INTRODUCTION
Periphytic ciliates are a primary component of the periphytic microfauna and play an important role in the functioning of microbial food webs by mediating the flux of organic matter and energy from the plankton to the benthos in many aquatic ecosystems (Fischer et al., Reference Fischer, Sachse, Steinberg and Pusch2002; Weitere et al., Reference Weitere, Schmidt-Denter and Arndt2003; Kathol et al., Reference Kathol, Norf, Arndt and Weitere2009; Xu et al., Reference Xu, Zhang, Jiang, Zhu, Al-Rasheid, Warren and Song2011c). Due to their short life cycle and delicate pellicles, they may respond more rapidly to environmental changes than any metazoa (Coppellotti & Matarazzo, Reference Coppellotti and Matarazzo2000; Ismael & Dorgham, Reference Ismael and Dorgham2003; Kchaou et al., Reference Kchaou, Elloumi, Drira, Hamza, Ayadi, Bouain and Aleya2009). Many forms can tolerate extremes of environmental conditions compared with macrofauna (Cairns & Henebry, Reference Cairns, Henebry and Cairns1982). Furthermore, with ease of sampling, relative immobility, increasing availability of user-friendly taxonomic references and standardized methodologies for temporal and spatial comparisons, the ciliates have widely been used as useful indicators to assess environmental stress and anthropogenic impact in many aquatic ecosystems, especially the freshwater environments (Xu et al., Reference Xu, Cao, Xie, Deng, Feng and Xu2005; Jiang et al., Reference Jiang, Wu and Shen2007; Morin et al., Reference Morin, Duong, Dabrin, Coynel, Herlory, Baudrimont, Delmas, Durrieu, Schäfer, Winterton, Blanc and Coste2008; Risse-Buhl & Küsel, Reference Risse-Buhl and Küsel2009).
To date, there have been several studies on community patterns and colonization dynamics of marine periphytic ciliates (Railkin, Reference Railkin1998; Gong et al., Reference Gong, Song and Warren2005; Xu et al., Reference Xu, Zhang, Jiang, Zhu and Al-Rasheid2012a, Reference Xu, Zhang, Jiang, Zhu and Al-Resheidc; Zhang et al., Reference Zhang, Xu, Jiang, Zhu and Al-Resheid2013). As regards their seasonal variation in community structures in coastal waters of the Yellow Sea, however, few studies have been carried out due to lack of taxonomic references although this field is important to both microbial ecological research and monitoring programmes (Xu et al., Reference Xu, Zhang, Jiang, Zhu and Al-Rasheid2012b; Zhang et al., Reference Zhang, Xu, Jiang, Zhu and Al-Resheid2012a, Reference Zhang, Xu, Jiang, Zhu and Al-Resheidb).
A 10-month baseline survey of biofilm-dwelling ciliate community patterns was conducted using an artificial substratum (glass slides) in coastal waters of the Yellow Sea, near Qingdao, northern China from August 2011 to July 2012. The main objectives of this study were: (1) to document the taxonomic composition and community structure of periphytic ciliate communities over a four-season period; (2) to reveal the seasonal variation in community structures of the ciliates; and (3) to analyse their relationships to environmental conditions in coastal waters.
MATERIALS AND METHODS
Study area and dataset collection
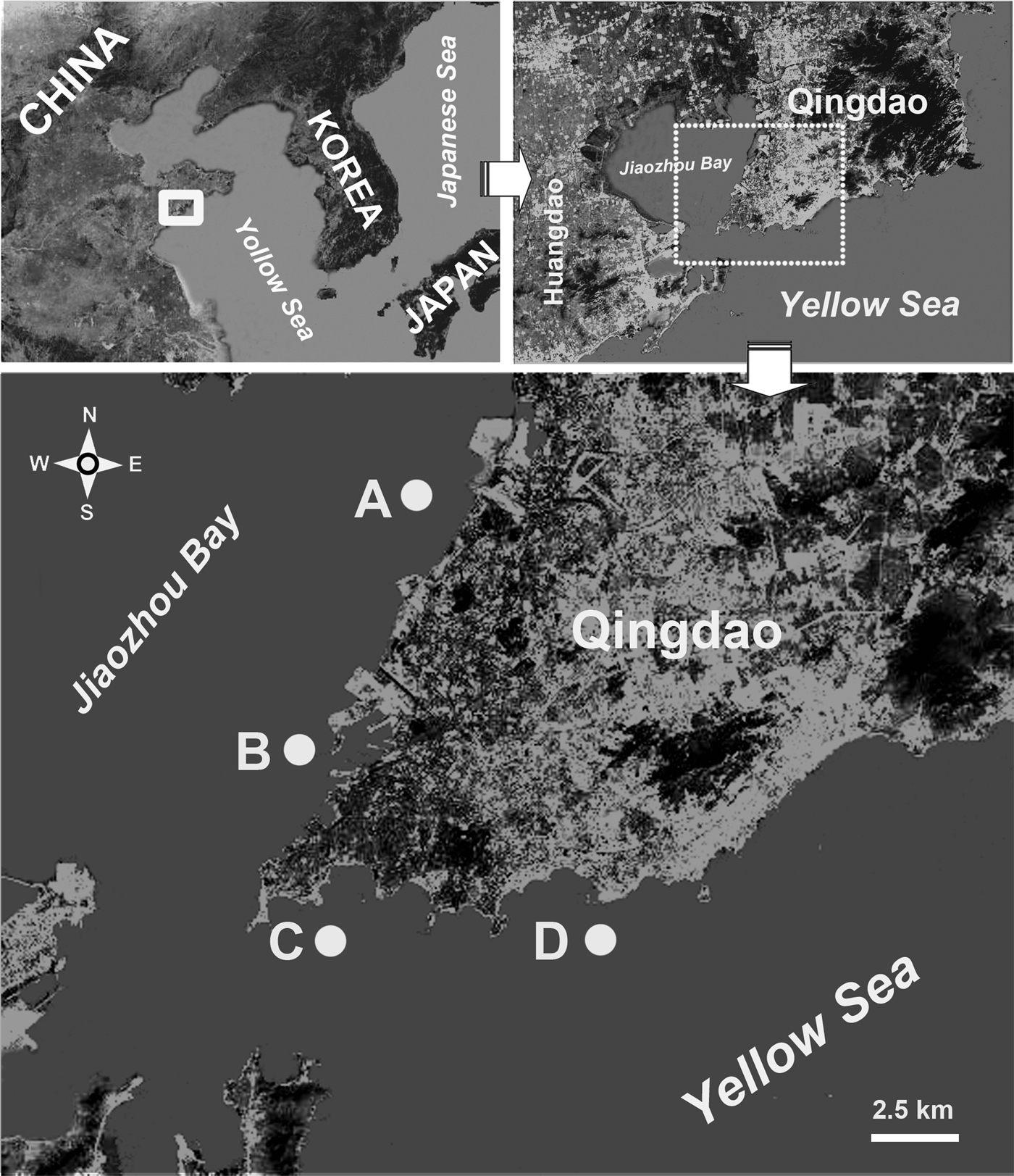
Four sampling stations were selected in coastal waters of the Yellow Sea, near Qingdao (population 7 × 106), northern China (Figure 1A–D). Station A was located in a mariculture area in Jiaozhou Bay; station B near Lundu harbour; station C near Zhongyuan harbour; and station D near the Olympic Sailing Centre (Figure 1).

