscarinic, and 5-HT1A receptor antagonists
A galangin 50 mg/kg dose was selected for the combined administrations because there was no significant difference between the 50 and 100 mg/kg doses of galangin in PPI%. There was no significant alteration in the effects of galangin (50 mg/kg) on PPI% at the three prepulse intensity levels (74, 78, and 86 dB) when galangin was administered with the nicotinic receptor antagonist mecamylamine, muscarinic receptor antagonist scopolamine, and 5-HT1A receptor antagonist WAY-100635 (p > 0.05) (Fig. 2).
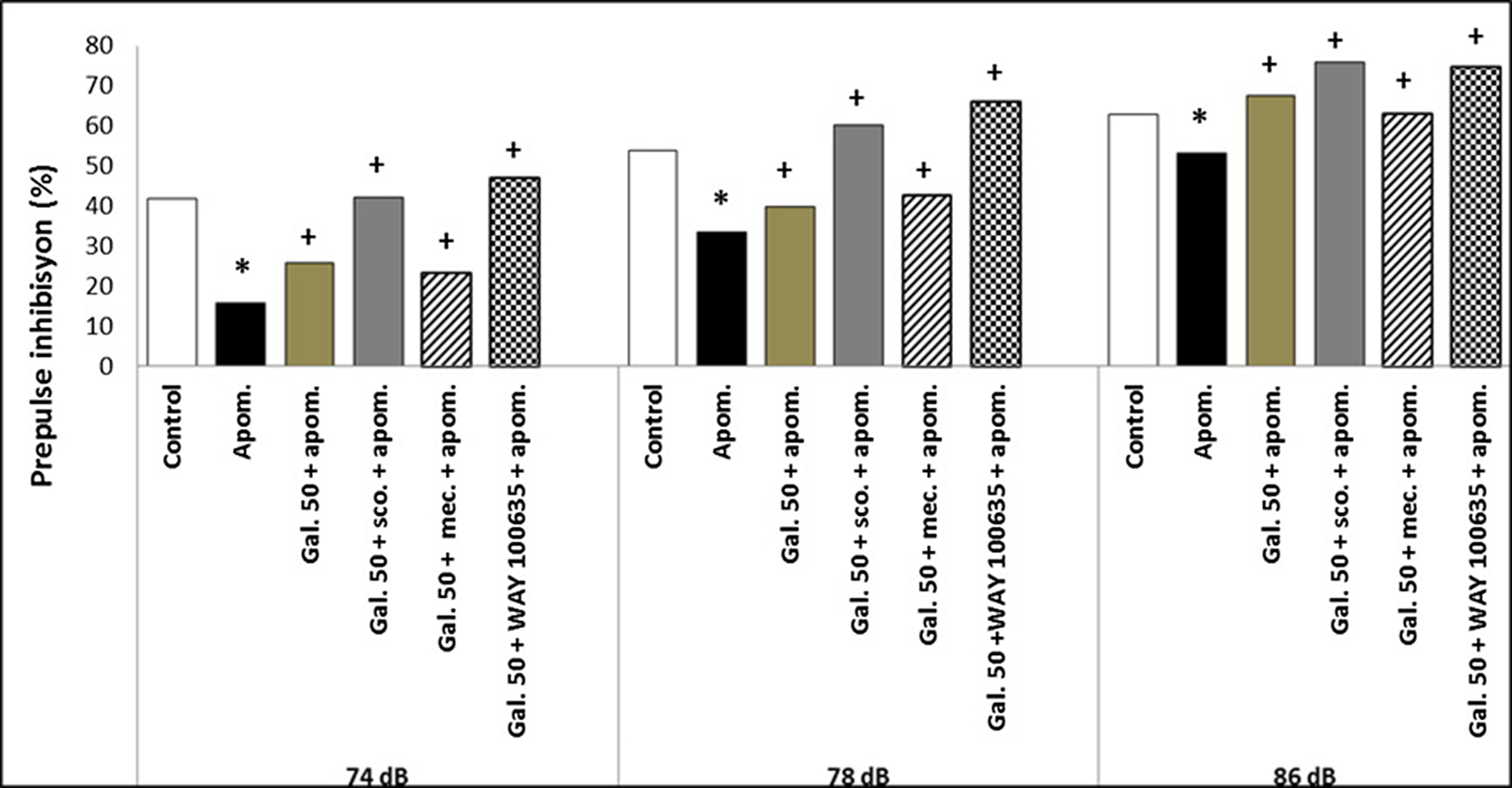
Fig. 2. The effects of galangin with nicotinic, muscarinic, 5-HT1A receptor antagonist combinations on apomorphine-induced PPI disruption at three prepulse intensity levels (74, 78, and 86 dB) in rats. The results are expressed as median. Apom., apomorphine; Gal. 50, galangin 50 mg/kg; sco., scopolamine; mec., mecamylamine. * p < 0.05 refers to a statistically significant difference compared with the control group. + p < 0.05 refers to a statistically significant difference compared with the apomorphine group.
