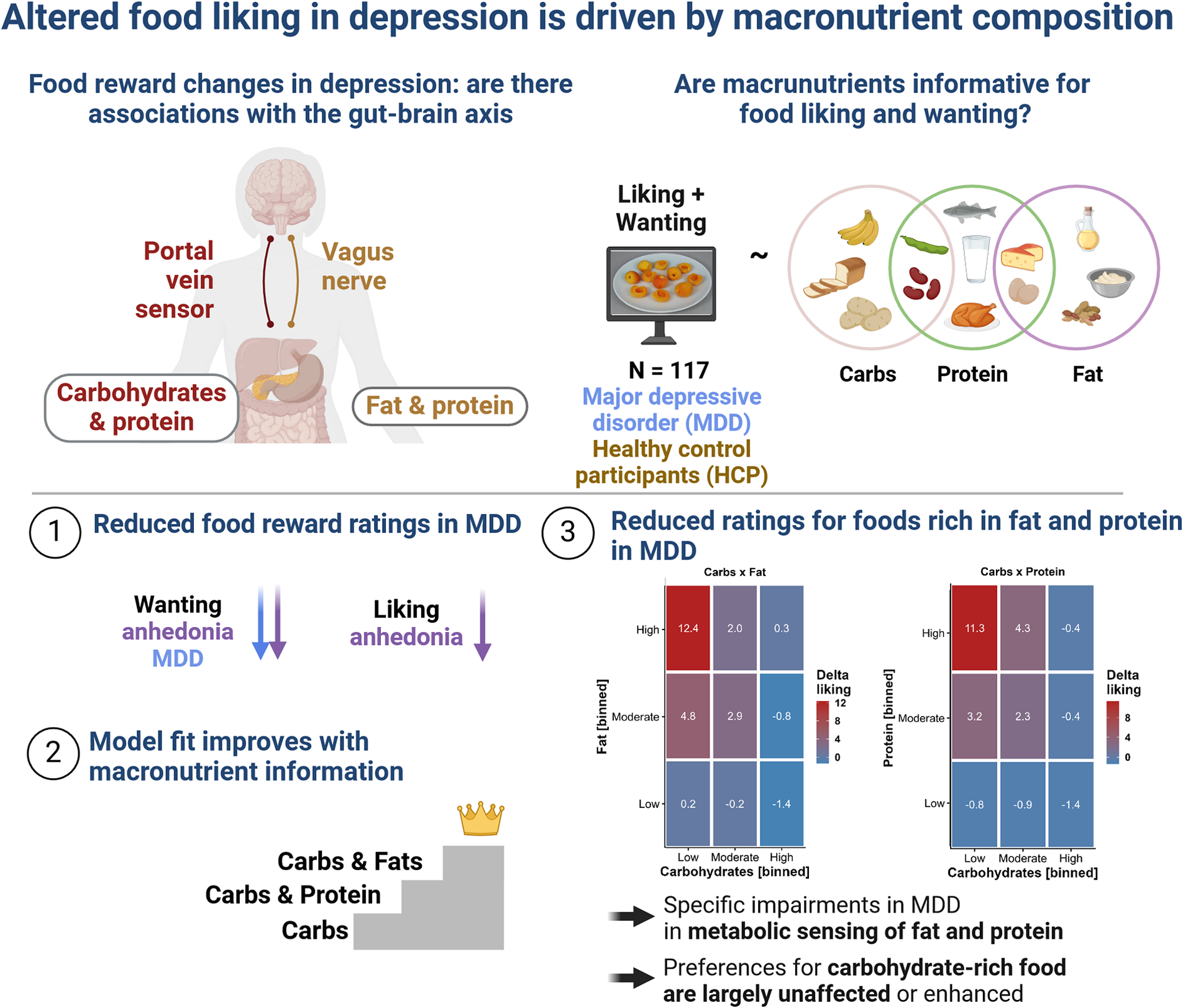
Introduction
Major depressive disorder (MDD) affects about 280 million people worldwide (Institute of Health Metrics and Evaluation, 2023). It is characterized by diverse symptoms (Fried, Flake, & Robinaugh, Reference Fried, Flake and Robinaugh2022; Fried & Nesse, Reference Fried and Nesse2015b), such as depressed mood and loss of interest or pleasure (‘anhedonia’) as cardinal symptoms. MDD also includes opposing somatic symptoms, such as increased versus decreased appetite and body weight, during a depressive episode (Milaneschi, Simmons, van Rossum, & Penninx, Reference Milaneschi, Simmons, van Rossum and Penninx2019; Polivy & Herman, Reference Polivy and Herman1976). Although appetite-related symptoms are often not directly treated and may even worsen with antidepressant medication (Himmerich, Minkwitz, & Kirkby, Reference Himmerich, Minkwitz and Kirkby2015; Kazes et al., Reference Kazes, Danion, Grangé, Pradignac, Simon, Burrus-Mehl, Schlienger and L1994), they are comparatively stable across episodes (Stunkard, Fernstrom, Price, Frank, & Kupfer, Reference Stunkard, Fernstrom, Price, Frank and Kupfer1990) and possibly reflect mechanistically distinct subtypes of depression (Cuijpers et al., Reference Cuijpers, Weitz, Lamers, Penninx, Twisk, DeRubeis, Dimidjian, Dunlop, Jarrett, Segal and Hollon2017; Milaneschi et al., Reference Milaneschi2017; Milaneschi, Lamers, Bot, Drent, & Penninx, Reference Milaneschi, Lamers, Bot, Drent and Penninx2017; Penninx, Milaneschi, Lamers, & Vogelzangs, Reference Penninx, Milaneschi, Lamers and Vogelzangs2013; Simmons et al., Reference Simmons, Burrows, Avery, Kerr, Taylor, Bodurka, Potter, Teague and Drevets2020). Consequently, such opposing changes in appetite and body weight have been associated with differential brain responses to food cues (Simmons et al., Reference Simmons, Burrows, Avery, Kerr, Bodurka, Savage and Drevets2016) and distinct functional connectivity profiles of the reward circuit (Kroemer et al., Reference Kroemer, Opel, Teckentrup, Li, Grotegerd, Meinert, Lemke, Kircher, Nenadic, Krug, Jansen, Sommer, Steinstrater, Small, Dannlowski and Walter2022). These emerging results suggest that an improved phenotyping of appetite-related symptoms in depression promises a more refined diagnosis of reward dysfunction (Fried & Nesse, Reference Fried and Nesse2015a; Fried, Nesse, Zivin, Guille, & Sen, Reference Fried, Nesse, Zivin, Guille and Sen2014; Schulz et al., Reference Schulz, Klaus, Peglow, Ellinger, Kühnel, Walter and Kroemer2024). In the long run, such insights may improve the allocation of suitable treatments because effective therapies for somatic symptoms of MDD should conceivably consider the direction of changes in appetite and body weight.
