Introduction
Medication errors are common and a vast majority of these errors occur as a result of prescription writing (Nirodi & Mitchell, Reference Nirodi and Mitchell2002; Stubbs et al. Reference Stubbs, Haw and Taylor2006; Maidment et al. Reference Maidment, Haw, Stubbs, Fox, Katona and Franklin2008). A recent study conducted in the United Kingdom which examined the causes and prevalence of prescribing errors made by Foundation Year 1 Doctors, revealed a prescription error rate of 8.9% in all prescription orders (General Medical Council, 2009 – EQUIP Study). Prescribing errors can have serious consequences and potentially impact on patient morbidity (Bates et al. Reference Bates, Boyle, Vander Vliet, Schneider and Leape1995a, Reference Bates, Cullen, Laird, Petersen, Small, Servi, Laffel, Sweitzer, Shea and Hallisey1995b). Poor prescription writing accounts for 70% of medication errors that could result in adverse effects (Velo & Minuz, Reference Velo and Minuz2009).
Prescription errors frequently occur among elderly psychiatric inpatients. In a UK study examining the quality of drug prescribing for psychiatric inpatients over the age of 65 years, correct and legible prescriptions were found in only 39% of cases (Nirodi & Mitchell, Reference Nirodi and Mitchell2002). Omissions were discovered in 36% of all regular prescriptions and in 78% of ‘as required’ medications.
Many medication errors are deemed preventable (Leape et al. Reference Leape, Brennan, Laird, Lawthers, Localio, Barnes, Herbert, Newhouse, Weiler and Hiatt1991; Bates et al. Reference Bates, Boyle, Vander Vliet, Schneider and Leape1995a). Regular auditing of the quality of prescribing in hospital medication charts can help to identify deficiencies in prescribing practice, facilitate interventions specifically designed to address these and monitor their influence (Kripalani et al. Reference Kripalani, Badanapuram and Bell2007; Ved & Coupe, Reference Ved and Coupe2007). One Australian study showed that by implementing a standard medication chart in public hospitals, the prescribing error rate decreased from 20% to 15.8% (Coombes et al. Reference Coombes, Stowasser, Reid and Mitchell2009). In another study, a series of audits conducted in New Zealand over a 10-year period demonstrated that hospital prescribing practice can improve dramatically with the introduction of tailored interventions (Gommans et al. Reference Gommans, McIntosh, Bee and Allan2008). Effective methods to improve prescribing included educational strategies (e.g. feedback of audit results, education sessions for doctors and nurses on prescribing and medication errors) and changes to systems (e.g. modifications to medication charts, development of hospital wide prescribing standards and an alert notification system).
In Ireland, the Health Information and Quality Authority (HIQA) stipulates that a medication management policy must be in place for the administration of medication to older patients in residential care (HIQA, 2008). All medication errors, suspected adverse reactions and incidents should be recorded, reported and analysed within an open culture of reporting. Feedback can then be used to improve patient safety and prevent reoccurrence. HIQA also advises that nursing, medical and pharmacy staff jointly reviews each resident on long-term psychotropic medication on a 3-monthly basis. Similarly, the Mental Health Act 2001 states that if ‘medicine has been administered to a patient for the purposes of ameliorating his or her mental disorder for a continuous period of 3 months, the administration of that medication shall not be continued’ unless the patient gives his consent in writing (Mental Health Act, 2001). Where the patient is ‘unwilling or unable’ to provide consent, the medication can only continue to be administered where it is approved by the treating consultant psychiatrist and one other psychiatrist.
Informed consent implies that the patient is made aware of the condition being treated, the reason for recommending the proposed treatment, what they can expect in terms of improvement, side effects that may occur and the need for any monitoring. Medical practitioners are obliged to ensure that any medication prescribed for a patient is safe, evidence-based and in the patient’s best interests (Medical Council, 2009).