The effects of galangin on apomorphine-induced climbing test
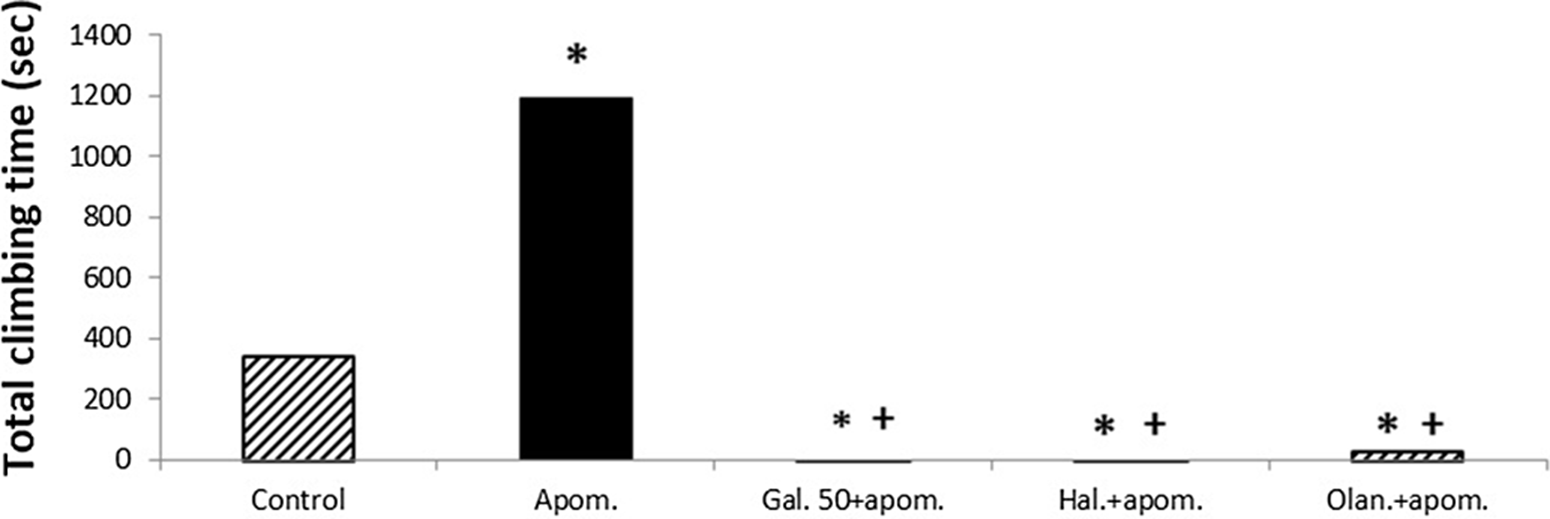
Apomorphine significantly increased the total climbing time compared with the control group (p < 0.05) (Fig. 3). Galangin 50 mg/kg significantly reduced the apomorphine-induced total climbing time compared with both the single apomorphine group and the control group (p < 0.05) (Fig. 3). Haloperidol (1 mg/kg) and olanzapine (1 mg/kg) also significantly reduced the apomorphine-induced total climbing time compared with both the single apomorphine group and the control group (p < 0.05) (Fig. 3). No significant difference was observed in the total climbing time between galangin 50 mg/kg, haloperidol, and olanzapine (p > 0.05) (Fig. 3).
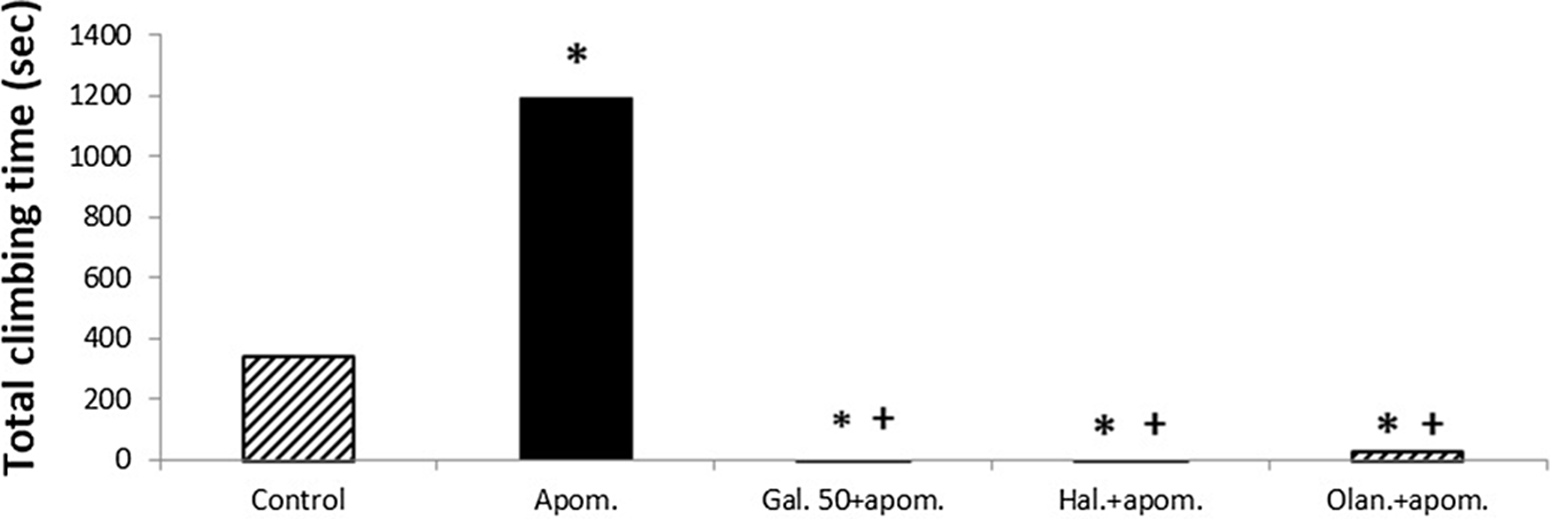
Fig. 3. The effects of galangin on apomorphine-induced climbing test in mice. The results are expressed as median. * p < 0.05 refers to a statistically significant difference compared with the control group. + p < 0.05 refers to a statistically significant difference compared with the apomorphine group. Haloperidol and olanzapine are used as reference antipsychotic drugs. Apom., apomorphine; Gal. 50, galangin 50 mg/kg; Hal., haloperidol; Olan., olanzapine.