To better characterize appetite-related processes and symptoms, many studies use food cue reactivity (FCR) paradigms (Smeets, Charbonnier, van Meer, van der Laan, & Spetter, Reference Smeets, Charbonnier, van Meer, van der Laan and Spetter2012; van der Laan, de Ridder, Viergever, & Smeets, Reference van der Laan, de Ridder, Viergever and Smeets2011). During these FCR tasks, participants view various food images while concurrent brain (Charbonnier et al., Reference Charbonnier, van Meer, Johnstone, Crabtree, Buosi, Manios, Androutsos, Giannopoulou, Viergever and Smeets2018; Frank et al., Reference Frank, Laharnar, Kullmann, Veit, Canova, Hegner, Fritsche and Preissl2010; Guthoff et al., Reference Guthoff, Grichisch, Canova, Tschritter, Veit, Hallschmid, Haring, Preissl, Hennige and Fritsche2010; Hare, Camerer, & Rangel, Reference Hare, Camerer and Rangel2009; Kroemer et al., Reference Kroemer, Krebs, Kobiella, Grimm, Pilhatsch, Bidlingmaier, Zimmermann and Smolka2013a; Kroemer et al., Reference Kroemer, Krebs, Kobiella, Grimm, Vollstadt-Klein, Wolfensteller, Kling, Bidlingmaier, Zimmermann and Smolka2013b) or physiological responses (Rebollo, Schmidt, Longren, & Park, Reference Rebollo, Schmidt, Longren and Park2021) are collected, or ratings scales are embedded to capture cue-elicited responses guiding eating behavior (e.g., taste, healthiness, wanting, or liking; (Charbonnier, van Meer, van der Laan, Viergever, & Smeets, Reference Charbonnier, van Meer, van der Laan, Viergever and Smeets2015; Müller, Teckentrup, Kühnel, Ferstl, & Kroemer, Reference Müller, Teckentrup, Kühnel, Ferstl and Kroemer2022). Food items from the standardized food battery are typical European foods and have been selected based on recognizability (Charbonnier et al., Reference Charbonnier, van Meer, van der Laan, Viergever and Smeets2015). Food images are typically contrasted against non-food images (Koepp et al., Reference Koepp, Klaus, Ferstl, Müller, Kühnel and Kroemer2021; Müller et al., Reference Müller, Teckentrup, Kühnel, Ferstl and Kroemer2022), or grouped into categories, for example, based on high versus low energy density (Dimitropoulos, Tkach, Ho, & Kennedy, Reference Dimitropoulos, Tkach, Ho and Kennedy2012; Mehta et al., Reference Mehta, Melhorn, Smeraglio, Tyagi, Grabowski, Schwartz and Schur2012). Recent studies have demonstrated that FCR tasks help reveal nuanced information about the encoding of food reward by considering the macronutrient composition of the depicted food (Motoki, Saito, Suzuki, & Sugiura, Reference Motoki, Saito, Suzuki and Sugiura2021; Small & DiFeliceantonio, Reference Small and DiFeliceantonio2019). For example, DiFeliceantonio et al. (Reference DiFeliceantonio, Coppin, Rigoux, Edwin Thanarajah, Dagher, Tittgemeyer and Small2018) have shown supra-additive effects of fat and carbohydrate content on food reward using brain responses and willingness to pay as outcomes. These findings extrapolate previous work on the correspondence between willingness to pay and the true energy density of food but not the perceived energy content (Tang, Fellows, & Dagher, Reference Tang, Fellows and Dagher2014). Such nutritional information is estimated surprisingly quickly as the accuracy of ratings does not improve if participants have more time than 100 ms to evaluate the food (Motoki et al., Reference Motoki, Saito, Suzuki and Sugiura2021). Therefore, conventional FCR tasks may provide useful insights about altered food reward processing of macronutrients in depression, even though a comprehensive assessment is lacking to date.
Associating the macronutrient composition of food with markers of reward dysfunction in depression is also promising in light of plausible neurobiological links with disturbed energy metabolism (Borgmann & Fenselau, Reference Borgmann and Fenselau2024; Kenny, Reference Kenny2011; Milaneschi et al., Reference Milaneschi, Simmons, van Rossum and Penninx2019). Relating subjective reinforcing values of food to nutritive values of its fat and carbohydrates content relies on parallel neural afferent signaling pathways (de Araujo, Schatzker, & Small, Reference de Araujo, Schatzker and Small2020; McDougle et al., Reference McDougle, de Araujo, Singh, Yang, Braga, Paille, Mendez-Hernandez, Vergara, Woodie, Gour, Sharma, Urs, Warren and de Lartigue2024; Small & DiFeliceantonio, Reference Small and DiFeliceantonio2019). Whereas fat-induced reward signals are mainly transmitted via vagal afferents (de Araujo, Reference de Araujo2016; de Araujo et al., Reference de Araujo, Schatzker and Small2020; Goldstein et al., Reference Goldstein, McKnight, Carty, Arnold, Betley and Alhadeff2021; Tellez et al., Reference Tellez, Medina, Han, Ferreira, Licona-Limon, Ren, Lam, Schwartz and de Araujo2013), carbohydrate-induced reward signals are primarily conveyed via portal vein sensors and spinal afferents (Bacharach, Tordoff, & Alhadeff, Reference Bacharach, Tordoff and Alhadeff2023; Goldstein et al., Reference Goldstein, McKnight, Carty, Arnold, Betley and Alhadeff2021) although vagal afferents contribute as well (Fernandes et al., Reference Fernandes, Alves da Silva, Almeida, Cui, Gerfen, Costa and Oliveira-Maia2020). For protein-induced effects, there is mostly indirect evidence pointing to vagal afferent signals (de Lartigue & Diepenbroek, Reference de Lartigue and Diepenbroek2016), although subdiaphragmatic vagotomies in rats show that it is not an obligatory pathway for the transfer of energy-related information to the brain (L’Heureux-Bouron et al., Reference L’Heureux-Bouron, Tome, Rampin, Even, Larue-Achagiotis and Fromentin2003). Moreover, distinct gut-innervating sensory neurons differentially control feeding and glucoregulatory neurocircuits, potentially pointing to specific targets for improved metabolic control (Bai et al., Reference Bai, Mesgarzadeh, Ramesh, Huey, Liu, Gray, Aitken, Chen, Beutler, Ahn, Madisen, Zeng, Krasnow and Knight2019; Borgmann et al., Reference Borgmann, Ciglieri, Biglari, Brandt, Cremer, Backes, Tittgemeyer, Wunderlich, Bruning and Fenselau2021; Li et al., Reference Li, Tan, Lu, Tsang, Chung and Zuker2022; Tan et al., Reference Tan, Sisti, Jin, Vignovich, Villavicencio, Tsang, Goffer and Zuker2020).
