INTRODUCTION
Benzimidazoles (BZ) are a major group of anthelmintics which are widely used to treat gastro-intestinal nematode infections of humans and other animals, and represent an important component of plans to eliminate major parasitic diseases of humans, such as lymphatic filariasis. BZs act by inhibiting microtubule assembly (Prichard, Reference Prichard1994) and are highly cost efficient for worm control in geographical areas where resistance is not present. However, the emergence of widespread resistance in parasites of veterinary importance is now severely limiting the use of BZs in some countries, particularly in ruminants and horses (Kaplan, Reference Kaplan2004; Kaplan et al. Reference Kaplan, Klei, Lyons, Lester, Courtney, French, Tolliver, Vidyashankar and Zhao2004; Wolstenholme et al. Reference Wolstenholme, Fairweather, Prichard, von Samson-Himmelstjerna and Sangster2004; von Samson-Himmelstjerna and Blackhall, Reference von Samson-Himmelstjerna and Blackhall2005). If similar levels of resistance were to appear in parasites of humans following mass drug administration, this could have an impact on current control strategies. An understanding of possible resistance mechanisms and the development of tests for the monitoring and spread of resistance alleles would be useful tools to support such programmes (Albonico et al. Reference Albonico, Engels and Savioli2004a, Reference Albonico, Wright and Bickle2008). Since BZ-resistance has become a serious problem in the veterinary field, a number of tests have been developed for assessing the BZ-resistance phenotype or genotype of such parasites. The sustainable use of anthelmintics requires a regular monitoring of drug efficacy on individual farms. The phenotypic test most often used is the egg hatch test (EHT), in which the drug concentration required to inhibit hatching of 50% of nematode eggs is determined (Coles et al. Reference Coles, Jackson, Pomroy, Prichard, von Samson-Himmelstjerna, Silvestre, Taylor and Vercruysse2006). The sensitivity of such in vitro tests is relatively poor, and they are considered to permit the detection of resistance in isolates with at least 25% resistant individuals (Martin et al. Reference Martin, Anderson and Jarrett1989). On the other hand, molecular tests, based on the analysis of resistance-associated target gene polymorphisms through the use of PCR, are usually highly sensitive.
In various nematode species, BZ-resistance is caused by single nucleotide polymorphisms (SNPs) in the gene encoding isotype 1 of β-tubulin. In Haemonchus contortus, the SNP most commonly associated with resistance is present at codon 200 (TTC to TAC), resulting in a phenylalanine to tyrosine amino acid change (F200Y) (Kwa et al. Reference Kwa, Veenstra and Roos1994; Elard et al. Reference Elard, Comes and Humbert1996; Elard and Humbert, Reference Elard and Humbert1999). However, other polymorphisms have also been identified. An SNP at codon 167 (TTC to TAC – F167Y) has been detected in different species (Silvestre and Cabaret, Reference Silvestre and Cabaret2002; Drogemuller et al. Reference Drogemuller, Schnieder and von Samson-Himmelstjerna2004; Schwab et al. Reference Schwab, Boakye, Kyelem and Prichard2005; Melville et al. Reference Melville, Sykes and McCarthy2006; Hodgkinson et al. Reference Hodgkinson, Clark, Kaplan, Lake and Matthews2008) and another SNP at codon 198 has been reported recently in H. contortus (GAA to GCA – E198A) from both Switzerland and Australia (Ghisi et al. Reference Ghisi, Kaminsky and Maser2007; Mottier and Prichard, Reference Mottier and Prichard2008). One of these ‘multiple’ polymorphisms is present at higher frequencies in populations of BZ-resistant H. contortus (see Kwa et al. Reference Kwa, Veenstra and Roos1994; Mottier and Prichard, Reference Mottier and Prichard2008). This heterogeneity in resistance-associated SNPs could make it difficult to design a simple molecular test for the reliable detection of parasites resistant to BZs, and the relative importance of these polymorphisms has not been thoroughly assessed.