Previous studies have examined the quality of prescription writing in psychiatric units both within the United Kingdom and also in Ireland (Hallahan et al. Reference Hallahan, Murray and McDonald2007; Ved & Coupe, Reference Ved and Coupe2007). To our knowledge, the standards of prescription writing within an Irish long-term psychogeriatric unit have not been examined before.
Methods
We undertook three clinical audits of prescription sheets to improve the quality of the prescriptions written for elderly psychiatric inpatients. The first audit was carried out in the long-term psychogeriatric unit of St Ita’s hospital, Portrane. Before its closure in 2011, this approved centre provided inpatient care for elderly residents of North East Dublin under the care of the psychiatry of old age team. It consisted of three wards with a total of 36 beds. This unit was closed following concerns expressed by the Mental Health Commission with regard to the poor physical environment. Patients were subsequently transferred to the O’Casey rooms, Fairview, a 24-bed unit providing continuing care for residents with severe and enduring mental illness and dementia. We conducted the second and third audits in the O’Casey rooms.
Data collection for each of the three audits took place on three separate afternoons over the course of 30 months. The review of patient prescription records and collection of information was undertaken by one member of the medical team. The initial audit was carried out by a consultant psychiatrist, audits two and three were conducted by a non-consultant hospital doctor (NCHD). The medication charts of each patient were assessed against a predetermined checklist of prescribing standards. Information was recorded anonymously on a pro-forma before being entered into a database for analysis.
The standard of prescription writing was assessed by examining each of the 28 items listed in Tables 1–3. Documentation of patient identification details, regular medication and ‘as required’ medication was noted. The numbers of prescriptions per patients was also recorded. Our findings were compared with standards of prescription writing as recommended by the British National Formulary (Joint Formulary Committee 2010) (see Appendix A) and by the Royal College of Physicians of Edinburgh (Maxwell & Wilkinson, Reference Maxwell and Wilkinson2007) (see Appendix B). The use of the Medical Council Registration Number (MCRN) when writing and discontinuing prescriptions, as required under the Medical Practitioners Act, was also examined (Office of the Attorney General, 2007). The Act clearly states that the MCRN should be ‘included on all medical prescriptions and all other documentation and records, whether in paper of electronic format, relating to that practitioner’s practice as a registered medical practitioner’.
Table 1 Recording of patient identification details on prescription sheet
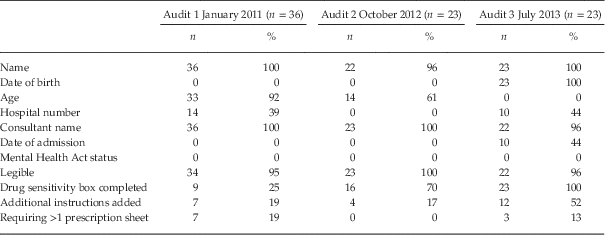
Table 2 Completeness of regular prescriptions

a Prescribing standard is not relevant to all prescription sheets included in audit. This is represented by x/y, where x is the number of prescription sheets correctly conforming to the standard and y the applicable sample size.
Table 3 Completeness of ‘as required’ prescriptions

The first audit was undertaken in January 2011. The prescription sheets of all 36 patients in the long-term psychogeriatric units in St Ita’s Hospital were reviewed in one day.
In April 2011 the long stay patients in St Ita’s hospital were relocated to the O’Casey rooms, Fairview. This was an ideal opportunity to offer feedback from the first audit and to improve the standards of prescription writing. A set of prescribing guidelines was developed for the new unit based on the results of our initial review (Fig. 1). These guidelines were distributed to ward staff and a copy was placed on the drug dispensing trolley.

Fig. 1 Prescribing guidelines developed for the O’Casey rooms.
Following implementation of the new prescribing guidelines, a second audit of the quality of prescription writing was undertaken in October 2012. The prescription sheets of the 23 inpatients in the O’Casey rooms were examined.
Based on the findings from audit two, the Nurse Practice Development Unit and the Drugs and Therapeutic Committee in St Ita’s hospital developed a new prescription sheet for the O’Casey rooms. The format of the new prescription sheet was designed to account for the needs of the unit and to adhere to Irish and UK best practise guidelines.