The effects of galangin on MK-801-induced hyperlocomotion test
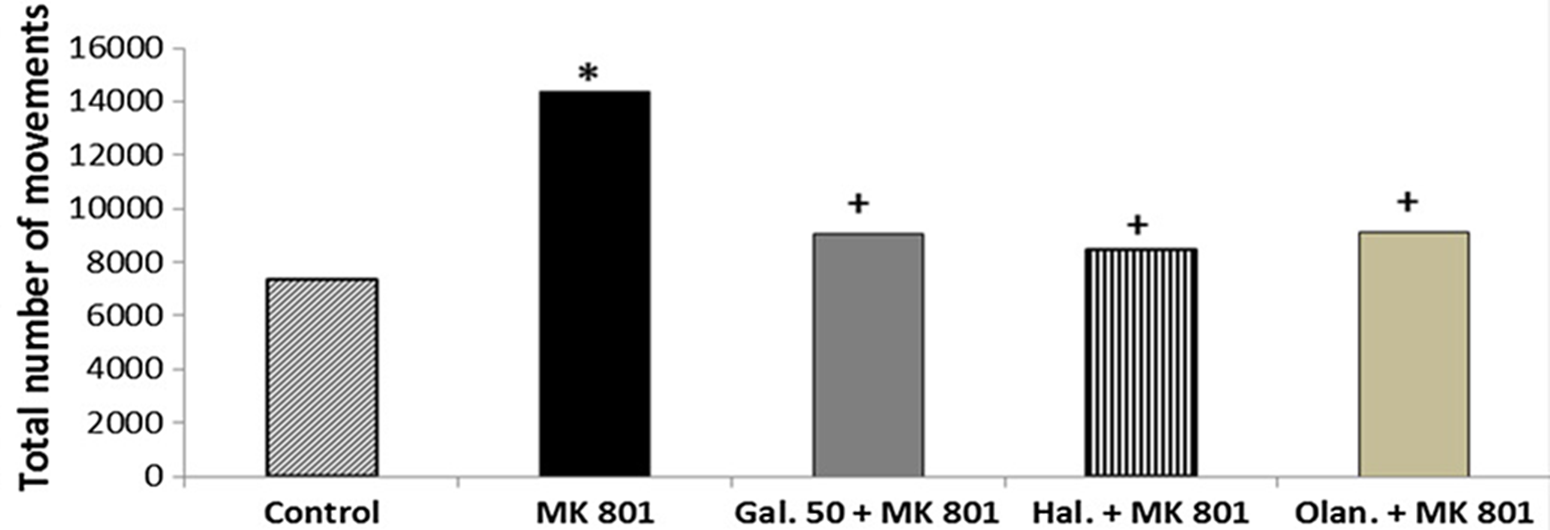
MK-801 significantly increased the total number of movements compared with the control group, indicating hyperlocomotion (p < 0.05) (Fig. 4). Galangin 50 mg/kg significantly decreased the total number of movements compared with MK-801 (p < 0.05) (Fig. 4). Haloperidol (1 mg/kg) and olanzapine (1 mg/kg) also significantly decreased the total number of movements compared with MK-801 (p < 0.05) (Fig. 4). No significant difference was observed in the total number of movements between galangin 50 mg/kg, haloperidol, and olanzapine (p > 0.05) (Fig. 4).
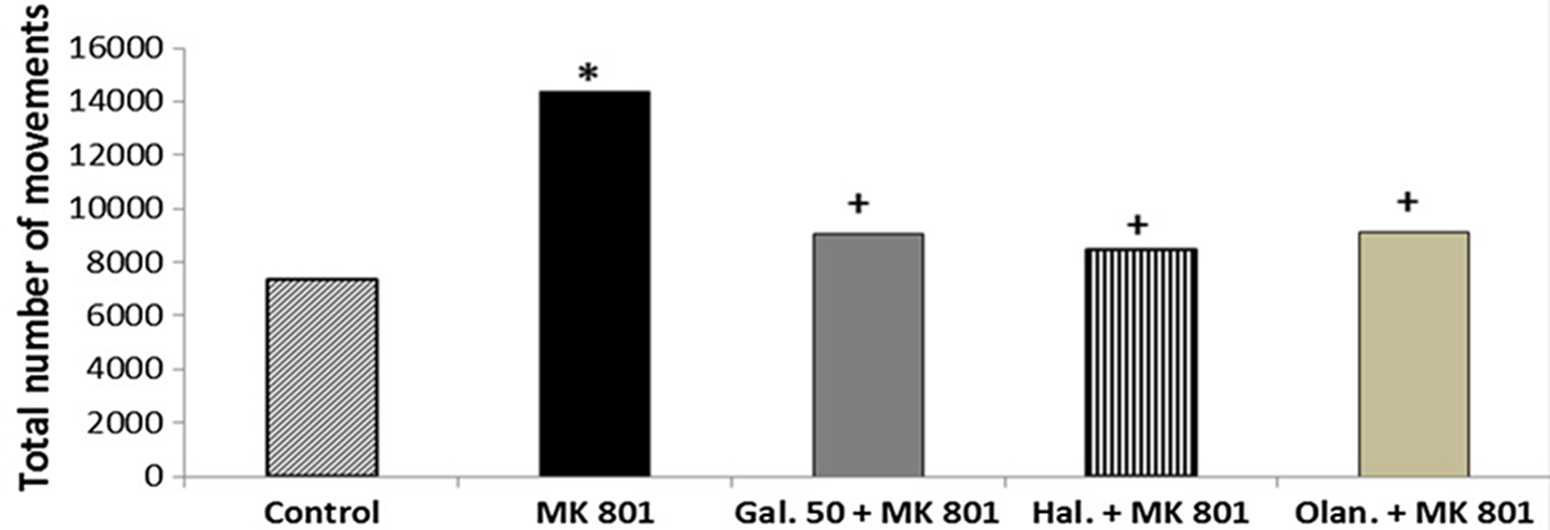
Fig. 4. The effects of galangin on MK-801-induced hyperlocomotion test in mice. The results are expressed as median. * p < 0.05 refers to a statistically significant difference compared with the control group. + p < 0.05 refers to a statistically significant difference compared with the MK-801 group. Gal. 50, galangin 50 mg/kg; Hal., haloperidol; Olan., olanzapine.