Since the molecular basis of BZ-resistance is relatively well characterized, this has allowed the development of PCR-based detection methods for resistance-associated polymorphisms. A number of such methods have been described to identify individual resistant trichostrongyloid parasites (Silvestre and Humbert, Reference Silvestre and Humbert2000; von Samson-Himmelstjerna et al. Reference von Samson-Himmelstjerna, Buschbaum, Wirtherle, Pape and Schnieder2003; Alvarez-Sanchez et al. Reference Alvarez-Sanchez, Perez-Garcia, Cruz-Rojo and Rojo-Vazquez2005). Previous studies have described methods for measuring H. contortus allele frequency and genotyping individual worms using real-time PCR with locked nucleic acid (LNA) (Braasch and Corey, Reference Braasch and Corey2001; Petersen and Wengel, Reference Petersen and Wengel2003) Taqman probes (Walsh et al. Reference Walsh, Donnan, Jackson, Skuce and Wolstenholme2007). These approaches allow allele frequencies to be quantified both in pooled samples, containing large numbers of nematodes, and in individual nematodes. Furthermore, a specific pyrosequencing (Ronaghi et al. Reference Ronaghi, Karamohamed, Pettersson, Uhlen and Nyren1996, Reference Ronaghi, Uhlen and Nyren1998) assay has been established to detect and measure all of the previously reported BZ-resistance associated SNPs and applied to a number of laboratory maintained and recent field isolates of H. contortus. In the present study, we demonstrate that measuring the frequency of the resistance-associated SNPs provides a reliable prediction of the BZ-resistance status of H. contortus and that the SNP at position 200 was the most important in the isolates examined.
MATERIALS AND METHODS
Isolates of H. contortus
Eleven different laboratory-maintained isolates of H. contortus were used. Isolates MHco1, 3 and 4 were described by Redman et al. (Reference Redman, Packard, Grillo, Smith, Jackson and Gilleard2008). MHco5 (IRE) is a BZ-resistant derivative of MHco3 (ISE). MHco6 was kindly provided by Dr R. Kaminsky (Novartis, Switzerland) and was originally isolated in South Africa prior to 1984. MHco8 was originally isolated in Kenya and was a kind donation from Dr J. Högland (Uppsala, Sweden), who received it in 1999. MHco9 was isolated in Germany, MHco11 was originally isolated in France and kindly donated by Dr H. Hoste (INRA, Toulouse, France) and MHCo12 as well as MHco13 were recently isolated in Switzerland and provided by Dr R. Kaminsky. The SWE isolate was from Sweden and provided by Dr J. Höglund. Seven isolates (denoted HHco/x/7) were isolated from the field in Germany in 2007.
‘Genotyping’ of individual larvae and determination of allele frequencies using real-time PCR
Genomic DNA was isolated from H. contortus third-stage larvae (L3s) using the method developed by Silvestre and Humbert (Reference Silvestre and Humbert2000). Single, ex-sheathed larvae were collected in 2 μl of water and digested overnight in 30 μl digestion buffer (50 mm KCl, 10 mm Tris pH 8·3, 2·5 mm MgCl2, 0·45% (v/v) Nonidet P-40, 0·45% (v/v) Tween-20, 0·01% (w/v) gelatine and proteinase K (100 μg/ml) at 60°C, and the suspension used without purification. Also, ~100 L3s (following ex-sheathment) were suspended in 75 μl of water; 200 μl of the digestion buffer were then added, and the lysates incubated at 60°C for 12–14 h. Following the incubation, the DNA was purified using the Gene Elute genomic DNA kit (Sigma-Aldrich) and resuspended in 100 μl of resuspension buffer (10 mm Tris-HCl, 0·5 mm EDTA, pH 9·0).
LNA Taqman based real-time PCR assays were carried out as described previously (Walsh et al. Reference Walsh, Donnan, Jackson, Skuce and Wolstenholme2007). Briefly, both probe (Proligo, Paris, France) and primer concentrations (MWG Biotech, Ebersberg, Germany) were 150 μm for the resistant allele (TAC) probe, 50 μm for the susceptible allele (TTC) probe and 100 μm of each primer. Cycling conditions were: an initial denaturation step at 95°C for 2 min followed by 40 cycles of 95°C for 30 s, 64°C for 1 min. Fluorescence data were captured at the end of the annealing phase, once per cycle.