The old prescription sheet consisted of two separate documents: a medication list and a recording sheet. This increases the risk of drug errors. In particular, medications, which are dispensed infrequently, are more likely to be overlooked. The old prescription sheet was therefore replaced by a combined drug prescription and administration record, in the format of a booklet. Other changes included the introduction of a distinct allocated space for documentation of patient date of birth, date of admission, special instructions and a medication stop/review date. In addition, the new prescription sheet has a more comprehensive layout with separate sections for regular, ‘as required’, ‘once only’ and depot medications. Finally, as the O’Casey rooms did not have a patient hospital number system in place, the routine use of unique patient identifiers was established. These are recorded on all clinical documents and patient prescription sheets.
The new prescription sheet was introduced to the unit in May 2013. Two months later we undertook a third audit.
This series of audits was performed in accordance with the Declaration of Helsinki (World Medical Association, 2008) and Data Protection Guidelines on Research in the Health Sector (Data Protection Commissioner, 2007). Ethical approval was not required by local protocol. Information was recorded anonymously and patient confidentiality was protected at all times. Data were recorded, stored and analysed using Predictive Analytics SoftWare (Version 18).
Results
We reviewed the drug prescription sheets of all 36 psychogeriatric inpatients in St Ita’s for the first audit. The drug prescription sheets of the 23 inpatients in the O’Casey rooms were examined for the second and third audits. The three audits essentially comprised of the same client group. In terms of demographics, all patients were over the age of 65 years, living within the HSE Dublin North East catchment area and suffering from a severe and enduring mental illness and cognitive impairment.
The median number of regular drugs prescribed per patient at the first audit (10, with a range of 3–27) was comparable to the number prescribed at the second audit (10, with a range of 5–15). This had increased slightly following the third audit (12, with a range of 6–19).
Similarly, there was very little difference in the mean number of ‘as required’ drugs prescribed at audit one (3, with a range of 0–7) and audit two (4, with a range of 1–8). There was a small reduction following the third audit (1, with a range of 0–7).
Documentation of patient information details on hospital prescription sheets was relatively poor for audits one and two (Table 1). No patients had their date of admission recorded (there was no allocated space for this information on the prescription sheets in use at that time). This had improved by 44% at audit three, following implementation of the new prescription sheet. Additional instructions in relation to drug administration were included in <20% of cases at audits one and two. This had more than doubled for audit three. Patient age was recorded in 92% of cases at audit one and 61% of cases at audit two. Even though this information was not documented for any patients at audit three, patient date of birth was recorded in all cases.
Recording of patient drug allergies improved dramatically throughout the three audits, increasing by 75%.
There was no improvement in the recording of patient hospital numbers. This occurred in less than half of cases at audits one and three. (Patient identification numbers were not in use in the O’Casey rooms at the time of cycle two.) In addition, none of the prescription sheets documented patient mental health act status.
At audit one, 19% of patients required two prescription sheets to be stapled together due to lack of space. No patient required more than one prescription sheet at audit two which can be attributed to the smaller range in the number of regular medications prescribed. With the use of the new prescription sheet at audit three, 13% of patients still required two separate sheets.
With regard to the writing of regular prescriptions (Table 2), there was consistent improvement throughout the three audits. Overall, prescribing of generic psychotropic drugs improved by 69%, the use of block letters improved by 22%, the use of acceptable abbreviations improved by 50%, inclusion of the prescribers MCRN improved by 78% and the rewriting of prescription alterations improved by 50%.
Although the correct completion of drug cancellations had improved by 27% at audit three, only 17% of the cancellations were accompanied by the prescribers MCRN.
By audit three, a mere 4% (n=1) of prescription sheets included the frequency of drug administration. However, the drug administration times were ticked in 100% of cases throughout.
There was a significant decrease in the prescription of ‘as required’ medications following the introduction of the new prescription sheet. Only 14 of the 23 patients were being prescribed ‘as required’ medications at the time of the third audit.