The effects of galangin on catalepsy test
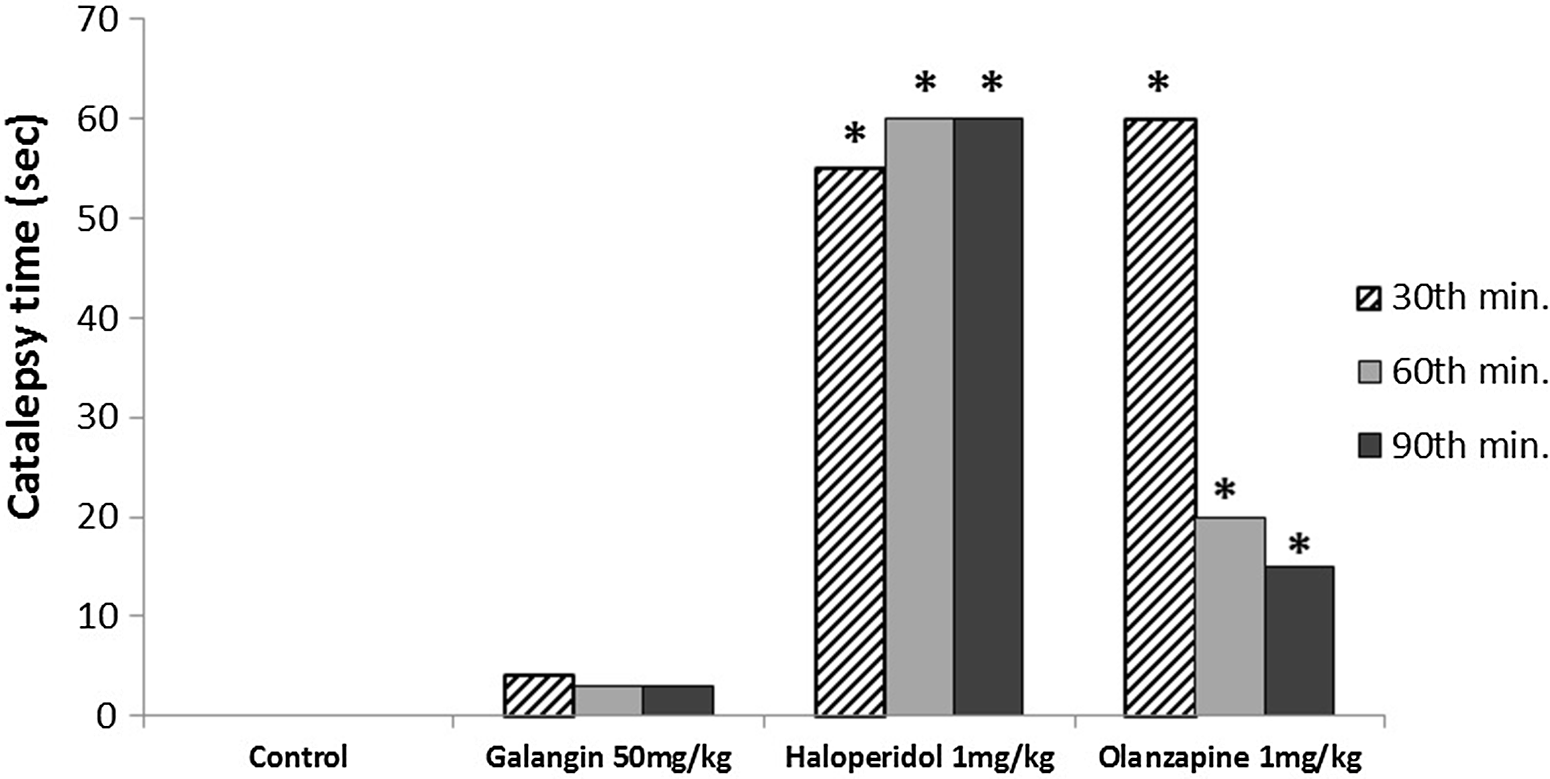
Galangin 50 mg/kg did not significantly increase the catalepsy time compared with the control group at the 30th, 60th, and 90th min of the test (p > 0.05) (Fig. 5). On the other hand, haloperidol and olanzapine significantly increased the catalepsy time compared with both the galangin 50 mg/kg and control groups at the 30th, 60th, and 90th min of the test (p < 0.05) (Fig. 5). There was no significant difference between haloperidol and olanzapine in terms of catalepsy time at all time points in the test (p > 0.05) (Fig. 5).