Allele frequency determination for β-tubulin from pooled larvae using pyrosequencing
To develop suitable pyrosequencing assays for the analysis of H. contortus β-tubulin codon 167, 198 and 200 for isotypes 1 and 2 of β-tubulin, two, isotype-specific pyrosequencing primers were designed based on an alignment of the sequences of isotype 1 from H. contortus (GenBank Accession number M76493) and 2 (Accession number M76491). The Pyrosequencing™ Assay Design Software v.1.0.6 was used for this purpose. The amplification of both β-tubulin isotypes 1 and 2 from DNA was achieved using the forward primer HcPy2PCR For (5′-GAC GCA TTC ACT TGG AGG AG-3′) together with the biotinylated reverse primer HcPy2PCR Rev (5′Biotin-CAT AGG TTG GAT TTG TGA GTT-3′). For the subsequent analysis of the β-tubulin isotype 1 at codons 167, 198 and 200 or determination of allele frequencies, the sequencing primers Hc167PySeq1 (5′-ATA GAA TTA TGG CTT CGT-3′), Haem.con. 198 SeqPr (5′-GGT AGA GAA CAC CGA TG-3′) and Hc200PySeq1 (5′-TAG AGA ACA CCG ATG AAA CAT-3′), respectively, were used. The corresponding primers used for the sequencing of the β-tubulin isotype 2 were H.c. 167PySeq 1 Iso 2 (5′-ATA GAA TTA TGT CAT CCT-3′) and H.c. 200PySeq 1 Iso 2 (5′-TGG AAA ATA CTG ACG AAA CGT-3′). The suitability of the universal primer set and the specificity of primers for pyrosequencing was confirmed by testing them on cloned fragments of β-tubulin isotype 1 or 2 from H. contortus. The isotype 1-region (899 bp) was generated using primers H.c. Iso 1 For (5′-CGT TCT GGA CCG TAT GGA CAG-3′) and H.c. Iso 1 Rev (5′-GTG TAA GCT CAG CAA CTG TCG AA-3′), whereas the isotype 2-region (640 bp) was amplified using H.c. Iso 2 For (5′-TCC GGA CCT TTT GGT GCT-3′) and H.c. Iso 2 Rev (5′-GCG TGA GCT CAG AAA CAG TTA GT-3′). These primers were designed using the Primerselect™ 5.06 software (DNASTAR). For the analysis of codon 368, the PCR primers H.c. Codon 368 For (5′-GGT CGT ATG AGC ATG CGA GAA GTA-3′) and H.c. Codon 368 Rev+Biotin (5′biotin-ATT GCT CCG AAA TAC GCT TGA AC-3′) were used. The pyrosequencing-primer was H.c.beta-tubulin Codon368seqPr (5′-AAA TGG CGG CTA CCT-3′).
For each isolate, genomic DNA, isolated from a pool of 10 000 H. contortus L3s, was used as the template for the PCR. The amplicons were cloned into pCR4®-TOPO® vector (Invitrogen) and their sequences determined by conventional sequencing (Seqlab, Göttingen) to confirm their isotype 1 or 2 status. The isotype status was concordant with that determined by pyrosequencing (not shown).
For the analysis of SNP allele frequencies inferred to be linked to BZ-resistance, genomic DNA samples isolated from pools of 1000 or 10 000 H. contortus L3s were used. One μl DNA was amplified in a mix containing 5 U HotFire polymerase (Solis Biodyne), 1·5 mm MgCl2, 80 nm dNTPs and 200 μm of each forward and reverse primer. Amplification was performed, following a 15 min 95°C polymerase activation step, for 40 cycles at 95°C for 1 min, 53°C for 1 min and 72°C for 1 min, followed by a final elongation step at 72°C for 10 min. The pyrosequencing assays were conducted according to the manufacturer's (Biotage, Hamburg) protocols using the PSQ™ MA-96A (Biotage) instrument. In brief, following PCR amplification, the amount of amplicon was assessed by electrophoresis using Gelstar® (Lonzy) for staining, and abundant products were used for pyrosequencing (Ronaghi et al. Reference Ronaghi, Karamohamed, Pettersson, Uhlen and Nyren1996, Reference Ronaghi, Uhlen and Nyren1998). Up to 40 μl of amplicon were added to 40 μl 2×binding buffer (Biotage), 3 μl Streptavidin Sepharose™ beads (Biotage) and the solution mixed for 5 min to allow the binding of the biotinylated DNA fragments to the Streptavidin Sepharose™ beads. Using a Pyrosequencing Vacuum Prep Tool (Biotage), the beads together with the DNA were aspirated to a 96 format filter tool and then passaged through wash buffers as well as a sodium hydroxide buffer to remove the non-biotinylated DNA strand. Subsequently, the beads were released on to the 96-well sequencing plate and to each sample well, 40 μl of sequencing primer (0·4 μm), dissolved in annealing buffer, were added. Initially, for each assay, 2 control samples were performed, i.e. sequencing primer only, sequencing plus biotinylated primer and biotinylated primer only samples, to demonstrate specificity. Following the completion of the pyrosequencing reaction, the software of the instrument produces a ‘pyrogram’ and, based on the relative peak heights for the different alleles, a percent quantification for the frequency of each can be established.
Egg hatch test (EHT)
To determine the drug susceptibility or resistance of the various nematode populations, the EHT was carried out essentially as described by Coles et al. (Reference Coles, Jackson, Pomroy, Prichard, von Samson-Himmelstjerna, Silvestre, Taylor and Vercruysse2006). This test has been standardized by ‘ring testing’ (von Samson-Himmelstjerna et al. unpublished). The modifications in the optimized protocol were: (i) thiabendazole (TBZ) was dissolved in dimethyl sulphoxide (DMSO) and (ii) tests were set up using deionized water. The calculation of the effective concentration required to inhibit 50% and 95% of the eggs from hatching (EC50 and EC95, respectively) was performed using the computer program GraphPad Prism 5.0 for Windows. To fit the achieved dose-response-data by non-linear regression, a 4-parameter logistic equation with a variable slope was chosen. All analyses were done following logarithmic transformation of the data (X=log X) and defining the lowest value as 0.