During the audit cycle there was also a progressive increase in the percentage of prescription sheets containing the indication for ‘as required’ medication (Table 3). (Of note, there was no allocated space on the prescription sheets to document this information at audits one and two.) In addition, there was a small improvement in the recording of maximum drug dose allowed in 24 hours. However documentation of the stop/review date for ‘as required’ medication dropped considerably by audit three, with none of the prescription sheets detailing this information.
The minimum dose interval for ‘as required’ medication was not stipulated on any of the prescription sheets during the audit process.
Discussion
The results of this study suggest that clinical audit and feedback can improve the quality of prescriptions in an in-patient setting. Completion of the drug sensitivity box had reached 100% at audit three. This is reassuring given that drug allergies are believed to occur in 10–15% of hospitalised patients, resulting in morbidity, prolonged admissions and risk of mortality (Thong & Tan, Reference Thong and Tan2011). Other specific aspects of prescription writing that had been poor at the beginning of the audit cycle also showed improvement, most noticeably prescribing of generic psychotropic drugs and inclusion of the prescribers MCRN. However, some basic aspects of prescription writing remained weak such as frequency of drug administration and documentation of the stop/review date for ‘as required’ medication. In addition, throughout the three audits none of the prescription sheets recorded patient mental health act status. A clear record of whether a patient is admitted on a voluntary or involuntary basis is important in the context of drug administration. Under section 57 of the Mental Health Act, an involuntary patient may be treated without consent where ‘in the opinion of the consultant psychiatrist responsible for the care and treatment of the patient, the treatment is necessary to safeguard the life of the patient, to restore his or her health, to alleviate his or her condition, or to relieve his or her suffering, and, by reason of his or her mental disorder, the patient concerned is incapable of giving consent’ (Mental Health Act, 2001).
Although the introduction of a new prescription sheet appears to have contributed to better standards of prescription writing, some flaws still remain. The prescription sheet only provides room for a limited number of medications. Following its implementation, three of the 23 patients still required two prescription sheets to be stapled together. Lack of adequate space and poor design of drug prescription sheets increases the likelihood of drug administration errors and omissions (Paton & Wallace, Reference Paton and Wallace1997). Currently there is space for 18 regular medications and 21 ‘as required’ medications. Therefore a possible solution could be to re-allocate some of the sections provided for ‘as required medications’ to regular medications.
Some aspects of substandard prescription writing can be attributed to the poor quality of prescribing among doctors. The PROTECT study recently examined the prevalence of prescribing errors in eight different Scottish hospitals (Ryan et al. Reference Ryan, Ross, Davey, Duncan, Francis, Fielding, Johnston, Ker, Lee, Macleod, Maxwell, McKay, McLay, Webb and Bond2014). Of the 44 726 prescriptions written, 3364 errors were identified. The error rates for all grades of doctor were comparable: 7.4% per item prescribed for junior doctors in their first year of post-graduate training, 8.6% for junior doctors in their second year of training, and 6.3% for consultants. Upon completion of the third audit in the O’Casey rooms, a regular induction programme for NCHD’s was implemented. It involves reviewing basic prescribing skills and educating on the correct and appropriate use of the new prescription sheet. This is in accordance with the recommendations of the Equip study, that prescribing practice be included not only as part of the undergraduate medical curriculum, but should be provided for all grades of doctors (General Medical Council, 2009).
There are also future plans to provide a ward pharmacist who will regularly review and oversee medication prescribing and dispensing. This has been shown to effectively reduce adverse drug effects (Leape et al. Reference Leape, Cullen, Clapp, Burdick, Demonaco, Erickson and Bates1999; Finley et al. Reference Finley, Crismon and Rush2003; Holland et al. Reference Holland, Desborough, Goodyer, Hall, Wright and Loke2008).