Fig. 1. Sampling stations (A–D) in coastal waters of the Yellow Sea, near Qingdao, northern China.
Samplings occurred monthly, at the four stations, from August 2011 to July 2012. The glass slide systems were designed, deployed, anchored and sampled as described by Xu et al. (Reference Xu, Min, Choi, Jung and Park2009a, Reference Xu, Min, Choi, Kim, Jung and Limb). A total of 960 glass slides (2.5 × 7.5 cm) were used as artificial substrata for collecting the ciliates at 1 m below the surface. For each sampling station, two PVC frames were used to hold the 20 slide replicates and all of the slides were collected at the exposure time of 14 days. For each replicate, 10 slides were randomly collected from each PVC frame after 14 days; these were transferred to Petri dishes containing water from the sampling station and then stored in a cooling box before transporting to the laboratory within 2 h for identification and enumeration (Xu et al., Reference Xu, Min, Choi, Kim, Jung and Lim2009b).
Ciliate identification and enumeration were conducted following the methods of Xu et al. (Reference Xu, Min, Choi, Jung and Park2009a, Reference Xu, Min, Choi, Kim, Jung and Limb). Protargol staining was performed for species identification (Berger, Reference Berger1999). Ciliate identification was based on the published references to keys and guides such as Song et al. (Reference Song, Warren and Hu2009) and the taxonomic scheme followed Lynn (Reference Lynn2008).
Enumeration of ciliates in vivo was conducted at a 100-fold magnification under an inverted microscope within 4 to 8 h after sampling, preventing significant changes in species composition (Xu et al., Reference Xu, Min, Choi, Jung and Park2009a, Reference Xu, Min, Choi, Kim, Jung and Limb). The entire slide (17.5 cm2) was examined to record occurrences and abundances of all species, using bright field illumination. After 14-day exposure time, for counting the numbers of dominant ciliates, 20 fields of view per slide were randomly chosen, and the ciliate abundances were calculated from two replicates with 10 glass slides each to confirm the average abundance of ciliates (ind cm−2) (Xu et al., Reference Xu, Zhang, Jiang, Zhu, Al-Rasheid, Warren and Song2011c; Zhang et al., Reference Zhang, Xu, Jiang, Zhu and Al-Resheid2013).
A 500 ml water sample for nutrient analyses was collected simultaneously at 1 m from each sampling station. All water samples were immediately stored at −20°C, in the dark. Dissolved inorganic nitrogen (nitrate nitrogen NO3-N, nitrite nitrogen NO2-N and ammonium nitrogen NH3-N) and soluble reactive phosphate (SRP) were determined according to the ‘Standard Methods for the Examination of Water and Wastewater’ (APHA, 1992; Xu et al., Reference Xu, Song, Warren, Al-Rasheid, Al-Farraj, Gong and Hu2008). Water temperature (T), salinity (Sal), pH and dissolved oxygen (DO) were recorded with appropriate sensors (WTW Multi 3500i sensor), and transparency (Tra) was measured in situ using a transparent scale.
Data analysis
Species diversity (H′), evenness (J′) and richness (D) of samples were computed following the equations:
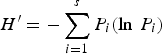 $$H^{\prime} = -\sum\limits_{i = 1}^s {{P_i}\lpar \ln \, {P_i}\rpar }$$
$$H^{\prime} = -\sum\limits_{i = 1}^s {{P_i}\lpar \ln \, {P_i}\rpar }$$where H′ = observed diversity index; P i = proportion of the total count arising from the ith species; S = total number of species; and N = total number of individuals.
Boxplots were produced to summarize the variations in diversity measures in four seasons by whiskers (minimum and maximum), boxes (±25%) and lines (medians) (Xu et al., Reference Xu, Zhang, Jiang, Zhu, Al-Rasheid, Warren and Song2011c).
Multivariate analyses of seasonal variations in the ciliate communities were analysed using the PRIMER v6.1.16 package (Clarke & Gorley, Reference Clarke and Gorley2006) and the PERMANOVA + v1.0.6 for PRIMER (Anderson et al., Reference Anderson, Gorley and Clarke2008). Bray–Curtis similarity matrices were computed on four-root transformed data. The species distribution among four seasons was analysed using the submodule CLUSTER on Bray–Curtis similarities from the standardized/transformed species-abundance data. The seasonal differences of communities among the four seasons were summarized using the submodule CAP (canonical analysis of principal coordinates) of PERMANOVA+ on Bray–Curtis similarities from the transformed species-abundance data (Anderson et al., Reference Anderson, Gorley and Clarke2008). Differences between groups of samples were tested by the submodule ANOSIM (analysis of similarities) (Clarke & Gorley, Reference Clarke and Gorley2006). The seasonal environmental status of the four seasons was summarized using the CAP based on log-transformed/normalized abiotic data (Clarke & Gorley, Reference Clarke and Gorley2006). The contribution of each species to the average Bray–Curtis similarity among four seasons, as well as to the similarity within one season, was analysed using the SIMPER (similarity percentage analysis) program (Clarke & Gorley, Reference Clarke and Gorley2006). The submodule BIOENV (biota-environment correlation analysis) was used to explore potential relationships between biotic parameters and the abiotic data. The significance of biota-environment correlations was tested using the routine RELATE (Clarke & Gorley, Reference Clarke and Gorley2006).