Enhanced craving for carbohydrates has often been observed in patients with MDD with an alleged link to increased tryptophan metabolism and brain serotonin levels (Fernstrom, Krowinski, & Kupfer, Reference Fernstrom, Krowinski and Kupfer1987; Fernstrom & Wurtman, Reference Fernstrom and Wurtman1971; Markus, Reference Markus2007; Wurtman & Wurtman, Reference Wurtman and Wurtman1995). Accordingly, self-reported differences in macronutrient consumption are associated with a risk for depression in large cross-sectional studies (Cheng, Fu, Lian, Zhan, & Zhang, Reference Cheng, Fu, Lian, Zhan and Zhang2024), and Mendelian randomization studies even suggest a protective effect of carbohydrate intake (Yao et al., Reference Yao, Zhang, Dong, Wang, Zhang, Guo, Guo and Yang2022). Because depression is often comorbid with obesity (Milaneschi et al., Reference Milaneschi, Simmons, van Rossum and Penninx2019) and type 2 diabetes (Anderson, Freedland, Clouse, & Lustman, Reference Anderson, Freedland, Clouse and Lustman2001; Geraets et al., Reference Geraets, Kohler, Muzambi, Schalkwijk, Oenema, Eussen, Dagnelie, Stehouwer, Schaper, Henry, van der Kallen, Wesselius, Koster, Verhey and Schram2020; Lustman et al., Reference Lustman, Anderson, Freedland, de Groot, Carney and Clouse2000), altered energy metabolism could contribute to reward deficits and somatic symptoms (Pistis et al., Reference Pistis, Milaneschi, Vandeleur, Lasserre, Penninx, Lamers, Boomsma, Hottenga, Marques-Vidal, Vollenweider, Waeber, Aubry, Preisig and Kutalik2021), and an improved knowledge of the pathways contributing to specific depressive symptoms may help identify suitable therapies. Illustratively, recent studies have demonstrated that patients with MDD show altered lipid metabolism (Tkachev et al., Reference Tkachev2023), and targeting vagal afferent projections with vagus nerve stimulation could potentially alleviate key symptoms of depression (Edwin Thanarajah & Reif, Reference Edwin Thanarajah and Reif2022; Ferstl, Kühnel, Klaus, Lin, & Kroemer, Reference Ferstl, Kühnel, Klaus, Lin and Kroemer2024; Teckentrup & Kroemer, Reference Teckentrup and Kroemer2024), including food reward deficits (Bodenlos et al., Reference Bodenlos, Kose, Borckardt, Nahas, Shaw, O’Neil and George2007; Han et al., Reference Han, Tellez, Perkins, Perez, Qu, Ferreira, Ferreira, Quinn, Liu, Gao, Kaelberer, Bohorquez, Shammah-Lagnado, de Lartigue and de Araujo2018; Koepp et al., Reference Koepp, Klaus, Ferstl, Müller, Kühnel and Kroemer2021; McDougle et al., Reference McDougle, de Araujo, Singh, Yang, Braga, Paille, Mendez-Hernandez, Vergara, Woodie, Gour, Sharma, Urs, Warren and de Lartigue2024). In sum, evaluating whether altered appetite in depression is specifically correlated with the macronutrient composition of food may reveal physiological relevant insights.
To address the pressing question of whether altered food reward in depression is associated with the macronutrient composition of the depicted food, we investigated patients with MDD and healthy control participants (HCPs) using a conventional FCR task with 60 familiar food and 20 non-food images (Koepp et al., Reference Koepp, Klaus, Ferstl, Müller, Kühnel and Kroemer2021; Müller et al., Reference Müller, Teckentrup, Kühnel, Ferstl and Kroemer2022). In addition, we characterized changes in appetite and body weight in patients with MDD using the SIGH-ADS interview (Williams & Terman, Reference Williams and Terman2003) that covers atypical symptoms of depression. Crucially, we also collected fasting levels of glucose, insulin, and triglycerides to estimate individual differences in insulin sensitivity as measures of metabolic disturbances. To evaluate associations with the macronutrient composition of food, we used linear mixed-effects models predicting wanting and liking ratings using the true nutritional value of the rated food images (Charbonnier et al., Reference Charbonnier, van Meer, van der Laan, Viergever and Smeets2015). By demonstrating specific associations of reward deficits in depression with foods rich in fat, but not carbohydrates, our study hints at the potential of incorporating macronutrient information into the research of somatic symptoms.
Methods
Participants
As part of an ongoing cross-sectional study, we analyzed a sample of 117 participants (54 diagnosed with MDD and 63 HCPs). The reported results are part of a larger preregistered study about the effects of the gut–brain axis on depression (https://clinicaltrials.gov/study/NCT05318924, Fahed et al. (Reference Fahed, Schulz, Klaus, Ellinger, Walter and Kroemer2024), Schulz et al. (Reference Schulz, Klaus, Peglow, Ellinger, Kühnel, Walter and Kroemer2024)). On average, participants had a mean age of 30 ± 8 years, and a mean body mass index (BMI) of 23.6 ± 3.2 kg/m2 (see Table 1). Compared to the group of patients with MDD, we oversampled older HCPs for an additional part of the study involving PET/fMRI (in which only a subset (N = 26) of healthy participants were invited). To avoid confounding effects of age, we accounted for age in all mixed-effects analyses (with additional confounds).
Table 1. Sample characteristics

a Mean ± SD; n (%).
b Two Sample t-test or Pearson’s Chi-squared test.
All participants were screened for eligibility prior to investigation by phone. Individuals were excluded when over the age of 50 or younger than 20 or if their BMI exceeded 30.0 kg/m2 or was below 18.5 kg/ m2. Furthermore, they were excluded if they had the comorbid disorder schizophrenia, bipolar disorder, severe substance abuse, or a severe neurological condition. HCPs were excluded if a mood or anxiety disorder was present. Due to high comorbidities with anxiety disorders (Kaufman & Charney, Reference Kaufman and Charney2000), this criterion was not applied to patients with MDD. Individuals were also excluded if they suffered from one of the following conditions within 12 months before screening: eating disorder, obsessive-compulsive disorder, unprocessed trauma and stressor-related disorder, and somatic symptom disorder. Moreover, they were screened for medication or illnesses influencing body weight and current pregnancy or nursing were additional exclusion criteria for women. Assignment to the MDD group required having MDD at the time of screening (i.e., within 3 months of enrollment) according to DSM-5 criteria. To generalize results to typical patients with MDD, there was no restriction concerning the type of therapy or medication except for changes of medication type or dosage within the preceding 2 months (Supplementary Materials SI1). Furthermore, participants were asked to be healthy at the time of testing, and no participants reported the use of antibiotics.