Sequencing from pooled cDNA
PCR-primers were designed to amplify β-tubulin cDNA fragments of ~500 bp, and 3 pairs of primers were used to give complete coverage and sufficient overlap in the coding sequence. RNA was isolated from pooled L3 and eggs as described previously (Laughton et al. Reference Laughton, Amar, Thomas, Towner, Harris, Lunt and Wolstenholme1994). Reverse transcription PCRs were performed to amplify the coding sequence of β tubulin isotype 1 using AffinityScript™ Multiple Temperature Reverse Transcriptase (Stratagene, La Jolla, California). PCR products were purified using the QIAquick® PCR purification kit (Qiagen, Crawley, UK) and sequenced directly using the primers 5′-ATGCGTGAAATCGTTCATGTGC-3′, 5′-ATGCTGCAATTGGAAGCCCTG-3′, 5′-CTCGAGCCTGGAACGATGGACT-3′, 5′-AACCGGGCATGAAGAAGTGAAGAC-3′, 5′-CTGCCTTCGATTCCCTGGACA-3′ and 5′-TCCTCGGGATATGCCTCTTCTCC-3′ (MWG Biotech, Ebersberg, Germany).
Statistical analyses
A key objective of the present study was to investigate the diagnostic effectiveness of the two molecular methods, i.e. the beta-tubulin codon 200 real-time PCR (Walsh et al. Reference Walsh, Donnan, Jackson, Skuce and Wolstenholme2007) and pyrosequencing assays as methods for detection of resistance-related allele frequencies in comparison to the EHT EC50 data. For statistical analysis, the EHT, as the current in vitro-reference method (Coles et al. Reference Coles, Jackson, Pomroy, Prichard, von Samson-Himmelstjerna, Silvestre, Taylor and Vercruysse2006), was considered as the standard, with a cut-off point of 0·1 μg TBZ per ml for the detection of resistance (i.e. all samples with an EC50>0·1 μg/ml were considered as ‘true positives’). The sensitivity, specificity, positive and negative predictive values were calculated using data resulting from the real-time PCR and pyrosequencing analyses, respectively, using the definitions and terms defined in the following. For the molecular assays, those with beta-tubulin isotype 1 codon 200 TTC allele frequencies of <90% were defined as ‘true-positives’.
Sensitivity is the probability that an isolate which is resistant is indeed tested as resistant:
Specificity is the probability that an isolate which is not resistant is tested as not resistant:
The positive predictive value is the proportion of isolates with positive test results that are correctly diagnosed:
The negative predictive value is the proportion of isolates with negative test results that are correctly diagnosed:
RESULTS
Pyrosequencing has been developed as a rapid molecular technique for the detection of polymorphisms in a variety of organisms. Here, we compared a previously described LNA-based real-time PCR procedure (Walsh et al. Reference Walsh, Donnan, Jackson, Skuce and Wolstenholme2007) with a newly developed pyrosequencing method for the quantitative analysis of the β-tubulin isotype 1 codon 200 alleles in H. contortus. Various laboratory H. contortus isolates were examined using both methods, and some field isolates were tested using pyrosequencing alone. Pyrosequencing was also used to measure the ‘susceptible’ and ‘resistant’ allele frequencies at codons 167, 198 and 368 of the H. contortus β-tubulin isotype 1. Pyrosequencing requires previous PCR-amplification of the DNA fragment within which the polymorphism of interest is located. In order to test the reproducibility and accuracy of the pyrosequencing assay for β-tubulin isotype codon 200, and to compare the results obtained using this method to those from the LNA Taqman real-time PCR assay, serial mixtures of both alleles were established. For this purpose, using the universal pyrosequencing PCR primer set with genomic DNA from 1 worm of a BZ-susceptible and 1 of a BZ-resistant isolate, the 2 respective beta-tubulin codon 200 alleles were obtained and cloned into pCR4-TOPO (Invitrogen). Custom sequencing (Seqlab) confirmed the codon 200 TTC and TAC alleles, respectively. Ratios of 1:0, 9:1, 4:1, 7:3, 3:2 and 1:1 of both alleles were assessed. In three independent experiments, high regression of expected and observed allele frequencies (R2=0·996) as well as a low standard deviation of less than 11% were recorded (Fig. 1).

Fig. 1. Frequencies of TTC Haemonchus contortus β-tubulin isotype 1 codon 200 alleles using mixtures of cloned cDNA with either TTC or TAC allele determined by pyrosequencing. Results given as arithmetic means of 3 independent analyses; error bars show standard deviations.