Univariate correlation analyses were conducted using the statistical program SPSS v16.0. Data were log-transformed before analyses (Jiang et al., Reference Jiang, Xu, Hu, Zhu, Al-Rasheid and Warren2011; Xu et al., Reference Xu, Jiang, Al-Rasheid, Al-Farraj and Song2011a, Reference Xu, Zhang, Jiang, Min and Choib).
It should be noted that in February and March, samples were lost in some stations, and thus a total of 40 datasets were used to analyse in this study.
RESULTS
Environmental variables
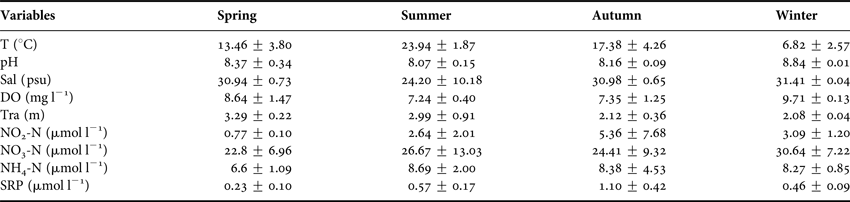
The mean values of the 11 environmental variables are summarized in Table 1. Water temperature followed a clear seasonal pattern, mean values ranging from 6.82 to 23.94°C from spring to winter. Salinity was ~31.0 psu and maintained relatively stable levels in three seasons with a sharp drop in summer (24.42 psu) due to the heavy rainfall. pH ranged from 8.07 to 8.84. DO varied inversely with temperature. SRP ranged from 0.23 to 1.10 mg l−1 with a minor peak in autumn. Concentrations of NO2-N varied synchronously with SRP. NH4-N and NO3-N peaked in summer and winter, respectively with a drop in spring (Table 1).
Table 1. Average values of environmental variables for each season monitored at the four sampling stations in coastal water of the Yellow Sea, near Qingdao, northern China during a 1-year cycle (August 2011–July 2012).

T, water temperature; Sal, salinity; DO, dissolved oxygen; COD, chemical oxygen demand (COD); Tra, transparency; SRP, soluble active phosphate; NO3-N, nitrate nitrogen; NO2-N, nitrite nitrogen; NH4-N, ammonium nitrogen.
Taxonomic composition and species distribution
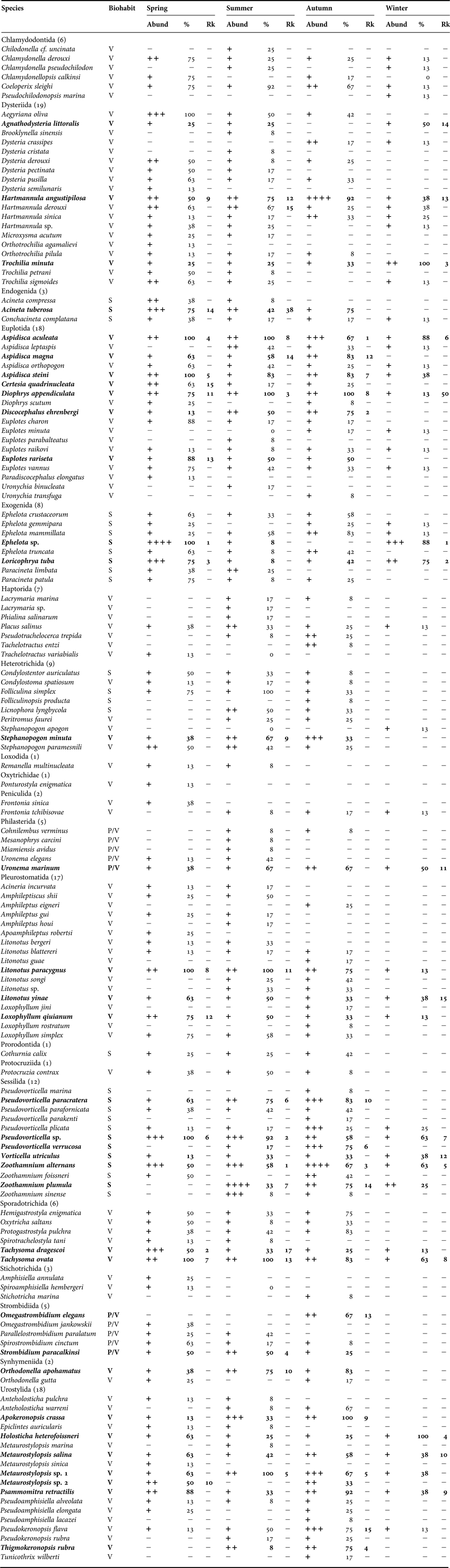
A list of species, their average abundances, occurrence and the ranks of the top 15 contributors in each season (as determined by the contribution of each species to the average Bray–Curtis similarity within each season using the SIMPER analysis) are given in Table 2. A total of 144 ciliate species representing 78 genera, 43 families, 18 orders and eight classes were recorded. Of these, 31 species occurred in all seasons and were defined as ‘common’, while 36 species that occurred in the top 15 ranked contributors at each season were defined ‘dominant’ (Table 2). Note that some species seemed to be endemic to seasons: 11, 11, 13 and two forms occurred only in spring, summer, autumn and winter season, respectively.
Table 2. List of periphytic ciliates recorded at the four sampling stations, including average abundance (ind cm−2), occurrence and top 15 ranks by contribution of each species to the average Bray–Curtis similarity during each of the four seasons in coastal waters of the Yellow Sea, near Qingdao, northern China during a 1-year cycle (August 2011–July 2012).