Recruitment took place at the Department of Psychiatry and Psychotherapy, University of Tübingen, Germany and on social media using online advertisements and flyers. Participants were asked to provide written consent prior to the first session. Compensation was given in the form of monetary and food-related rewards (snacks) resulting from the performance in decision-making games. Furthermore, participants received €50 or 5 credit points (if they were students and selected them) for full study completion. The study was approved by the institutional review board that is the local ethics committee of the University of Tübingen, Faculty of Medicine, in accordance with the Declaration of Helsinki (as revised in 2008).
Experimental procedure
The study was conducted at the Department of Psychiatry and Psychotherapy in Tübingen, Germany and consisted of two parts, completed either on one or two separate days. Participants gave written informed consent before beginning the study. Participation included an online assessment with questionnaires, for example, to assess symptoms of depression [Becks Depression Inventory, BDI-II (Hautzinger et al., 2009)] and anhedonia [Snaith-Hamilton Please Scale in German, SHAPS-D (Franz et al., 1998)]. During the first session, we conducted the Structured Clinical Interview for DSM-5 (SCID) (First, Reference First2014). In addition, we used the Structured Interview Guide for the Hamilton Rating Depression Scale with Atypical Depression Supplement (SIGH-ADS) (Williams & Terman, Reference Williams and Terman2003) within the MDD group to calculate delta appetite scores (>0 increase; <0 decrease) that reflect opposing appetite-related symptoms (Kroemer et al., Reference Kroemer, Opel, Teckentrup, Li, Grotegerd, Meinert, Lemke, Kircher, Nenadic, Krug, Jansen, Sommer, Steinstrater, Small, Dannlowski and Walter2022; Schulz et al., Reference Schulz, Klaus, Peglow, Ellinger, Kühnel, Walter and Kroemer2024). As this part took approximately 30 min for HCP and up to 2 h for patients with MDD, HCPs were offered to complete both parts within 1 day, whereas patients with MDD were invited to come for the second part on another day. The second part consisted of blood draws and a battery of reward-related tasks. On this day, participants were investigated after an overnight fast (~12 h) in which they were only allowed to consume unsweetened beverages (e.g., water, tea). First, participants had to answer questions in their current subjective metabolic and affective state using visual analogue scales (VAS; Fahed et al. (Reference Fahed, Schulz, Klaus, Ellinger, Walter and Kroemer2024)). Second, fasting blood samples to determine insulin, glucose, ghrelin, and triglyceride levels were analyzed as we reported previously (Fahed et al., Reference Fahed, Schulz, Klaus, Ellinger, Walter and Kroemer2024; Schulz et al., 2023, Supplementary Materials SI2). The experimenters also determined the participants’ height, weight, waist and hip circumference, and documented menstrual cycle (if applicable), and time of the last meal and drink. After receiving task-specific instructions, they were asked to complete a battery of reward-related tasks (Schulz et al., Reference Schulz, Klaus, Peglow, Ellinger, Kühnel, Walter and Kroemer2024), including an FCR task (Müller et al., Reference Müller, Teckentrup, Kühnel, Ferstl and Kroemer2022). The duration of the whole session was about 3.5 h and the FCR task was the first task, taking up about 20 min. Participants were provided water ad libitum. At the end of the session, they received their acquired food rewards and financial compensation.
Food cue reactivity task
To assess anticipatory aspects of food reward, we used an FCR task (Müller et al., Reference Müller, Teckentrup, Kühnel, Ferstl and Kroemer2022). The task consisted of standardized images by Charbonnier et al. (2016) optimized for visual characteristics (homogenous plate with gray background). From this set of images, 60 food images and 20 non-food images depicting office supplies, serving as a control condition, were drawn (Supplementary Materials SI3). The task consisted of cue and rating phases. Participants were shown one image before they were asked to evaluate it either based on (a) how much they like the item based on past experiences (liking) or (b) how much they want to receive the depicted item (wanting). Both answers were given on VAS; however, different orientations emphasized the difference in concepts. Liking was rated on a vertical scale (Lim, Wood, & Green, Reference Lim, Wood and Green2009) ranging from −100 (strongest disliking imaginable) to +100 (strongest liking imaginable), whereas wanting was rated on a horizontal scale ranging from 0 (not wanted at all) to 100 (strongly wanted). Each trial started with a black fixation cross on a white screen for 1 s, followed by a (non-) food image for 2 s. Afterward, another fixation cross was shown prior to the rating scale, which was presented for up to 2.8 s to induce fast decision making in participants. The participants could choose their answer using an XBox controller joystick (Microsoft Corporation, Redmond, WA) and confirm by pressing A, after which a new trial started. To assess liking and wanting separately, all participants viewed the images twice. The order of stimulus presentation and rating was pseudo-randomized. The tasks were completed on a laptop (Lenovo L580) and executed using Psychtoolbox v3.0.18 (Kleiner et al., Reference Kleiner, Brainard, Pelli, Ingling, Murray and Broussard2007) in MATLAB (2021b).
Data analysis
Liking and wanting ratings
To evaluate the associations of liking and wanting ratings with macronutrients, we conducted linear mixed-effects models. We divided liking ratings (original range − 100 to 100) by 2 to match the range of wanting ratings (from 0 to 100). These models used either liking or wanting as outcome and included the centered predictors group (HCP versus MDD), BMI, age, and sex (female versus male). Because we used model comparisons in the next step, these base models (M0) were fitted with full maximum likelihood estimation with random intercepts and slopes. Then, we tested three extended models including macronutrient content of the depicted food (z-standardized to ensure convergence) as predictors. Because carbohydrate craving has often been associated with depression, the first extended model (i.e., carbohydrate model, eM1) contained the term carbohydrate content. The second extended model contained the term carbohydrate content and fat content as well as their interaction (i.e., Carbohydrate × Fat model, eM2a). The third extended model contained the term carbohydrate content and protein content as well as their interaction (i.e., Carbohydrate × Protein model, eM2b). The latter two extended models were used as alternative models and compared to the base model as well as the carbohydrate model to ensure nested model comparisons. A potentially larger model including all macronutrients was not feasible with all random slopes for the interactions and would likely require more items for robust estimation of the interaction terms. We also evaluated the incremental effect of anhedonia symptoms by adding group-centered SHAPS scores as a predictor. To compare slopes within the linear-mixed effects models, we used the R function contest with a t-contrast vector comparing the interaction terms of group with carbohydrate, fat, and protein content. To calculate unbiased slope estimates, we ran separate models with restricted maximum likelihood estimation and only including the macronutrient content (z-standardized) as well as the interactions of Carbohydrates × Fat, or Carbohydrates × Protein, respectively. These models provide unbiased estimates because they did not include group or demographic variables as predictors while random intercepts and slopes capture individual differences from the group average (extracted with the coef function).