Our study has several limitations. The absence of a control group means that we cannot be certain that any improvements were a direct result of our audits or interventions, for example, some of the benefits may have been because of other coincidental and unmeasured activities on the ward or a general staff awareness of the ongoing audit and an increased appreciation of the issue of medication error. The actual writing of the prescription is only a small part of the process of prescribing medication. We did not investigate the clinical appropriateness of prescribing (i.e. whether the drug, its dose or its route were actually appropriate for the patient’s medical condition), nor did we look at potential drug interactions. We also did not attempt to correlate the improved quality of prescribing with any evidence of a reduction in actual medication incidents or adverse events and therefore cannot confirm that better prescribing actually benefited patients. Another weakness of the study is that the sample size is modest and only based on data from two different psychogeriatric inpatient units. Therefore the results are unable to be generalised.
Conclusion
Quality assurance requires on-going data collection, review of that data and action. It is hoped that continued auditing in the O’Casey rooms will improve prescription practise and reduce drug errors. The ongoing education of doctors and the increased involvement of a pharmacist at ward level are also likely to be of beneficial effect.
Acknowledgement
The authors would like to acknowledge the participation of all staff in the Department of Psychiatry of Old Age, St Ita’s Hospital, Portrane and the O’Casey rooms, Fairview. We are also very grateful to the peer-reviewers for their helpful comments and suggestions.
Appendix A.
Guidelines for prescription writing: British National Formulary (Joint Formulary Committee, 2010)
Prescriptions should be written legibly in ink or otherwise so as to be indelible, should be dated, should state the name and address of the patient, the address of the prescriber, an indication of the type of prescriber, and should be signed in ink by the prescriber. The age and the date of birth of the patient should preferably be stated, and it is a legal requirement in the case of prescription-only medicines to state the age for children under 12 years.
The following should be noted:
a. The strength or quantity to be contained in capsules, lozenges, tablets, etc. should be stated by the prescriber. In particular, strength of liquid preparations should be clearly stated (e.g. 125 mg/5 ml).
b. The unnecessary use of decimal points should be avoided, for example 3 mg, not 3.0 mg.
Quantities of 1 gram or more should be written as 1 g, etc.
Quantities <1 gram should be written in milligrams, for example 500 mg, not 0.5 g.
Quantities <1 mg should be written in micrograms, for example 100 micrograms, not 0.1 mg.
When decimals are unavoidable a zero should be written in front of the decimal point where there is no other figure, for example 0.5 ml, not .5 ml.
Use of the decimal point is acceptable to express a range, for example 0.5–1 g.
c. ‘Micrograms’ and ‘nanograms’ should not be abbreviated. Similarly ‘units’ should not be abbreviated.
d. The term ‘millilitre’ (ml or mL) is used in medicine and pharmacy, and cubic centimetre, c.c., or cm3 should not be used.
e. Dose and dose frequency should be stated; in the case of preparations to be taken ‘as required’ a minimum dose interval should be specified.
When doses other than multiples of 5 ml are prescribed for oral liquid preparations the dose-volume will be provided by means of an oral syringe, see Oral Syringes under General Guidance (except for preparations intended to be measured with a pipette).
Suitable quantities:
∙ Elixirs, linctuses and paediatric mixtures (5 ml dose), 50, 100, or 150 ml
∙ Adult Mixtures (10 ml dose), 200 or 300 ml
∙ Ear drops, eye drops and nasal drops, 10 ml (or the manufacturer’s pack)
∙ Eye lotions, gargles and mouthwashes, 200 ml
f. For suitable quantities of dermatological preparations, see section 13.1.2.
g. The names of drugs and preparations should be written clearly and not abbreviated, using approved titles only (see also General Guidance to avoid creating generic titles for modified-release preparations).
h. The quantity to be supplied may be stated by indicating the number of days of treatment required in the box provided on NHS forms. In most cases the exact amount will be supplied. This does not apply to items directed to be used as required – if the dose and frequency are not given then the quantity to be supplied needs to be stated.
When several items are ordered on one form the box can be marked with the number of days of treatment provided the quantity is added for any item for which the amount cannot be calculated.
i. Although directions should preferably be in English without abbreviation, it is recognised that some Latin abbreviations are used (for details see Latin abbreviations).