Rk, rank; V, vagile; S, sessile; P, planktonic; species in bold, top 15 dominant species by contribution to the ciliate communities at each sampling station; %, occurrence; N, average abundance (+: 0–1, ++: 1–10, +++: 10–100, ++++: 100–1000, +++++: over 1000 ind cm−2).
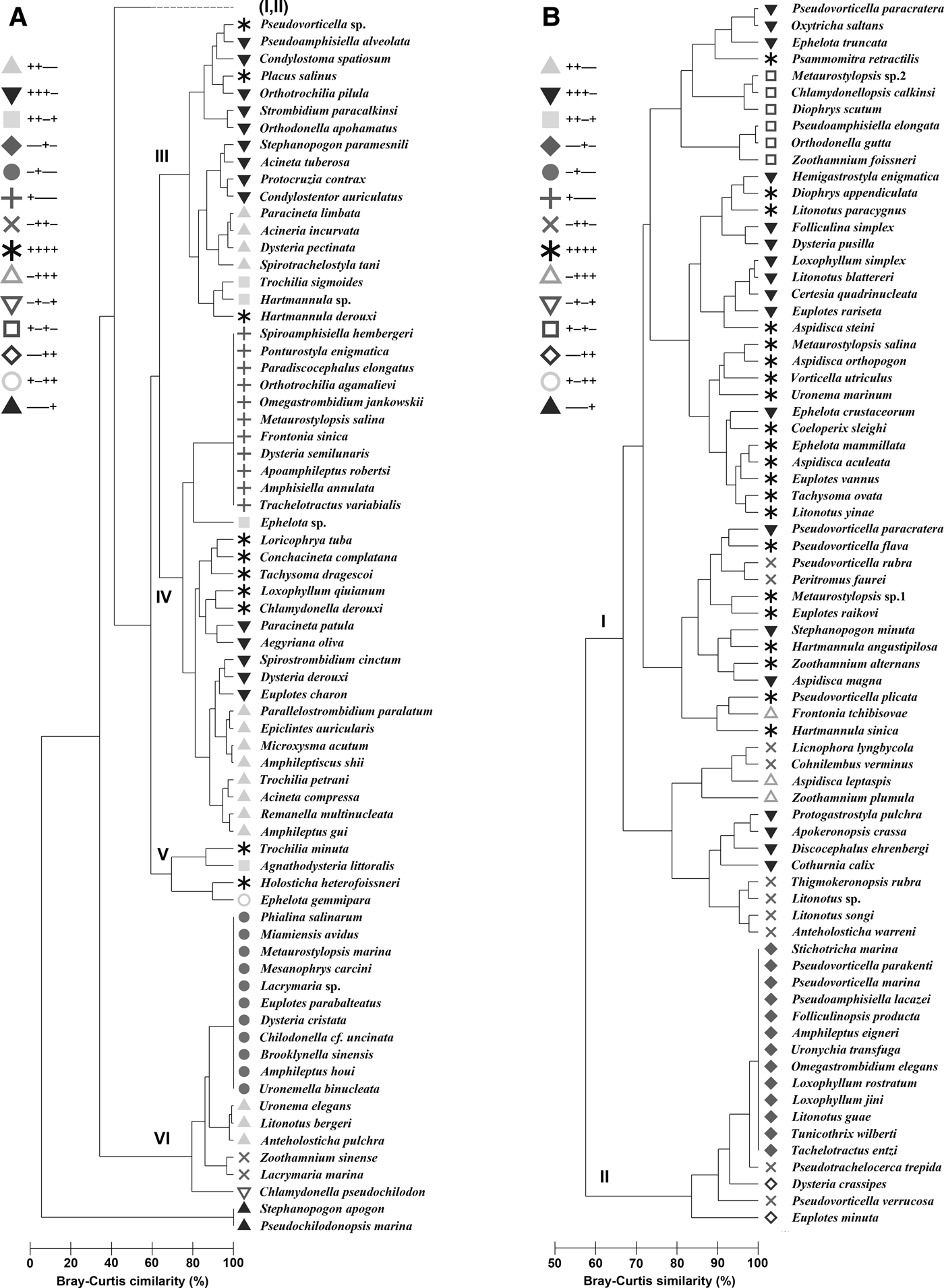
The dendrogram of the species distribution during four seasons revealed that the 144 species fell into six groups (I–VI) at a 65% similarity level (Figure 2). Group I contained 56 species with high occurrence frequency and abundance, including 21 common/dominant species, and thus represented the primary contributors to the succession of ciliate communities. The other five groups (II–VI) represented the rarer assemblages with low occurrence and/or abundance (Figure 2, Table 2). The analysis of similarities (ANOSIM) demonstrated a significant difference among the six groups (R = 0.716, P < 0.05).

Fig. 2. A dendrogram of the species distribution during four seasons using group-average clustering on Bray–Curtis similarities from standardized and fourth-root transformed species-abundance data of each species within periphytic ciliate communities in coastal waters of the Yellow Sea, near Qingdao, northern China. +, presence; −, absence; a, rare groups; b, common group; I–VI, group I–VI.