Fasting blood levels
As proxies of insulin sensitivity or resistance, we calculated the homeostasis model assessment of insulin resistance (HOMA-IR) using fasting glucose and insulin levels (Matthews et al., 1985; (insulin [pmol/l] /6,945) × glucose [mg/dl]/405), as well as the triglyceride–glucose index using fasting triglycerides and glucose levels (Unger et al., 2014; Ln (triglycerides [mg/dl] × glucose [mg/dl])/2). The samples were analyzed by the Central Laboratory of the Institute of Clinical Chemistry and Pathobiochemistry of the University Hospital Tübingen. The concentrations of both acylated and unacetylated ghrelin were determined in plasma using ELISA kits (#A05306 and #A05319; both from Bertin Bioreagent, Bertin Technologies, Montigny-le-Bretonneux, France; distributed by BioCat, Germany) at the Institute of Nutritional and Food Sciences, Human Nutrition, University of Bonn. To improve the distribution of the obtained blood levels, we used natural log transformation and inspected them using Q-Q plots, indicating that they were well approximated by normal distributions (Schulz et al., Reference Schulz, Klaus, Peglow, Ellinger, Kühnel, Walter and Kroemer2024).
Statistical threshold and software
All statistical analyses and visualizations were performed using R version 4.3.2 (R Core Team, 2023). The linear mixed-effects models were computed using Satterthwaite’s method with the R package lmertest (Kuznetsova, Brockhoff, & Christensen, Reference Kuznetsova, Brockhoff and Christensen2017). The significance threshold was set to p < .05.
Results
Patients with MDD report lower food wanting
First, both groups came in roughly at the same time of the day after an overnight fast and reported similar ratings of metabolic state (hunger – satiety) at the time of the blood draw, roughly 20 min before the FCR (Supplementary Figure SI4).
To evaluate group differences in food reward ratings, we first used two basal mixed-effects models as references for model comparisons. We predicted either liking or wanting using group and nuisance variables (i.e., BMI, age, and sex) as predictors (grand mean centered). Patients with MDD showed no differences in food liking (b = −1.68, p = .23; M HCP = 26.4 versus M MDD = 23.1 in untransformed units), but reported lower food wanting (b = −6.22, p = .003; M HCP = 64.4 versus M MDD = 58.9) compared to HCPs (for descriptive values of each food item, see Supplementary Materials SI5). Adding group-centered SHAPS scores as a predictor revealed that associations with food liking (b = −0.28, p = .010) and food wanting (b = −0.68, p < .001) were much more pronounced for participants that reported more symptoms of anhedonia.
Adding the macronutrient content of food items substantially improves the model fit of liking ratings
Next, we examined whether adding the carbohydrate content (including an interaction with group with a random slope to capture inter-individual variability) of the depicted food improved the fit of the base models. For food liking, modeling the food’s carbohydrate content improved fit considerably, χ 2(4) = 300.57, p < .001, ΔBIC = 266. Increasing the carbohydrate content by 1 SD unit (~26.5 g/100 g) led to a reduction in liking of b = −2.28 (p < .001). This association was non-significantly attenuated in patients with MDD (b = 0.91, p = .23).
We then added the food’s content of fat (including interactions with group and carbohydrate content with random slopes for all terms) and observed a considerable improvement compared to modeling only the carbohydrate content, χ 2(11) = 377.51, p < .001, ΔBIC = 280. Increasing the fat content by 1 SD unit (~13.4 g/100 g) led to a non-significant slight reduction in liking of b = −0.36 (p = .38). This association with fat was non-significantly amplified in patients with MDD (b = −1.51, p = .071) and after accounting for the fat content, patients with MDD reported higher liking for foods with a high content of carbohydrates (b = 1.75, p = .007). A contrast comparing the slopes for carbohydrates and fat revealed a strong relative preference for carbohydrates in patients with MDD (versus HCPs) compared to fat, t(117) = 3.44, p < .001. Fat also enhanced the liking of foods that were rich in carbohydrates, which was reflected in a strong interaction (b = 2.25, p < .001). Crucially, we also observed a significant interaction of group with the term Carbohydrates × Fat, reflecting that patients with MDD reported higher liking (b = 1.64, p = .015; Figure 1a).

Figure 1. Patients with major depressive disorder (MDD) report lower liking for foods with high content of fat and protein unless they contain carbohydrates as well. To visualize the interaction of different macronutrients and MDD, we binned macronutrient contents for display (A-D). Low corresponds to the lower quartile, moderate to the second and third quartile, and high to the upper quartile. All reported statistics refer to the interaction term derived from liner mixed-effects models using the continuous macronutrient content (corresponding individual estimates are shown in Figure 4). A: Liking ratings split by group and the macronutrients carbohydrates and fat. For display, we binned macronutrient contents so that low corresponds to the lower quartile, moderate to the second and third quartile, and high to the upper quartile. B: Differences between healthy control participants (HCP) and patients with MDD for foods according to their carbohydrate and fat content. Patients with MDD report lower liking for high-fat foods (interaction contrast: p < .001). C: Liking ratings split by group and the macronutrients carbohydrates and protein. D: Similar to fat, patients with MDD report lower liking for high-protein foods (interaction contrast: p = .003).
Alternatively, we evaluated the interaction of carbohydrates with the protein content of foods. The addition of protein instead of fat led to a smaller improvement in model fit compared to modeling the content of carbohydrates and fat, χ 2(11) = 271.62, p < .001, ΔBIC = 174 (with the carbohydrate model as reference). Increasing the protein content by 1 SD unit (~6.0 g/100 g) led to a reduction in liking of b = −0.91 (p = .027). This association with protein was amplified in patients with MDD (b = −1.86, p = .024). A contrast comparing the slopes for carbohydrates and protein revealed a strong relative preference for carbohydrates in patients with MDD compared to protein and HCPs, t(117) = 3.06, p = .003. Similar to fat, protein also enhanced the liking of foods rich in carbohydrates, leading to a significant interaction term (b = 0.84, p = .002). However, patients with MDD did not show an enhanced interaction effect in contrast to fat (b = 0.55, p = .31; Figure 1b).
Wanting ratings similarly reflect the macronutrient content of food
For food wanting, modeling the food’s carbohydrate content improved fit comparable to food liking, χ 2(4) = 268.91, p < .001, ΔBIC = 233. Increasing the carbohydrate content by 1 SD unit (~26.5 g/100 g) led to a reduction in wanting of b = −2.72 (p < .001). This association was non-significantly attenuated in patients with MDD (b = 0.75, p = .52).