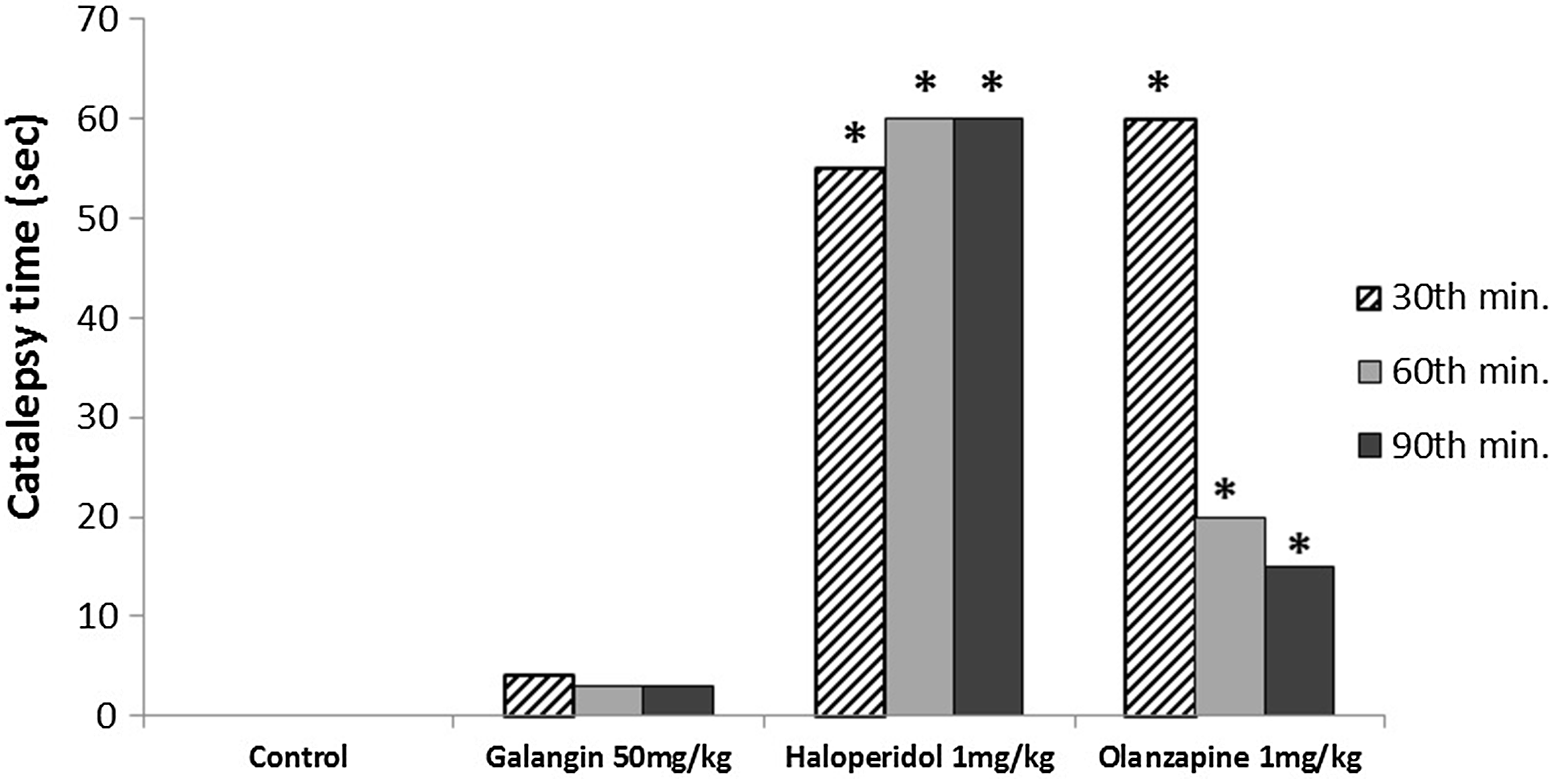
Fig. 5. The effects of galangin in catalepsy test in mice. The results are expressed as median. *p < 0.05 refers to a statistically significant difference compared with the galangin 50 mg/kg and control groups.
The effects of galangin on brain acetylcholine concentrations
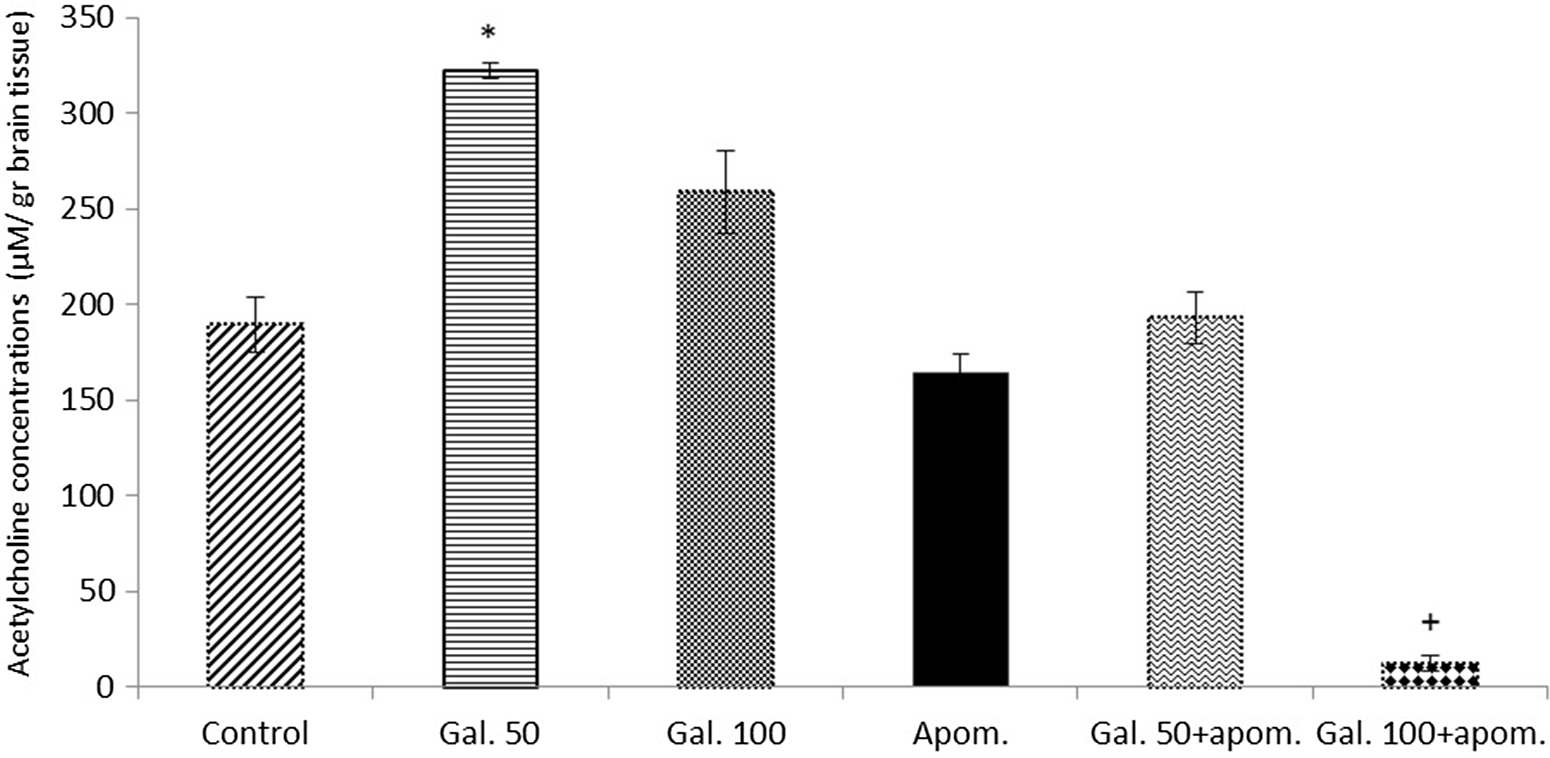
Galangin 50 mg/kg significantly increased brain acetylcholine concentrations compared with the control when administered alone (p < 0.001) (Fig. 6). An increase in the acetylcholine concentrations was also observed with galangin 100 mg/kg alone administration compared with the control; however, this increase was not statistically significant (p > 0.05) (Fig. 6). When galangin 50 and 100 mg/kg were administered in combination with apomorphine, a significant decrease in acetylcholine concentrations was observed compared with galangin 50 and 100 mg/kg alone (p < 0.001) (Fig. 6).