Seasonal patterns of periphytic ciliate communities
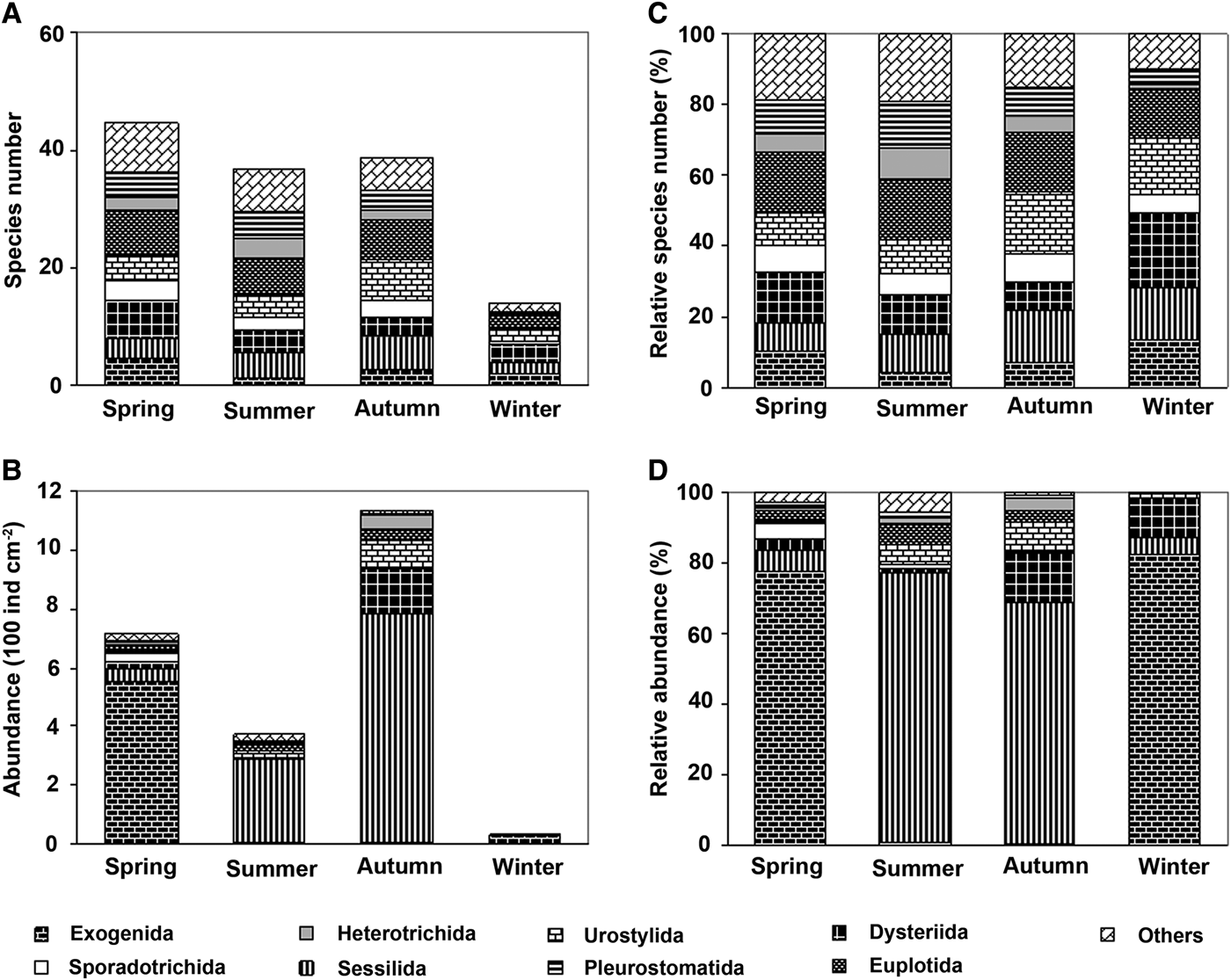
Average values of species number and abundance of the ciliate communities in each of the four seasons are shown in Figure 3A, B. The species count and mean abundances indicated two peaks, one in spring and one in the autumn, with minimum mean values in winter (Figure 3A, C).

Fig. 3. Species number (A), abundance (B, ind cm−2), relative species number (C, %) and relative abundance (D, %) of periphytic ciliate communities in four seasons in coastal waters of the Yellow Sea, near Qingdao, northern China.
The ciliate communities in the four seasons represented clear seasonal patterns of relative species composition and abundances (Figure 3C, D). It is noteworthy that the Sessilida, Exogenida, Dysteriida, Urostylida and Euplotida were the primary contributors to the taxonomic compositions of the communities (Figure 3C). In terms of the relative abundances, four structural types of communities can be recognized: (1) those dominated by exogenids (mainly Ephelota sp.) and sessilids (mainly Zoothaminium spp. and Pseudovorticella spp.) with the former being the primary contributor in spring; (2) those dominated by sessilids, euplotids and urostylids with sessilids the greatest contributor in summer; (3) those dominated by sessilids and dysteriids, and urostylids with sessilids the greatest contributor in autumn; and (4) those dominated by exogenids and dysteriids with the former the primary contributor in winter (Figure 3D, Table 2).
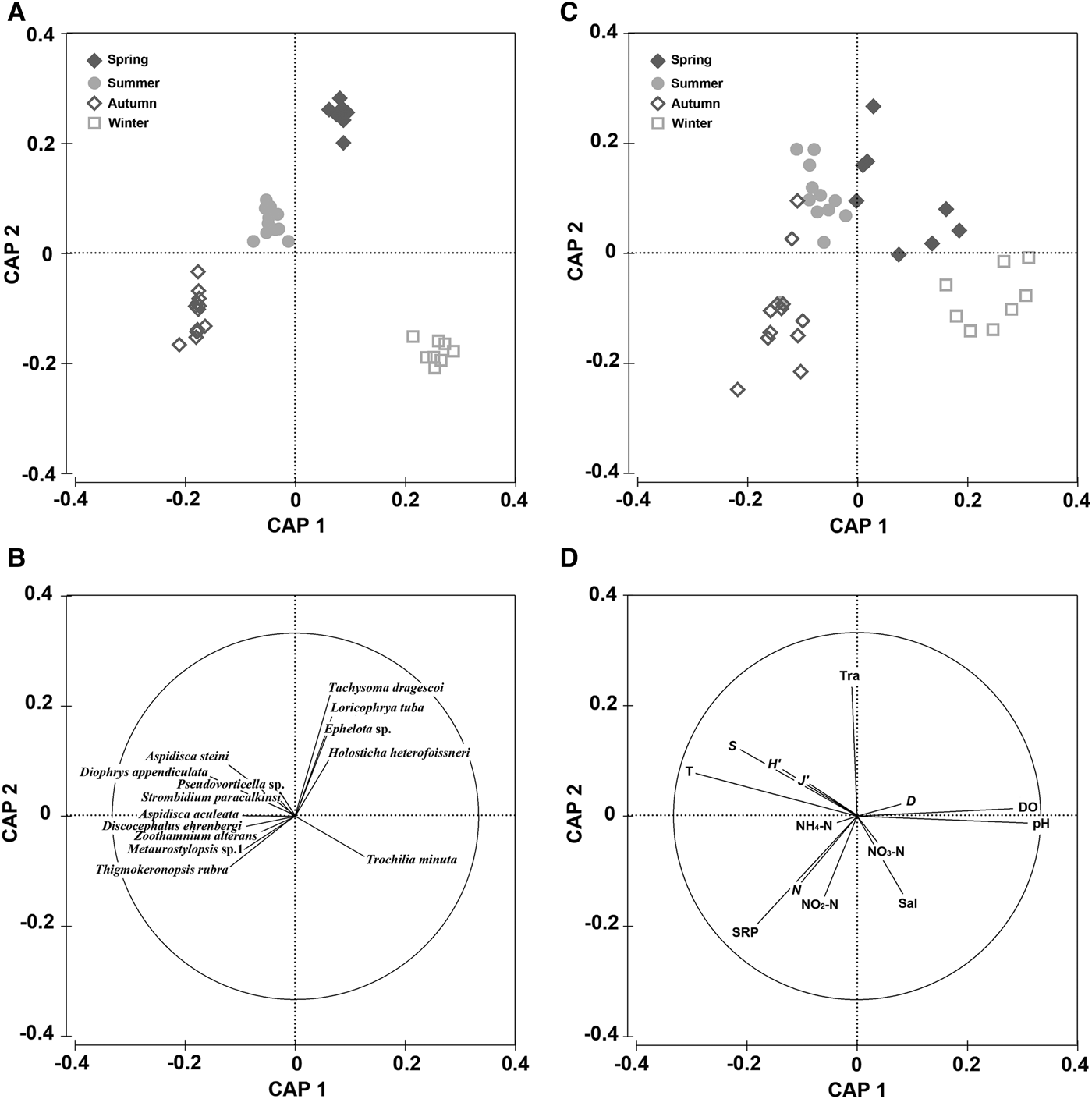
Discriminating among 40 data points from four stations (A–D) was performed by CAP on Bray–Curtis similarities and indicated seasonal pattern of the communities (Figure 4A). The first canonical axis (CAP 1) separated the ciliate communities sampled in summer and autumn (on the left) from those in spring and winter (on the right), while the second canonical axis (CAP 2) discriminated the samples in spring and summer (upper) from those at the other two seasons (lower) (Figure 4A). ANOSIM test revealed that there were significant differences among the four seasons (R = 0.684, P < 0.05) and between each pair of seasons (P < 0.05) apart from seasons summer and autumn, between which there were no significant differences (R = 0.213, P > 0.05).