Analogous to liking, adding the food’s fat content led to a considerable improvement compared to modeling only the carbohydrate content for wanting, χ 2(11) = 422.20, p < .001, ΔBIC = 325. Increasing the fat content by 1 SD unit (~13.4 g/100 g) led to a non-significant reduction in wanting of b = −1.17 (p = .058). This association with fat was non-significantly amplified in patients with MDD (b = −1.54, p = .21). A contrast comparing the slopes for carbohydrates and fat revealed a moderate relative preference for carbohydrates compared to fat in patients with MDD versus HCPs, t(117) = 2.38, p = .019. Fat also enhanced the wanting of foods rich in carbohydrates, which was reflected in a strong interaction (b = 3.75, p < .001). We also observed a non-significant interaction of group with the term Carbohydrates × Fat, reflecting that patients with MDD reported marginally higher wanting (b = 1.96, p = .052; Figure 2a) that was numerically comparable to liking.

Figure 2. Patients with major depressive disorder (MDD) report lower wanting for foods with high content of fat and protein unless they contain carbohydrates as well. A: Wanting ratings split by group and the macronutrients carbohydrates and fat. For display, we binned content so that low corresponds to the lower quartile, moderate to the second and third quartile, and high to the upper quartile. B: Differences between healthy control participants (HCP) and patients with MDD for foods according to their carbohydrate and fat content. Patients with MDD report lower wanting for high-fat foods (interaction contrast: p = .019). C: Wanting ratings split by group and the macronutrients carbohydrates and protein. D: Similar to fat, patients with MDD report lower wanting for high-protein foods (interaction contrast: p = .028).
The addition of protein instead of fat to the carbohydrate model led to a smaller improvement in model fit compared to modeling the carbohydrate and fat content for food wanting, χ 2(11) = 292.73, p < .001, ΔBIC = 196 (with the carbohydrate model as reference). Increasing the protein content by 1 SD unit (~6.0 g/100 g) led to a reduction in wanting of b = −1.39 (p = .022) and this reduction was more pronounced in patients with MDD (b = −2.48, p = .042). A contrast comparing the slopes for carbohydrates and protein revealed a moderate relative preference for carbohydrates compared to fat in patients with MDD versus HCPs, t(117) = 2.22, p = .028. Analogous to liking, protein enhanced the wanting of carbohydrate-rich food, leading to a significant interaction term (b = 2.03, p < .001), and patients with MDD showed no differences compared to HCPs (b = 0.33, p = .68; Figure 2b; for item-level results, see Figure 3).

Figure 3. Differences in ratings between patients with major depressive disorder (MDD) and healthy control participants at the item level. First, each dot corresponds to a food item. Second, the size of the dot shows the absolute differences in wanting ratings between the groups, with larger dots indicating larger group differences. In addition, the color of the dot codes the direction of those differences (HCP-MDD, i.e., lighter colors show deficits in patients with MDD). For additional information on the selected items, see Supplementary Table SI3. Items are arranged on a scale from 0–100 (carbohydrates versus fat) indicating distribution of carbohydrates and fat per 100 g. A: Group differences in wanting ratings. MDDs show deficits in wanting for items low in carbohydrates and high in fat or protein. B: Group differences in liking ratings. MDDs show deficits in liking for items low in carbohydrates and high in fat or protein.
Carbohydrate cravings have previously been associated with depression. Hence, we started model comparison by adding carbohydrate content and then the interaction with either fat or protein content. A larger model including all macronutrients with random slopes for the interactions was not feasible and would likely require more items for robust estimation of the interaction terms. An exploratory post hoc analysis showed that patients with MDD gave lower ratings for wanting (b = −4.50, p = 0.004) and liking (b = −2.89, p = 0.005) for foods rich in protein. Nevertheless, patients with MDD gave higher ratings for wanting (b = 1.75, p = .019) but not liking (b = 0.70, p = .15) if foods were rich in both protein and fat (Supplementary Materials SI6).
Patients with MDD show a reduced correlation between macronutrient slopes
To examine whether group differences in food ratings are driven by a disturbed correspondence between macronutrients, we ran additional analyses using unbiased individual estimates of effects (i.e., random slopes reflecting the increase of ratings by one unit of the macronutrient content) derived from modified linear mixed-effects models (see Methods). Notably, patients with MDD showed an attenuated association of individual carbohydrate estimates with fat estimates, t(113) = −3.78, p < .001, and an attenuated association with protein estimates (each predicted as outcomes by carbohydrate slope estimates), t(113) = −3.70, p < .001 (Figure 4). This difference in the correspondence between the macronutrient slopes suggests that patients with MDD have specific impairments in the metabolic sensing of fat and protein content, while preferences for carbohydrate-rich foods are largely unaffected or even enhanced.

Figure 4. Patients with major depressive disorder (MDD) show attenuated associations between liking slopes for carbohydrates and fat as well as protein. A: Patients with MDD show a weaker correlation between individual estimates of increases in liking per one unit of carbohydrate content (i.e., liking slopes) and increases in liking for fat, t(113) = −3.78, p < .001. B: Patients with MDD show a negative correlation between liking slopes for carbohydrates and protein t(113) = −3.70, p < .001. C: Individual estimates per macronutrient split by group. The unbiased empirical Bayes (EB) estimates were derived from linear mixed-effects models that did not include group as a regressor. Patients with MDD show higher estimates in likings slopes for carbohydrates and Carbohydrates × Fat.

Figure 5. Results of the sensitivity analyses show associations with overall liking (L) and liking slopes reflecting associations of individual ratings with the macronutrient composition of the rated food items. Correlations ± .24 are significant at p < .01 (N = 117; for ghrelin N = 97, thus correlations ± .26 are significant. INT = intercept, BDI = Beck Depression Inventory, BMI = body mass index, F = fasting, AG = acyl ghrelin, DG = desacyl ghrelin, HOMA = Homeostasis Model Assessment, SHAPS = Snaith-Hamilton Pleasure Scale, STAI-T = State–Trait Anxiety Inventory – Trait, TyG = Triglyceride-Glucose Index.
Sensitivity and exploratory analyses
After establishing the associations of liking and wanting ratings with the macronutrient content of food, we conducted additional sensitivity analyses to explore associations of food liking ratings with severity, demographic, and metabolic variables (Figure 5). Participants with higher self-reported severity of depressive symptoms, as indexed by the BDI-II, reported lower overall liking (r = .197, p = .034) as well as higher liking for food rich in carbohydrates (r = .250, p = .007) and Carbohydrates × Fat (r = .302, p < .001). Similarly, participants with more severe anhedonia, as indexed by the SHAPS, reported lower overall liking (r = .277, p = .003) and higher liking for food rich in Carbohydrates × Fat (r = .204, p = .027). In contrast, changes in appetite and body weight, as indexed by the delta appetite score, did not correlate with any of the liking estimates. Additional follow-up analyses using the STAI-T to assess trait-like anxiety symptoms suggested robust associations between greater anxiety and liking of food rich in carbohydrates (r = .321, p < .001) and Carbohydrates × Fat (r = .352, p < .001). Splitting patients with MDD into low and high anxiety group (Supplementary Materials SI7) reveals overall similar patterns to HCPs versus MDD, further highlighting the unexpected role of anxiety in macronutrient preferences.