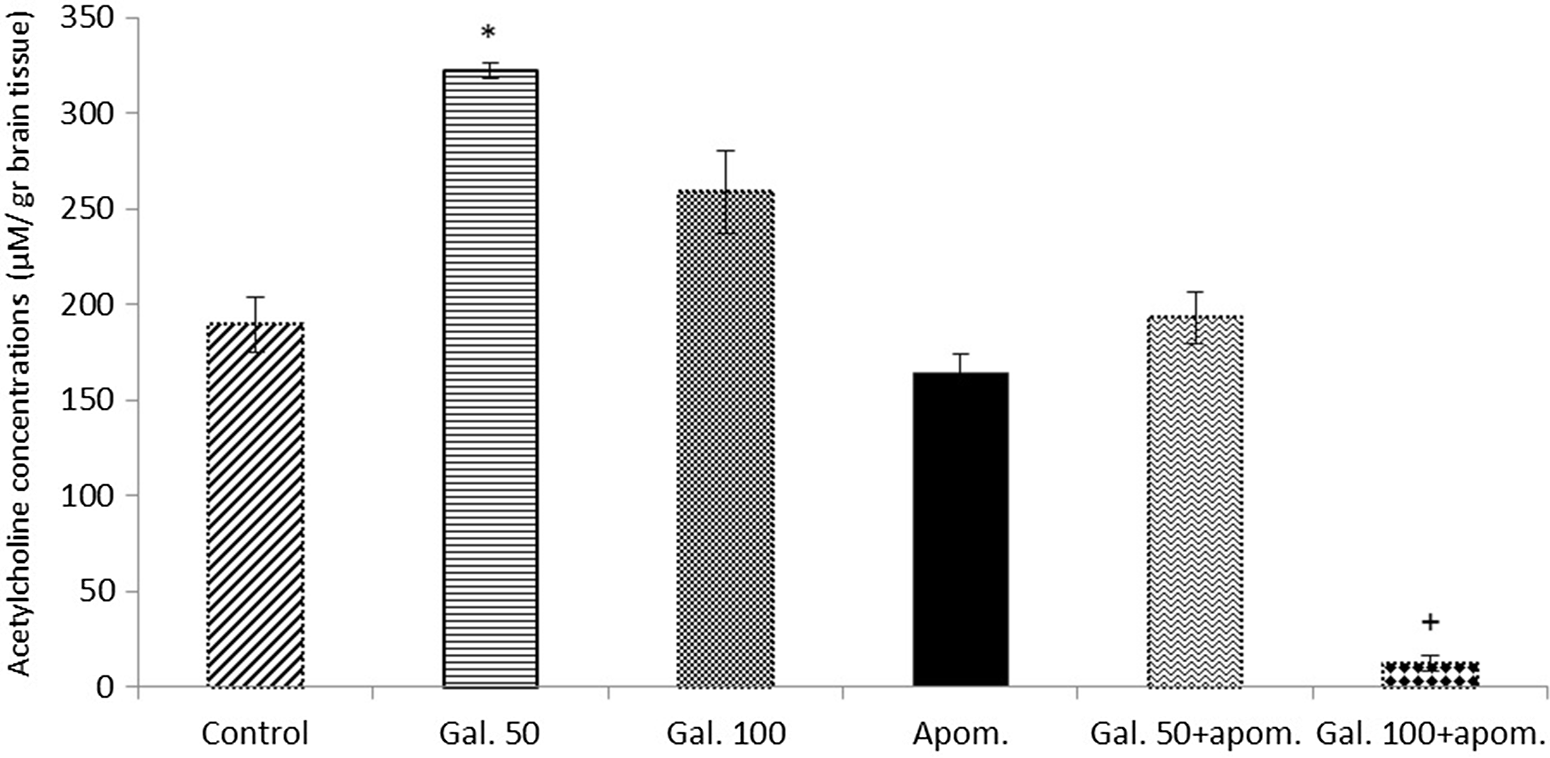
Fig. 6. The effects of galangin alone and in combination with apomorphine on brain acetylcholine concentrations. The results are expressed as mean ± SEM. Apom., apomorphine; Gal. 50, galangin 50 mg/kg; Gal. 100, galangin 100 mg/kg. * p < 0.001 refers to a statistically significant difference compared with the control, Apom., Gal. 50+apom., Gal. 100+apom.; + p < 0.001 refers to a statistically significant difference compared with all groups. Acetylcholine concentrations were calculated as µM/g brain tissue.