Fig. 4. Canonical analysis of principal coordinates (CAP) for the biotic matrices on Bray–Curtis similarities from four-root transformed species abundance data with correlations of 14 dominant species with the CAP axes (A, B), and for the abiotic matrices on Euclidean distance from log-transformed/normalized abiotic data with correlations of both environmental variables and ciliate community structural parameters with the CAP axes (C, D). All datasets were obtained from the four sampling stations (A–D) in coastal waters of the Yellow Sea, near Qingdao, northern China during the study period.
Vector overlay of Pearson correlations of the 14 dominant species with the CAP axes is shown in Figure 4B. Although these taxa were the top five ranked contributors in each season, vectors for four ciliate species (e.g. Loricophrya tuba and Ephelota sp.) pointed toward the sample cloud in spring (upper right), four (e.g. Diophrys appendiculata and Pseudovorticella sp.) toward that in summer (upper left), four (e.g. Discocephalus ehrenbergi and Zoothamnium alterans) toward that in autumn (lower left), and one Trochilia minuta toward that in winter (lower right) (Figure 4B).
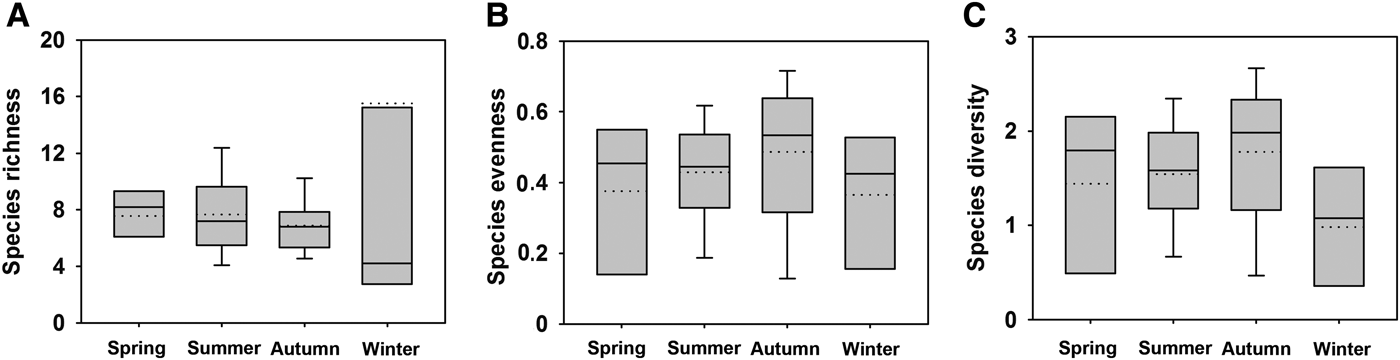
The seasonal variation in species richness (D), evenness (J′) and diversity (H′) indices during all four seasons is shown in Figure 5. The species richness peaked in winter and dropped in autumn, whereas the species evenness and diversity showed a similar temporal variation with peaks corresponding to autumn and a distinct low in winter (Figure 5).

Fig. 5. Species richness (A), evenness (B) and diversity (C) of periphytic ciliate communities during four seasons in coastal waters of the Yellow Sea, northern China. Dotted line, mean value.
Linkage between biota and abiota
The relationships between the seasonal patterns of the communities and environmental variables among the four seasons were summarized by CAP analyses on Euclidean distance from log-transformed/normalized abiotic data (Figure 4C, D). This multivariate approach revealed that seasonal patterns of the communities were consistent with the variations in environmental variables (Figure 4). ANOSIM demonstrated significant differences among the four seasons (R = 0.435, P < 0.05) and between each pair of seasons (P < 0.05). RELATE analysis revealed a significant correlation between seasonal variations of abundances and changes of environmental variables (ρ = 0.600, P < 0.05).