The next step in the process of the validation of this allele-specific quantitation procedure included pyrosequencing-based analysis of genomic DNA isolated from different mixtures of L3s from H. contortus isolates with known BZ-resistance phenotype. The combinations MHco3/MHco4, MHco12/MHc o3 and MHco13/MHco3 in the ratios 1:0, 9:1, 7:3 and 1:1 were prepared. Pools of either 1000 or 10 000 L3s were used for each DNA extraction, and each of the combinations was tested in at least 2 independent experiments. Fig. 2 displays the regression between the expected and the observed (β-tubulin isotype 1) codon 200 TTC allele frequencies. Using the Pearson product moment correlation (SigmaStat 3.11) for the comparison of all 4 isolates, correlation coefficients of >0·95 were calculated, showing high statistical significance (P<0·001).

Fig. 2. Regression of observed and calculated β-tubulin isotype 1 codon 200 TTC allele frequencies using various mixtures of genomic DNA from Haemonchus contortus isolates with different BZ-resistance phenotype. ○- -○ (dashed line) isolate MHco4 versus MHco3: Δ – Δ (solid line) isolate MHco12 versus MHco3: □ – □ (long dashed line) isolate MHco13 versus MHco3.
Subsequently, the pyrosequencing approach was applied to the 11 H. contortus laboratory isolates to determine β-tubulin isotype 1 allele frequencies at codons 167, 198, 200 and 368 as well as the codon 167 and 200 frequencies in isotype 2 i.e. all of the polymorphisms previously suggested to be involved in BZ-resistance (Kwa et al. Reference Kwa, Veenstra and Roos1994; Elard et al. Reference Elard, Comes and Humbert1996; Elard and Humbert, Reference Elard and Humbert1999; Silvestre and Cabaret, Reference Silvestre and Cabaret2002; Drogemuller et al. Reference Drogemuller, Schnieder and von Samson-Himmelstjerna2004; Schwab et al. Reference Schwab, Boakye, Kyelem and Prichard2005; Melville et al. Reference Melville, Sykes and McCarthy2006; Hodgkinson et al. Reference Hodgkinson, Clark, Kaplan, Lake and Matthews2008; Ghisi et al. Reference Ghisi, Kaminsky and Maser2007; Mottier and Prichard, Reference Mottier and Prichard2008). At codon 198, only the ‘susceptible’ alleles were present and no polymorphism was detected, whereas at position 368 only GTT/GTC, encoding valine, was detected. The results for codons 167 and 200 are given in Table 1. The β-tubulin isotype 1 codon 200 TTC allele frequencies were ⩽20% for highly BZ-resistant isolates. For isotype 2, the codon 200 TTC frequency was not reduced to the same extent in the resistant isolates and on average differed only ~10% between the BZ-susceptible (i.e. MHco1, 3, 6 and 9) and highly resistant (i.e. MHco5, 12 and SWE) isolates. Interestingly, repeated attempts to amplify the β-tubulin isotype 2 from 2 of the resistant isolates (MHco11, MHco13) did not succeed. We detected the TAC allele at codon 167 of isotype 1 in the MCco4 isolate, in which it was present at low frequency (7·2%), whereas in the other phenotypically resistant isolates only the TTC allele was detected.
Table 1. EC50 values for TBZ-treated Haemonchus contortus isolates and β tubulin allele frequencies for SNPs determined by real-time PCR and pyrosequencing

* Populations that show a clinical level of resistance with an EC50 concentration of >0·1 μg TBZ mL−1.
n.d., Isotype not detectable.
Since the resistance-related polymorphism at codon 200 of the β-tubulin isotype 1 was the only one that occurred at high frequencies (>30%) in the resistant isolates, the allele frequency at this site was also determined in each of the 11 isolates of H. contortus by the LNA Taqman real-time PCR assay using genomic DNA from 100 L3s (Table 1). The results obtained were very similar to those from pyrosequencing, with the exception of the SWE isolate, for which a slightly higher TTC percentage (19% instead of 9%) was obtained using the pyrosequencing approach. Overall, a statistically highly significant (P<0·001) correlation coefficient (Pearson product moment correlation) of 0·981 was obtained by comparing the results obtained using the 2 molecular methods. Furthermore, EC50 (Table 1) and EC95 values (data not shown) for TBZ in the EHT were determined for the same isolates. In this test, isolate MHco12 was most resistant, whereas isolates MHco5, MHco12, MHco13 and SWE were clearly resistant, and MHco4 and MHco8 were shown to be outside of the suggested cut-off value for ‘resistance’ (EC50=0·1 μg TBZ per ml) (Coles et al. Reference Coles, Bauer, Borgsteede, Geerts, Klei, Taylor and Waller1992). All other isolates were susceptible (Table 1), which was also confirmed by both the EC95 as well as the EC50 values (95% lower confidence limit being <0·1) for the susceptible isolates and >0·1 μg TBZ per ml for the resistant isolates, respectively.