Discussion
In this study, we observed that galangin reversed apomorphine-induced PPI disruption in rats and it seemed that muscarinic or nicotinic acetylcholine receptors, and 5-HT1A receptors were not involved in this effect. The reversal of apomorphine-induced PPI disruption by galangin was similar to that of the acetylcholinesterase inhibitor donepezil, typical antipsychotic haloperidol, and atypical antipsychotic olanzapine. Galangin increased brain acetylcholine concentrations when administered alone. On the other hand, the effect of galangin on brain acetylcholine concentrations was inhibited when galangin was administered with apomorphine. In addition, galangin reduced apomorphine-induced climbing and MK-801-induced hyperlocomotion in mice, similar to haloperidol and olanzapine. Furthermore, galangin did not produce cataleptic behaviour, unlike haloperidol and olanzapine.
It has been suggested that the cholinergic system contributes to the pathophysiology of schizophrenia and some other neurological diseases (Erskine et al., Reference Erskine, Taylor, Bakker, Brown, Tasker and Nathan2019). In addition, the cholinergic system plays an essential role in the modulation of both normal cognitive processes and schizophrenia-associated cognitive deficits (Erskine et al., Reference Erskine, Taylor, Bakker, Brown, Tasker and Nathan2019). This led researchers to focus on the management of cognitive deficits by enhancing cholinergic activity. Hohnadel et al. studied the effects of the acetylcholinesterase inhibitors galantamine and donepezil, indicated in the treatment of Alzheimer’s disease, on apomorphine, MK-801, and scopolamine-induced PPI disruption. They reported that galantamine reversed PPI disrupted by all three agents, whereas donepezil only reversed PPI disruption by apomorphine and scopolamine (Hohnadel et al., Reference Hohnadel, Bouchard and Terry2007). Koda et al. (Reference Koda, Ago, Kawasaki, Hashimoto, Baba and Matsuda2008) reported that the acetylcholinesterase inhibitors galantamine and donepezil (1 and 3 mg/kg) reversed apomorphine-induced PPI disruption. Ballmaier et al. (Reference Ballmaier, Casamenti, Scali, Mazzoncini, Zoli, Pepeu and Spano2002) reported that the acetylcholinesterase inhibitor rivastigmine reversed PPI disruption in rats with damaged cholinergic neurons. The results of these three studies are in line with our study outcomes. We also observed that galangin (50 and 100 mg/kg), which is reported to have acetylcholinesterase inhibitory activity, reversed apomorphine-induced PPI disruption in rats. We also compared the effects of galangin with donepezil as a reference anticholinesterase drug and observed that donepezil (1 mg/kg) reversed apomorphine-induced PPI disruption in rats and that this effect was comparable to galangin.
The PPI of acoustic startle reflex is a test that evaluates sensorimotor gating, which is disrupted in many neuropsychiatric disorders as well as schizophrenia (Sato, Reference Sato2020). The cholinergic system, especially M2 acetylcholine receptors, mediate functional PPI (Sato, Reference Sato2020). Disruption of PPI refers to abnormal sensorimotor gating functioning, which reflects a cognitive deficit (Koukouli et al., Reference Koukouli, Rooy, Tziotis, Sailor, OʼNeill, Levenga, Witte, Nilges, Changeux, Hoeffer, Stitzel, Gutkin, DiGregorio and Maskos2017). Apomorphine, a dopamine receptor agonist, is used to disrupt PPI in experimental schizophrenia models (Wang et al., Reference Wang, Zhang, Wang, Zhang, Wang, Liang, Alachkar and Civelli2019). In our study, galangin (both 50 and 100 mg/kg) alleviated apomorphine-induced PPI disruption. This suggested that galangin might possess antidopaminergic activity. Besides presenting various biological activities, galangin was also reported to have anticholinesterase activity (Guo et al., Reference Guo, Xie, Choi, Zheng, Bi, Xu, Dong and Tsim2010). It has also been shown that the cholinergic and the dopaminergic systems interacted in the brain and contributed to the pathophysiology of neuropsychiatric disorders (Zhang et al., Reference Zhang, Liu, Zhou, Zhang, He and Yuan2018). Extracellular acetylcholine levels are associated with dopamine release in the ventral tegmental area, and muscarinic and nicotinic receptors are involved in this process (Zhang et al., Reference Zhang, Liu, Zhou, Zhang, He and Yuan2018).
It was reported that muscarinic receptor antagonists disrupted PPI (Chʼng et al., Reference Chʼng, Walker, McCarthy, Le, Thomas, Gibbons, Udawela, Kusljic, Dean and Gogos2020). Jones et al. reported that the muscarinic acetylcholine M1/M4 receptor agonist xanomeline improved apomorphine-induced PPI disruption in rats and suggested an interaction between cholinergic and dopaminergic systems in the regulation of PPI. They offered xanomeline as a promising agent in the treatment of cognitive dysfunctions in schizophrenia (Jones et al., Reference Jones, Eberle, Shaw, McKinzie and Shannon2005). In this study, we administered galangin in combination with the muscarinic receptor antagonist scopolamine (0.05 mg/kg, a dose that does not disrupt PPI) to investigate the role of muscarinic receptors in the reversal effect of galangin on apomorphine-induced PPI disruption and observed that there was no alteration in this effect. Accordingly, we suggest that muscarinic receptors may not be involved in the effect of galangin.
In addition to muscarinic receptors, nicotinic acetylcholine receptors were also reported to mediate PPI and systemic nicotine administration was observed to enhance PPI (Pinnock et al., Reference Pinnock, Bosch, Brown, Simons, Yeomans, DeOliveira and Schmid2015). It was also reported that activation of nicotinic receptors alleviated the disrupted prepulse (Uslu et al., Reference Uslu, Savci, Buyukuysal and Goktalay2014). In this study, we administered galangin in combination with the nicotinic receptor antagonist mecamylamine to investigate the role of nicotinic receptors in the reversal effect of galangin on apomorphine-induced PPI disruption and observed that there was no alteration in this effect. Accordingly, we suggest that nicotinic receptors may not be involved in the effect of galangin.