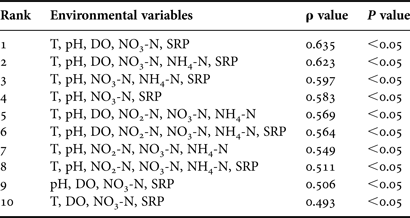
For all four seasons, the correlations between seasonal pattern of the ciliate communities in abundance and the changes of environmental variables were established by multivariate biota-environment (BIOENV) analysis, demonstrating the top 10 matching with the ciliates that occurred mainly with the combination of water temperature, pH, dissolved oxygen (DO) and nutrients (Table 3).
Table 3. Summary of results from biota-environment (BIOENV) analysis showing the 10 best matches of environmental variables with seasonal variations in the ciliate abundances during four seasons in coastal waters of the Yellow Sea, near Qingdao, northern China during the study period.

ρ value, Spearman correlation coefficient; P value, statistical significance level. See Table 1 for other abbreviations.
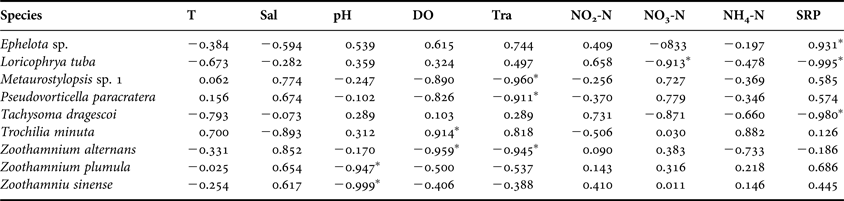
Univariate statistical correlations between environmental variables and abundances of the nine dominant ciliates are summarized in Table 4. Of these species, six forms (e.g. Trochilia minuta and Zoothaminium sp.) were significantly correlated with pH, DO or Tra, three (e.g. Loricophrya tuba and Tachysoma dragescoi) with the SRP alone or in combination with NO3-N (Table 4).
Table 4. Pearson correlations between average values of the nine dominant species abundances and average of environmental variables with temporal variations during four seasons in coastal waters of the Yellow Sea, near Qingdao, northern China during the study period.

*Significant difference at P < 0.05. See Table 1 for other abbreviations.
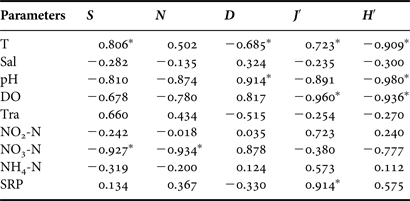
The species number, abundance, evenness and diversity were negatively correlated with the vectors for the physical-chemical variables (e.g., pH, DO and NO3-N) toward the winter (lower right), and positively correlated with the other variables (e.g., SRP) toward the autumn (Figure 4D). Univariate statistical correlation analysis determined that the species number was significantly negatively correlated with NO3-N (P < 0.01) (Table 5). However, the species evenness was not significantly correlated with nutrients. Note that the community structural parameters (except abundance) were significantly correlated with water temperature (Table 5).
Table 5. Pearson correlations between average values of the species number (S), abundance (N), richness (D), diversity (H′), evenness (J′) and average of environmental variables with temporal variations during four seasons in coastal waters of the Yellow Sea, near Qingdao, northern China during the study period.

*Significant difference at P < 0.05. See Table 1 for other abbreviations.
DISCUSSION
Compared with various natural substrates, such as stones and macrophytes, artificial substrates may provide a means of controlling community colonization, and thus they can be used to reduce the spatial heterogeneity (Coppellotti & Matarazzo, Reference Coppellotti and Matarazzo2000). Although many artificial substrates, such as polyurethane foam units (PFU), styrofoam spheres, plastic Petri dishes, glass and acrylic slides and glass coverslips, rods and tubes, have been used as useful tools to collect periphytic ciliate communities, glass slides seem to be the most convenient in the case of ciliates, since most species can be observed, counted and even identified in vivo by placing the whole slide under an inverted microscope or a stereomicroscope (Coppellotti & Matarazzo, Reference Coppellotti and Matarazzo2000; Gong et al., Reference Gong, Song and Warren2005; Xu et al., Reference Xu, Zhang, Jiang, Zhu and Al-Rasheid2012a, Reference Xu, Zhang, Jiang, Zhu and Al-Resheidc; Zhang et al., Reference Zhang, Xu, Jiang, Zhu and Al-Resheid2013).
Periphyton colonization of a glass slide commonly follows an asymptotic dynamic model, i.e. the number of species generally increases and then equilibrates (Xu et al., Reference Xu, Min, Choi, Jung and Park2009a, Reference Xu, Zhang, Jiang, Zhu and Al-Rasheid2012a; Zhang et al., Reference Zhang, Xu, Jiang, Zhu and Al-Resheid2013). Once the colonization reaches an equilibrium, the internal factors such as competition and predation pressure become more important (Railkin, Reference Railkin1998; Zhang et al., Reference Zhang, Xu, Jiang, Zhu and Al-Resheid2012a, Reference Zhang, Xu, Jiang, Zhu and Al-Resheidb). Our previous investigations demonstrated that the microphyton communities with colonization ages of more than 10 days could be considered as sufficiently mature samples in the coastal waters of the Yellow Sea (Gong et al., Reference Gong, Song and Warren2005; Zhang et al., Reference Zhang, Xu, Jiang, Zhu and Al-Resheid2012a, Reference Zhang, Xu, Jiang, Zhu and Al-Resheidb). In the present study, the glass slides were exposed for a fixed time period of 14 days, and thus the ciliate samples were analysed as mature communities.