The studies of the laboratory-maintained isolates (several of which were recently established from the field) suggested that measuring the frequency of the beta-tubulin isotype 1 codon 200 allele frequencies would be sufficient to predict the BZ-resistance status of the parasite population. In order to confirm that this observation was true for clinical samples, 7 nematode populations, containing a substantial proportion of H. contortus, were isolated from infected sheep in Germany (in 2007). We then carried out EHTs and analysed the frequency of the codon 200 polymorphism using pyrosequencing (the other SNPs were not analysed due to limited availability of DNA). Table 2 shows that there was a significant relationship (determined by 2nd degree polynomial regression) between the EC50 for TBZ, as determined in the EHT, and the frequency of the 200 TTC allele in β-tubulin isotype 1 (goodness of fit: 0·944, P=0·0032). Finally, to assess the diagnostic potential of the real-time PCR and pyrosequencing assays for the β-tubulin codon 200, a statistical comparison with the EHT data was performed, based on the results for all isolates. Taking the EHT as the reference and using the 0·1 μg TBZ per ml as the cut-off, the sensitivity for both assays was 1, whereas specificities of 0·57 and 0·71 for the PCR and pyrosequencing assays, respectively, were calculated. The corresponding positive predictive values were 78·57% and 84·81%, respectively, while the negative predictive value was 100% for both assays. For the opposite comparison, the pyrosequencing assay (since all isolates were tested by this assay) was regarded as standard using a β-tubulin codon 200 TTC allele frequency of <90% as the resistance threshold. In this case, the sensitivity of the EHT was 0·85, the specificity 1, the positive predictive value was 100% and the negative predictive value 71·4%. Fig. 3 shows a scatter-plot of the in vitro test data (i.e. the EHT EC50-data) plotted against the allele frequencies for codon 200 determined using real-time PCR and pyrosequencing. Using a putative threshold for resistance in the molecular assays of <90% TTC allele frequencies, all phenotypically BZ-resistant plus 2 susceptible (i.e. MHco8 and HHco/7/7) isolates were considered to be resistant.

Fig. 3. Comparison of TTC allele frequency (s) at codon 200 in isotype 1 of β tubulin with EHT TBZ EC50-values in Haemonchus contortus isolates (n=18). ▴ – allele frequency of isolates determined by real-time PCR: ○ – allele frequency of isolates determined by pyrosequencing. On the main graph, lines indicate the current cut-off for resistance in the egg hatch test (EHT) (EC50⩾0·1 μg/ml) and proposed allele frequency (⩽90% TTC). The inset is an expanded view of the bottom right- hand corner of the graph: in this case the dashed line indicates a cut-off for resistance in the EHT of EC50⩾0·05 μg/ml.
Table 2. Phenotypic and genotypic analysis of seven field samples from Northern Germany
(The EC50 for thiabendazole was obtained using the egg hatch test, as described, and the ‘TTC’ allele frequency obtained using the pyrosequencing assay.)
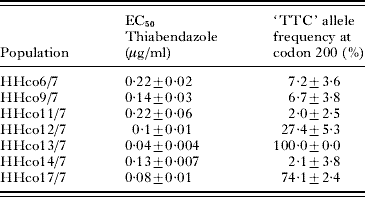
It was evident that the most resistant isolates had the highest resistance allele (i.e. TAC) frequencies, approaching 100%, whereas isolate MHco4 possessed a ‘TTC’ (susceptible) allele frequency of ~70%, as reported previously (Walsh et al. Reference Walsh, Donnan, Jackson, Skuce and Wolstenholme2007). Isolate MHco8 had a similar ‘TTC’ allele frequency to that of isolate MHco4, but was more sensitive to TBZ, with an EC50 of less than the accepted cut-off for resistance (0·1 μg TBZ per ml). This isolate was therefore classed as ‘susceptible’ based on the current guidelines of the World Association for the Advancement of Veterinary Parasitology (Coles et al. Reference Coles, Bauer, Borgsteede, Geerts, Klei, Taylor and Waller1992). In order to determine whether the difference in drug sensitivity between isolates MHco4 and MHco8 was due to the proportion of heterozygotes in the two isolates, we measured their individual genotype frequencies using the real-time PCR method (Walsh et al. Reference Walsh, Donnan, Jackson, Skuce and Wolstenholme2007). There was a slight difference in the number of heterozygotes, with isolate MHco8 (45%) having more than isolate MHco4 (38%). The number of homozygous resistant (10% for MHco4; 6% for MHco8) and susceptible individuals (52% for MHco4 and 49% for MHco8) were very similar in the two isolates.