Whereas age, sex, and BMI were not significantly associated with overall liking, they showed correlations with macronutrient liking estimates. A higher BMI was associated with higher liking for food rich in Carbohydrates × Protein (r = .218, p = .018). A higher age was associated with lower liking ratings for food rich in carbohydrates (r = −.370, p < .001) and Carbohydrates × Fat (r = −.297, p = .001), but higher liking ratings for Carbohydrates × Protein (r = .234, p = .011). Men reported higher liking ratings for food rich in fat (r = .242, p = .009) or protein (r = .252, p = .006) compared to women.
There were no differences in ghrelin, glucose, or triglycerides levels between participants with and without depression (Supplementary Materials SI8). However, insulin levels as well as insulin resistance as measured with the HOMA-IR were higher in participants with depression (t = −2.4, p = .020). Metabolic indices (transformed for parametric analyses) primarily showed associations with overall liking ratings. Both higher fasting levels of acyl (r = .257, p = .011) and desacyl ghrelin (r = .251, p = .013) were associated with higher overall liking ratings, with similar correlation coefficients in both groups (see Supplementary Materials SI9). Insulin sensitivity, as indexed by HOMA-IR and the triglyceride-glucose index, showed no significant associations with liking estimates. Associations with wanting slopes were qualitatively similar, and the use of antidepressant medication (antidepressant versus no antidepressant; Supplementary Materials SI1) did not affect the reported associations.
Discussion
Reward dysfunction (anhedonia) is a cardinal symptom of MDD, and somatic symptoms (e.g., altered appetite) are common in severe depression. Although carbohydrate craving has long been recognized as a symptom of depression (Wurtman & Wurtman, Reference Wurtman and Wurtman1995), a fully quantitative and experimental investigation of differences in perceived reward using the macronutrient composition of an extensive set of food items was missing to date. Using model comparisons, we demonstrate that the macronutrient composition of food explains substantial variance in individual ratings, providing an opportunity to dissect associations with individual estimates for mechanistic insights, bypassing recall-related biases of self-reported food intake (Ravelli & Schoeller, Reference Ravelli and Schoeller2020). Consequently, we show that patients with MDD prefer carbohydrate-rich foods and experience reduced reward from foods rich in fat or protein alone. These reward deficits can be compensated by high carbohydrate content though. Crucially, the reward-enhancing effect of carbohydrate-rich foods was associated with the severity of depressive symptoms and anhedonia. Perhaps surprisingly, we observed no robust associations with the direction of changes in appetite that differentiate subtypes of depression. Instead, we found strong associations with trait-like symptoms of anxiety. These results suggest that preferences for carbohydrate-rich foods may play a bigger role in the regulation of anxiety- or mood-related symptoms (Christensen & Pettijohn, Reference Christensen and Pettijohn2001), compared to somatic symptoms (i.e., delta appetite scores). This interpretation is supported by weak and non-significant associations of metabolic indices with individual estimates reflecting the effect of macronutrients on food reward ratings. To conclude, our results highlight the potential of considering the macronutrient composition of food to better understand candidate drivers of reward-related symptoms in MDD.
In accordance with clinical observations and earlier studies on depression (Paans et al., Reference Paans, Bot, Brouwer, Visser, Roca, Kohls, Watkins and Penninx2018; Paans et al., Reference Paans, Gibson-Smith, Bot, van Strien, Brouwer, Visser and Penninx2019; Rose, Koperski, & Golomb, Reference Rose, Koperski and Golomb2010), we observed a shift in liking and wanting ratings of patients with MDD toward foods rich in carbohydrates, compared to fat- or protein-rich foods alone. The significant interaction terms of carbohydrates with fat and protein further suggest that high carbohydrate content can compensate for the reduced perceived reward of foods rich in fat or protein. Notably, the estimated individual preferences for carbohydrates derived from the linear mixed-effects models (random slopes) were associated with symptom severity as indexed by the BDI-II, SHAPS, and STAI-T. Although our cross-sectional study design cannot address causality, preclinical experimental work has shown bidirectional links between dietary intake of carbohydrates and depressive symptoms. For example, a diet rich in refined carbohydrates facilitated anxiety and depressive-like behaviors after stress in mice (Santos et al., Reference Santos, Ferreira, Oliveira, Oliveira, Gomes and Aguiar2018), while a high-fat diet desensitizes the output of hypothalamic AgRP neurons, leading to dysregulation of behavior indicative of anxiety and depression (Xia et al., Reference Xia, Han, Meng, He, Srisai, Farias, Dang, Palmiter, Xu and Wu2021). Moreover, the administration of citalopram, one of the commonly prescribed SSRIs, reduced the preference for fat in male, but not female rats (De la Fuente-Reynoso, Barrios, & Juarez, Reference De la Fuente-Reynoso, Barrios De Tomasi and Juarez2022). In humans, dietary interventions seeking to reduce carbohydrate consumption have failed to induce robust antidepressive effects so far and, if anything, seem to increase anxiety, although the quality of the evidence is still low (Varaee, Darand, Hassanizadeh, & Hosseinzadeh, Reference Varaee, Darand, Hassanizadeh and Hosseinzadeh2023). In contrast, recent Mendelian randomization studies even suggest a protective effect of carbohydrate consumption on the risk for depression (Yao et al., Reference Yao, Zhang, Dong, Wang, Zhang, Guo, Guo and Yang2022). Therefore, longitudinal assessments of food liking and wanting in combination with interventions could be a highly promising approach to improve our understanding of the association between food preferences and depression.