5-HT1A receptor agonists were reported to disrupt PPI, and WAY-100635, an antagonist at 5-HT1A receptors, reversed this disruption (Conti, Reference Conti2012; Tai et al., Reference Tai, Ko, Lin, Wan, Chung and Liu2018). However, there are also contradictory results showing that 5-HT1A receptor agonists enhance PPI (Dulawa & Geyer, Reference Dulawa and Geyer2000). In our study, we administered galangin in combination with the 5-HT1A receptor antagonist WAY-100635 to investigate the role of 5-HT1A receptors in the reversal effect of galangin on apomorphine-induced PPI disruption and observed that there was no alteration in this effect. Accordingly, we suggest that 5-HT1A receptors may not be involved in the effect of galangin.
Functional disturbances of glutamate N-methyl-D-aspartate (NMDA) receptors are associated with the pathogenesis of schizophrenia (Uno & Coyle, Reference Uno and Coyle2019). In addition, it was suggested that cholinergic enhancement in the central nervous system alleviated the reduced NMDA receptor functions and improved cognitive functions (Jones et al., Reference Jones, Byun and Bubser2012). Furthermore, it was reported that nicotinic acetylcholine receptors and glutamate NMDA receptors were closely located on the nerves and there was a functional interaction between these two receptor types (Zappettini et al., Reference Zappettini, Grilli, Olivero, Chen, Padolecchia, Pittaluga, Tomé, Cunha and Marchi2014). MK-801 is a glutamate NMDA receptor antagonist that induces hyperactivity in rodents resembling the positive symptoms of schizophrenia (Fan et al., Reference Fan, Du, Zhu, Wu, Liu, Wang, Wang, Gu, Shan, Deng, Zhu, Xu, Ge, Li and Li2018). In this study, we used MK-801-induced hyperlocomotion test in mice as an experimental schizophrenia model and observed that galangin alleviated hyperlocomotion induced by MK-801. It seems that galangin may possess an activity through NMDA receptors.
We used the apomorphine-induced climbing test as one of the experimental schizophrenia models in the mice in our study. Apomorphine is a non-specific dopamine agonist that acts on dopamine D1 and D2 receptors and induces climbing behaviour in rodents (Sukhanov et al., Reference Sukhanov, Espinoza, Yakovlev, Hoener, Sotnikova and Gainetdinov2014). We observed that galangin reduced apomorphine-induced climbing, reflecting an antipsychotic-like effect. It has been suggested that positive symptoms of schizophrenia result from the overactivity of dopaminergic neurotransmission (Laruelle, Reference Laruelle2014). The interaction between cholinergic and dopaminergic systems has been shown (Abudukeyoumu et al., Reference Abudukeyoumu, Hernandez-Flores, Garcia-Munoz and Arbuthnott2019). It was reported that acetylcholine regulated dopaminergic neurotransmission via nicotinic acetylcholine receptors (6). In addition, a functional interconnection between muscarinic acetylcholine receptors and dopaminergic neurotransmission was also reported (Myslivecek, Reference Myslivecek2021). We may suggest that the effect of galangin on apomorphine-induced climbing may result from the interaction of dopaminergic and cholinergic mechanisms considering the reported anticholinesterase activity of galangin. However, in this study, we also observed that acetylcholine concentrations were not increased in rat brains when galangin and apomorphine were administered in combination. Therefore, it seems that galangin serves the antipsychotic-like effect by directly inhibiting dopaminergic neurotransmission.
Antipsychotics are the main treatment options for schizophrenia; however, they are associated with extrapyramidal adverse effects (Yoshida & Takeuchi, Reference Yoshida and Takeuchi2021). These adverse effects are less common with the use of atypical antipsychotics (Chow et al., Reference Chow, Kadouh, Bostwick and VandenBerg2020). The extrapyramidal adverse effects of antipsychotics can be modelled in animals (Gobira et al., Reference Gobira, Ropke, Aguiar, Crippa and Moreira2013). Catalepsy test is one of these models; catalepsy is defined as ‘the inability of the animal to change its posture (Hoffman & Donovan, Reference Hoffman and Donovan1995). In this study, we observed that galangin did not induce cataleptic behaviour in the catalepsy test in mice. We may suggest that galangin does not present an extrapyramidal adverse effect. This effect was superior to the typical antipsychotic haloperidol and antitypical antipsychotic olanzapine because both agents induced catalepsy in this study.
As a conclusion, we suggest that galangin may possess an antipsychotic-like effect considering its effects on apomorphine-induced PPI disruption in rats and experimental schizophrenia models in mice. These effects were comparable to the reference anticholinesterase donepezil and the antipsychotics haloperidol and olanzapine. Moreover, galangin may be advantageous because it did not induce catalepsy, unlike haloperidol and olanzapine. It seems that direct antidopaminergic actions, as well as the activity on glutamate NMDA receptors, may contribute to the effects of galangin rather than its anticholinesterase activity and effects on cholinergic mechanisms and 5-HT1A receptors. Further studies are needed to elucidate detailed mechanisms of the antipsychotic-like effect of galangin, as discussed in the limitations section of this study.
Authors contributions
BK study conception and design, data acquisition, analysis and interpretation of data, draughting of the manuscript, critical revision and final approval of the manuscript. SA data acquisition, critical revision and final approval of the manuscript. EY study conception and design, analysis and interpretation of data, critical revision and final approval of the manuscript. AM analysis and interpretation of data, critical revision and final approval of the manuscript. KE study conception and design, critical revision and final approval of the manuscript. FSK study conception and design, analysis and interpretation of data, critical revision and final approval of the manuscript.
Financial Support
This study was supported by the ‘Commission of Scientific Research’ of Eskisehir Osmangazi University (Project number: 2012-11027).
Conflict of Interest
None.