DISCUSSION
BZ-resistance is recognized to be a serious problem in the veterinary field, and the use of these drugs for mass treatment against parasites in humans has the potential to lead to similar issues. The development of rapid and sensitive methods for detecting resistance-associated alleles would therefore be valuable for monitoring the appearance and spread of potentially BZ-resistant nematodes. In this paper, we have shown that both the LNA Taqman real-time PCR and the pyrosequencing assays are reliable for investigating resistance-associated allele frequencies in isolates and populations of H. contortus. Overall, there was a very good agreement between the allele frequencies of the various isolates determined using the two methods, and both assays readily identified all the populations in which TBZ had a reduced efficacy in vitro. Comparing the two molecular assays, our experience is that the pyrosequencing assay is quicker and easier to perform. Furthermore, it is suitable for the testing of multiple SNPs with limited extra time and effort. However, the equipment required is more expensive and less widely available than that needed for quantitative real-time PCR. The analysis and detection of drug resistance-associated alleles by pyrosequencing has been described for many different systems including viruses (Hoffmann et al. Reference Hoffmann, Minkah, Leipzig, Wang, Arens, Tebas and Bushman2007), bacteria (Zhu et al. Reference Zhu, Tenover, Limor, Lonsway, Prince, Dunne and Patel2007) and protozoa (Zhou et al. Reference Zhou, Poe, Limor, Grady, Goldman, McCollum, Escalante, Barnwell and Udhayakumar2006). Troell et al. (Reference Troell, Mattson, Alderborn and Hoglund2003) showed that pyrosequencing allows an efficient analysis of the genetic make-up of nematode populations of H. contortus individuals. This paper is the first report of a pyrosequencing-based method for the detection and measurement of specific SNPs in pooled genomic DNA from parasitic nematodes. We show that it is a useful alternative to real-time PCR for measuring allele frequencies in H. contortus populations, with the potential to be applied to other species of nematodes in livestock and humans.
Using both assays, we showed that the resistance-allele frequency increased with the EC50 for TBZ for all of the 18 isolates tested. One susceptible isolate, MHco8, had a relatively high resistance-allele frequency (~30–40%) – almost identical to that of the resistant MHco4, yet in the EHT, it was susceptible to drug concentrations below those considered to represent a resistant phenotype. However, amongst isolates with a susceptible EHT phenotype, isolate MHco4 showed the highest EC50 value. Furthermore, this was the only isolate to have a small percentage (7%) of potential resistance alleles at codon 167 of β-tubulin isotype 1. It may be that the relatively small differences in the genotype frequencies between isolates MHco4 and MHco8 account for the difference and that, while, based on current guidelines for the interpretation of the in vitro tests (Coles et al. Reference Coles, Bauer, Borgsteede, Geerts, Klei, Taylor and Waller1992), isolate MHco8 is currently considered ‘susceptible’, high-level resistance might develop very rapidly under further selection pressure. Measuring the resistance-allele frequency was shown to be a reliable means of identifying isolates that would be considered ‘resistant’ in the EHT, but the resistance allele needs to be present at a relatively high frequency (>30%) for the population as a whole to infer resistance to a normal therapeutic dose of TBZ, which might indicate some problems with the use of the in vitro test to detect low-level resistance in the field. This finding is in agreement with the findings of Martin et al. (Reference Martin, Anderson and Jarrett1989), who concluded that BZ-resistance would only be detected using in vitro tests if ⩾25% of the population carried resistance genotypes. However, the findings in Fig. 3 suggest that the EHT would perform better, at least for H. contortus, if the threshold for resistance was lowered to 0·05 μg of TBZ per ml, rather than 0·1 μg TBZ per ml. The ability to detect the presence of a significant proportion of resistance-alleles in the phenotypically susceptible isolates MHc08 and HHco17/7 confirms that molecular tests, such as real-time PCR and pyrosequencing, make it possible to genetically identify parasite populations in which resistance is emerging but has not yet manifested itself in treatment failures. Based on the EHT and molecular data obtained in this study for a total of 18 H. contortus isolates, an allele frequency of less than 90% at β-tubulin for codon 200 TTC appears to suggest that BZ-resistance selection has occurred. The lowest TTC frequency seen in an isolate with an EC50 <0·05 μg TBZ/ml was 92%; 10% is a conservative estimate for the sensitivity of the molecular techniques' ability to detect the resistance allele, an important consideration if the assays were to become automated and ‘high-throughput’. However, this speculative resistance threshold requires further evaluation using the molecular assays on a larger set of isolates with known BZ-resistance phenotypes and, ideally, including additional field isolates containing multiple species of nematodes.