A number of investigations have revealed that determining ecological parameters of a community is highly dependent on sampling effort or sample size (e.g. Warwick & Clarke, Reference Warwick and Clarke2001; Xu et al., Reference Xu, Zhang, Jiang, Zhu and Al-Rasheid2012a, Reference Xu, Zhang, Jiang, Zhu and Al-Rasheidb). Based on our previous research, the required samples size varied with both colonization times and exposure water depths; for example, to recover more than 90% species and to achieve less than 10% standard errors for recovering species in a mature community at a depth of 1 m, 10 slides (175 cm2) were needed (Xu et al., Reference Xu, Zhang, Jiang, Zhu and Al-Rasheid2012a). Thus, fewer than five slide-replicates, which are commonly used as the sampling effort strategy in some previous investigations, might achieve <85% of species, for example, just two glass slides for a 14-day sample of periphytic communities would result in <75% recovering rates of the species expected (Coppellotti & Matarazzo, Reference Coppellotti and Matarazzo2000; Gong et al., Reference Gong, Song and Warren2005; Xu et al., Reference Xu, Min, Choi, Jung and Park2009a, Reference Xu, Zhang, Jiang, Zhu and Al-Rasheid2012a). Thus, the sampling strategy of this study was available for ecological research on the mature ciliate communities with a sample size of 10 slides.
In the present study, a large number of 144 ciliate species were identified in 40 samples during all four seasons at four stations in coastal waters of the Yellow Sea. This species richness was similar to the previous reports with large number samples and wide range of sampling stations. For example, a total of 130 periphytic ciliates were also found on a combination of submerged objects and glass slides (500 samples) in the Caspian Sea (Agamaliev, Reference Agamaliev1974). However, this species count in our study is higher in comparison with those in previous reports with low sampling effort or small sample size although the taxonomic composition was similar to other previously published reports (e.g., Agamaliev, Reference Agamaliev1974; Gong et al., Reference Gong, Song and Warren2005). For example, Persoone (Reference Persoone1968) reported 30 ciliate species in a polluted harbour at Ostend, Belgium, and Gong et al. (Reference Gong, Song and Warren2005) found 37 ciliate taxa at only one sampling station in the scallop-farming area of Jiaozhou Bay near Qingdao (China) using the glass slide method during a 1-year period. Thus, we suggest that both sufficient sample sizes and numbers are required to detect species richness.
It has been suggested that multivariate analyses are more effective than univariate analyses in detecting changes in community structures. They are also extremely useful for analysing differences between communities and species distributions on spatial and temporal scales (Clarke & Ainsworth, Reference Clarke and Ainsworth1993; Jiang et al., Reference Jiang, Wu and Shen2007, Reference Jiang, Xu, Hu, Zhu, Al-Rasheid and Warren2011; Xu et al., Reference Xu, Jiang, Al-Rasheid, Al-Farraj and Song2011a). Based on these methodologies, this study revealed the periphytic ciliates presented a clear seasonal pattern in species distribution and community structure in coastal waters of the Yellow Sea, near Qingdao, China.
Of 144 ciliate species, based on clustering analysis, 31 distributed in all four seasons, while 11, 11, 13 and two forms occurred only in spring, summer, autumn and winter seasons, respectively. The species number and abundance peaked in spring and autumn with minimum values in winter, respectively. Among 36 dominant species, nine forms (e.g. Pseudovorticella paracratera, Trochilia minuta, Loricophrya tuba, Tachysoma dragescoi and Zoothamnium sp.) were significantly correlated with pH, DO or nutrients.
The CAP ordination and ANOSIM test revealed a clear seasonal variation in the ciliate community structure although four sampling stations have different environmental conditions. Multivariate correlation (RELATE) analysis demonstrated that the seasonal variation in ciliate communities was significantly correlated with the temporal status of environmental conditions. Furthermore, the BIOENV analysis revealed that the seasonal shift of the ciliate faunas was driven by water temperature, pH, dissolved oxygen (DO) and the nutrients. These findings imply that the periphytic ciliate communities reflect the water condition status and have the potential for use in bioassessment in marine ecosystems.
Species diversity indices (e.g. richness, diversity and evenness of species) have been commonly used to summarize the diversity of a community, and have widely been used to discriminate water quality status in aquatic environments (Magurran, Reference Magurran1991; Ismael & Dorgham, Reference Ismael and Dorgham2003; Jiang et al., Reference Jiang, Xu, Hu, Zhu, Al-Rasheid and Warren2011; Shi et al., Reference Shi, Liu, Liu, Sun and Xu2012; Xu et al., Reference Xu, Zhang, Jiang, Zhu and Al-Rasheid2012a, Reference Xu, Zhang, Jiang, Zhu and Al-Resheidc; Zhang et al., Reference Zhang, Xu, Jiang, Zhu and Al-Resheid2012a, Reference Zhang, Xu, Jiang, Zhu and Al-Resheidb). Generally, the higher these indices are, the better the water quality status is represented, with the possible exception of low organic pollution (Ismael & Dorgham, Reference Ismael and Dorgham2003). In our study, the species richness, evenness and diversity measures were significantly correlated with water temperature, pH, DO or the soluble reactive phosphates (SRP). These findings suggest that these diversity measures might be used as a potential indicator for assessing environmental status of marine ecosystems.
In summary, the species distribution and community structure of periphytic ciliates showed a clear seasonal pattern during four seasons. The species number and abundance peaked in spring and autumn with minimum values in winter, respectively. The community structures of the ciliates represented significant differences among the four seasons, and were significantly correlated with the changes of environmental variables, especially water temperature, pH, dissolved oxygen (DO) and the nutrients. Nine dominant species were significantly correlated with pH, DO or nutrients. The species richness, evenness and diversity measures were significantly correlated with water temperature, pH, DO or the soluble reactive phosphates (SRP). Based on our research, periphytic ciliates showed a clear seasonal variation in community structure in response to environmental conditions and might be used as a potentially useful bioindicator for assessing environmental condition status in coastal waters. However, further investigations on a range of marine habitats and over extended time periods are needed in order to verify this conclusion.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (grant number 41076089) and Scholarship Award for Excellent Doctoral Student granted by the Chinese Ministry of Education. Special thanks are due to Dr Hongbo Pan, Dr Xuming Pan and Ms Lu Zhao, Laboratory of Protozoology, Institute of Evolution and Marine Biodiversity, Ocean University of China, China, for their help with sampling and sample processing.