At the mechanistic level, our results are mixed and highlight the necessity for follow-up work. On the one hand, reduced food reward ratings of patients with MDD for fat- and protein-rich foods support the idea that vagal afferent feedback signals might be dysregulated in depression (de Araujo, Reference de Araujo2016; de Araujo et al., Reference de Araujo, Schatzker and Small2020; de Lartigue & Diepenbroek, Reference de Lartigue and Diepenbroek2016; Goldstein et al., Reference Goldstein, McKnight, Carty, Arnold, Betley and Alhadeff2021; Tellez et al., Reference Tellez, Medina, Han, Ferreira, Licona-Limon, Ren, Lam, Schwartz and de Araujo2013). This interpretation is supported by the altered correlation between carbohydrate slopes and fat as well as protein slopes in patients with MDD derived from individual ratings of participants. On the other hand, metabolic indices reflecting insulin sensitivity were not associated with the estimated macronutrient slopes, and fasting levels of ghrelin were only associated with overall liking and wanting ratings. Although insulin sensitivity was robustly correlated with self-reported symptoms of anhedonia in our sample (Schulz et al., Reference Schulz, Klaus, Peglow, Ellinger, Kühnel, Walter and Kroemer2024), it showed no significant associations with food reward ratings. One explanation might be that insulin sensitivity has a stronger effect on the contrast between food and non-food rewards (Kroemer et al., Reference Kroemer, Krebs, Kobiella, Grimm, Vollstadt-Klein, Wolfensteller, Kling, Bidlingmaier, Zimmermann and Smolka2013b; Tiedemann et al., Reference Tiedemann, Schmid, Hettel, Giesen, Francke, Buchel and Brassen2017), which we did not evaluate here due to the focus on macronutrients. To resolve such open questions, interventional studies using intranasal insulin (Schneider et al., Reference Schneider, Spetter, Martin, Sapey, Yip, Manolopoulos, Tahrani, Thomas, Lee, Hallschmid, Rotshtein, Dourish and Higgs2022) or ghrelin administration (Malik, McGlone, Bedrossian, & Dagher, Reference Malik, McGlone, Bedrossian and Dagher2008) in patients with MDD would be necessary. Our findings that carbohydrate-rich food preferences are preserved in individuals with MDD align with evidence that opioid receptor activity disproportionately alters fat intake compared to carbohydrate intake (Katsuura et al., 2011; Schusdziarra et al., 1983; Taha et al., 2009; Yeomans & Gray, 2002). Given that opioid signaling is often dysregulated in MDD (Jelen et al., 2022), this might explain larger differences in the ratings of foods rich in fat. However, studies measuring or manipulating the opioid system are needed to disentangle the potentially macronutrient-specific reward mechanisms in depression across different metabolic states (i.e., fasted versus sated).
Despite its notable strengths, such as the use of a representative set of well-known European foods that were independently rated in terms of liking and wanting leading to more than 7000 ratings per outcome, the study has several limitations that will need to be addressed in future work. First, due to the cross-sectional study design, it is not possible to answer whether food preferences for carbohydrate-rich foods in depression are functional (i.e., reducing symptoms) or dysfunctional (aggravating symptoms). An interventional design with longitudinal follow-up may help resolve this clinically highly relevant question. Second, we did not collect additional ratings to capture the assumed macronutrient composition of the depicted food items. It is conceivable that subjective evaluations beyond liking and wanting, such as estimated contents of fat or carbohydrates, would shed light on conscious versus subconscious processes related to food preferences (Tang et al., Reference Tang, Fellows and Dagher2014). Relatedly, we collected no data on the typical frequency of consumption of the sampled foods, which might help elucidate potential links with eating behavior and might explain discrepancies to studies that rely on self-reported food intake. Third, we did not measure other circulating peptides, such as leptin, which has been linked to depression (Cao et al., Reference Cao, Chen, Brietzke, Cha, Shaukat, Pan, Park, Subramaniapillai, Zuckerman and Grant2018) or LEAP-2, an antagonist of ghrelin which can influence the activity of ghrelin (Ge et al., Reference Ge, Yang, Bednarek, Galon-Tilleman, Chen, Chen, Lichtman, Wang, Dalmas and Yin2018; Mani et al., Reference Mani, Puzziferri, He, Rodriguez, Osborne-Lawrence, Metzger, Chhina, Gaylinn, Thorner and Thomas2019). Finally, our analysis did not further stratify macronutrient categories and future research could provide more nuanced answers by extending the set of pictures to resolve differences within broad macronutrient categories (e.g., sugars versus starch content, saturated versus unsaturated fat).
Depression is characterized by impaired reward sensitivity and opposing changes in appetite and body weight during depressive episodes. In line with previously reported carbohydrate cravings as a symptom of depression, we observed that patients with MDD show a marked preference for foods rich in carbohydrates, compared to foods rich in fat or protein. Moreover, the presence of carbohydrates in foods even compensated for the lower perceived reward in foods rich in fat in patients with MDD as indicated by the modeled interaction terms. Individual preferences for carbohydrates were further associated with depression severity, anhedonia, and trait-like anxiety symptoms, but unexpectedly not with the direction of changes in appetite during the current episode. Taken together, our study highlights that incorporating the macronutrient content of rated food items considerably improves our understanding of food reward deficits in depression and may pave the way for future interventional research. We anticipate that collecting liking and wanting ratings of food items could improve the longitudinal assessment of appetite-related symptoms compared to conventional food frequency questionnaires alone because it does not rely on potentially selective recall.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0033291724003581.
Acknowledgment
We thank Ebru Sarmisak, Nora Gerth, Yul Wegner, Anne Schiller, Antonia Schlaich, Johanna Voß, and Rauda Fahed for help with data acquisition. Stephanie Ebbinghaus kindly helped with running the ELISA tests. The study was supported by the German Research Foundation (DFG), KR 4555/7-1, KR 4555/9-1, KR 4555/10-1, and & WA 2673/15-1.
CRediT Author contributions
Lilly Thurn: Formal Analysis, Visualization, Writing- Original draft preparation, Reviewing and Editing. Corinna Schulz: Visualization, Project administration, Investigation, Writing- Reviewing and Editing. Diba Borgmann: Writing- Reviewing and Editing. Johannes Klaus: Project administration, Investigation, Writing- Reviewing and Editing. Sabine Ellinger: Investigation, Writing- Reviewing and Editing. Martin Walter: Conceptualization, Methodology, Funding acquisition. Nils B. Kroemer: Conceptualization, Methodology, Funding acquisition, Supervision, Formal Analysis, Visualization, Writing- Original draft preparation, Reviewing and Editing.
Financial disclosure
JK works as a study therapist in a multicenter phase IIb study by Beckley Psychtech Ltd on 5-MeO-DMT in patients with MDD, unrelated to this investigation. JK did not receive any financial compensation from the company. MW is a member of the following advisory boards and gave presentations to the following companies: Bayer AG, Germany; Boehringer Ingelheim, Germany; Novartis, Perception Neuroscience, HMNC and Biologische Heilmittel Heel GmbH, Germany. MW has further conducted studies with institutional research support from HEEL and Janssen Pharmaceutical Research for a clinical trial (IIT) on ketamine in patients with MDD, unrelated to this investigation. MW did not receive any financial compensation from the companies mentioned above. All other authors report no biomedical financial interests or other potential conflicts of interest.
Financial support
The study was supported by the German Research Foundation (DFG), KR 4555/7–1, KR 4555/9–1, KR 4555/10–1, and & WA 2673/15–1.