The F200Y SNP is the major determinant of BZ-resistance in H. contortus; however, this and other organisms possess multiple SNPs elsewhere in the coding sequence that contribute to the resistance phenotype. For example, in a number of fungal species, SNPs at codon positions 6, 50, 134, 165, 167, 198, 200 and 241 have been associated with BZ-resistance (Koenraadt et al. Reference Koenraadt, Somerville and Jones1992; Ma et al. Reference Ma, Yoshimura and Michailides2003), whereas for parasitic nematodes, codons 167 and 198 have also been implicated (Silvestre and Cabaret, Reference Silvestre and Cabaret2002; Ghisi et al. Reference Ghisi, Kaminsky and Maser2007). Recent studies of some isolates of H. contortus has shown that the BZ-resistance-related polymorphism was E198A and not F200Y (Ghisi et al. Reference Ghisi, Kaminsky and Maser2007) or both in combination (Mottier and Prichard, Reference Mottier and Prichard2008). We found no clear evidence of such variation, or of any other coding SNP in any resistant isolate examined, except for a small (7%) percentage of the resistance-associated sequence at codon 167 of MHc04, and the absence of significant levels of these alleles in the isolates we studied makes it difficult for us to assess their relative contribution to global resistance. Since we made no attempt to choose isolates carrying any specific SNPs for our study, one interpretation is that the SNPs at codons 167 and 198 are less common in H. contortus than that at codon 200. However, a much more extensive survey than undertaken here would be required to support any such interpretation. It is possible that the small number of resistance-alleles at codon 167 of isolate MHc04 accounts for its slightly higher EC50 than MHc08, but we have no direct evidence to support this proposal. Another interesting observation was that the attempts to amplify β-tubulin isotype 2 regions in 2 of the resistant isolates (MHco11 and MHco13) failed. This finding may reflect the finding/s of an earlier report on the elimination of this isotype following intensive BZ-selection in H. contortus (see Kwa et al. Reference Kwa, Kooyman, Boersema and Roos1993). However, this observation was not repeated in 3 additional highly resistant isolates (SWE, MHco12 and MHco5). Recently, it was reported that the treatment of parasitic nematodes with macrocyclic lactone anthelmintics selects for the BZ-‘resistance’ alleles at positions 167, 198, 200 and 368 (Eng et al. Reference Eng, Blackhall, Osei-Atweneboana, Bourguinat, Galazzo, Beech, Unnasch, Awadzi, Lubega and Prichard2006; Mottier and Prichard, Reference Mottier and Prichard2008). Some of the isolates used in our study, such as isolate MHc04 (Van Wyk and Malan, Reference Van Wyk and Malan1988), are also resistant to macrocyclic lactones. The relationship between resistance to the two classes of anthelmintic will clearly be of great interest, but the treatment history of the isolates does not seem to impact on our main conclusion, that the BZ-resistance status of H. contortus isolates can be predicted by measuring the allele frequency of 3 SNPs. In our experience, the SNP at position 200 is likely to be that most frequently encountered; nonetheless, all 3 positions should be included where possible. The pyrosequencing assay is well suited to this and would increase in cost by ~60% if all 3 positions were included in it.
In conclusion, we have demonstrated that the benzimidazole-susceptibility of H. contortus can be determined by measuring a relatively small number of SNPs. If the mechanism/s of resistance present in human filarial or soil-transmitted helminths is/are similar, then such methods might be useful in ensuring that resistance does not become as great a problem in humans as is the case in parasites of ruminants. Previous attempts to detect these specific polymorphisms in possible BZ-resistant human hookworms were not successful (Albonico et al. Reference Albonico, Wright and Bickle2004b; Schwenkenbecher et al. Reference Schwenkenbecher, Albonico, Bickle and Kaplan2007), although they have been inferred for Wucheria bancrofti (see Schwab et al. Reference Schwab, Boakye, Kyelem and Prichard2005) and may reflect the difficulty of identifying true anthelmintic resistance in human parasites. Irrespective, the molecular approaches evaluated herein should be able to be adapted for the detection of resistance in a range of nematodes of animal and/or human health importance.
We thank Ronald Kaminsky (Novartis, Switzerland), Johan Höglund (SWEPAR, Uppsala, Sweden) and Herve Hoste (INRA, Toulouse, France) for their donations of H. contortus isolates. The excellent technical assistance by Ulla Küttler is thankfully acknowledged. This work was funded by grants from the Framework 6 programme of the European Commission (FOOD-CT-2005-022851), the Biotechnology and Biological Sciences Research Council (BBS/B/07594) and the Flexible Fund of the Scottish Executive Environmental and Rural Affairs Division.