Appendix B.
Guidelines for prescription writing: Royal College of Physicians of Edinburgh (Maxwell & Wilkinson, Reference Maxwell and Wilkinson2007)
∙ Write in block capitals, legibly, with black ballpoint pen. Most medicines will be administered by nursing staff in the absence of the prescriber, so clarity is essential.
∙ The beginning of every prescribing process should be the clear and unambiguous labelling of the kardex (or any other prescription chart) with the details of the intended recipient. Essential identifying details such as the patient’s name, hospital number, and date of birth (and age if under 12 years) should be written on every sheet. Patient’s weight and height may be required to calculate safe doses for many drugs with narrow therapeutic indices.
∙ The drug sensitivities/allergies box should be checked and further details of the drug history obtained if there are any doubts about its accuracy.
∙ Use generic drug names rather than brand names (e.g. simvastatin, not Zocor®). The only exceptions to this rule are if there is variation in the properties of different brands (mainly in lithium, theophylline and phenytoin) or the drug is a combination product with no generic name, for example Kliovance® and other HRT preparations. Avoid abbreviations such as ‘ISMN’ (for isosorbide mononitrate).
∙ Write the drug dose clearly. The only acceptable abbreviations are ‘g’ and ‘mg’. ‘Micrograms’ must always be written in full, never as ‘μg’. ‘Units’ (with regard to insulin, heparin, etc.) must always be written in full. Avoid decimal points, that is use 500 mg not 0.5 g. If a decimal point cannot be avoided, always put a ‘0’ in front of it, for example ‘0.5 micrograms’ not ‘.5 micrograms’. It is not necessary to use a decimal point if the number is a round number, for example 7 mg not 7.0 mg. For liquid preparations write dose in mg. The only exceptions when ‘ml’ can be written are if the product is a combination product (e.g. Gaviscon® liquid), or if the strength is not expressed in weight, for example adrenaline 1 in 1000. Use numbers/figure (e.g. 1 or ‘one’) to denote use of a sachet/enema. Always include dose of inhaled drugs (e.g. corticosteroids) in addition to stating ‘2 puffs’, as strengths can vary.
∙ Widely accepted Latin abbreviations for dose frequency are: once daily – ‘OD’; twice daily – ‘BD’; three times daily – ‘TDS’; and four times daily – ‘QDS’. The hospital kardex usually requires specific times to be identified that coincide with nursing drug rounds.
∙ Widely accepted abbreviations for route of administration are: intravenous – ‘IV’; intramuscular – ‘IM’; subcutaneous – ‘SC’; sublingual – ‘SL’; per rectum – ‘PR’; per vagina – ‘PV’; nasogastric – ‘NG’; intradermal – ‘ID’; and topical – ‘TOP’. Never abbreviate ‘oral’ or ‘intrathecal’. Care should be taken in specifying ‘right’ or ‘left’ for eye drops and ear drops.
∙ Space is provided for notes on important administration advice not detailed elsewhere (e.g. whether a medicine should be taken with food, type of inhaler device used, and anything else that relevant the drug dispenser should know). It is also important to state here the times for peak/trough plasma levels for drugs requiring therapeutic monitoring.
∙ If a course of treatment is for a known time period, cross off subsequent days when the medicine is not required. For example, for a 7-day course of antibiotics, put a vertical line through the 8th day to ‘gate’ the prescription. Similarly, if a drug is not to be given every day, cross off the days it is not required. For example, drugs such as alendronic acid and methotrexate usually have a once-weekly schedule.
∙ Always sign and print your name, and date each prescription. If a prescription record runs out and needs to be re-written, the start date is the day noted on the original card.
∙ Discontinuation of an individual prescription should be done carefully with a vertical line at the point of discontinuation, horizontal lines through the remaining days on the kardex, and diagonal lines through the prescription details and administration boxes. Sign and date this action and consider writing a supplementary note to inform colleagues about this action. The underlying details should remain legible.






