Intensification of the heat-transfer processes is one of the greatest problems in modern industry (nuclear industry, rocket engineering, electronics, laser emitters, etc.) and energetics. In these technologies, there is a need for intensive heat removal. One of the ways to solve this problem is the use of nanofluids (NFs) as coolants capable of operating at high critical heat fluxes (CHF). It is not surprising, therefore, that over the past two decades, considerable attention has been paid to colloidal dispersion of nanoparticles in base liquids (water, ethylene glycol, machine oil, etc.), known as “nanofluids” (Choi, Reference Choi, Signer and Wang1995). The nanoparticles used in NFs consist mainly of metals, oxides, carbides or various modifications of carbon (Das et al., Reference Das, Choi, Yu and Pradeep2007). Intensive research is being carried out worldwide on the development and use of NFs in power engineering, in particular, for cooling power transformers, internal combustion engines, cooling of powerful computer servers, etc. (Das et al., Reference Das, Choi, Yu and Pradeep2007). Numerous research groups are actively engaged in studying the properties of NFs and the number of publications devoted to NFs has increased exponentially in the past decade (Choi, Reference Choi2009). For example, monographs (Das et al., Reference Das, Choi, Yu and Pradeep2007) and a number of reviews have been published, in which a wide range of questions has been studied, from the thermophysical properties of NFs to their practical application (Eastman et al., Reference Eastman, Phillpot, Choi and Keblinski2004; Das et al., Reference Das, Choi and Patel2006; Ding et al., Reference Ding, Chen, Wang, Yang, He, Yang, Lee, Zhang and Huo2007; Wang & Mujumdar, Reference Wang and Mujumdar2007; Yu et al., Reference Yu, France, Choi and Routbort2007, Reference Yu, France, Routbort and Choi2008; Choi Reference Choi2008, Reference Choi2009; Wang & Wei, Reference Wang and Wei2009; Chandrasekar & Suresh, Reference Chandrasekar and Suresh2009; Terekhov et al., Reference Terekhov, Kalinina and Lemanov2010; Kim, Reference Kim2011, among others).
Previous work has shown that NFs exhibited greater (by 10–40%) thermal conductivities than those obtained for the base fluids (Choi, Reference Choi, Signer and Wang1995; Nan et al., Reference Nan, Birringer, Clarke and Gleiter1997; Eastman et al., Reference Eastman, Choi, Li, Yu and Thompson2001; Keblinski et al., Reference Keblinski, Phillpot, Choi and Eastman2002; Yu & Choi, Reference Yu and Choi2003; Assael et al., Reference Assael, Chen, Metaxa and Wakeham2004; Yu et al., Reference Yu, France, Routbort and Choi2008), and that the use of nanofluids as coolants allows high CHF and HTC values to be obtained at boiling (You et al., Reference You, Kim and Kim2003; Das et al., Reference Das, Putra and Roetzel2003; Vassallo, Reference Vassallo2004; Milanova & Kumar, Reference Milanova and Kumar2005; Bang & Chang, Reference Bang and Chang2005; Kim et al., Reference Kim, Bang, Buongiorno and Hu2006, Reference Kim, Kim and Kim2007a,Reference Kim and Kimb; Jo et al., Reference Jo, Jeon, Yoo and Kim2009; Bondarenko et al., Reference Bondarenko, Moraru, Sydorenko, Komysh and Khovavko2012, Reference Bondarenko, Moraru, Ilyenko, Khovavko, Komysh, Panov, Sydorenko and Snigur2013, Reference Bondarenko, Moraru, Sydorenko and Komysh2015, Reference Bondarenko, Moraru, Sydorenko and Komysh2016a,Reference Bondarenko, Moraru, Sydorenko, Komysh and Khovavkob; Pham et al., Reference Pham, Kim, Lee and Chang2012). However, the mechanisms for increasing the CHF and the effective thermal conductivity of the NFs are not fully understood. It has been suggested that the increase in thermal conductivity may be due to clustering of nanoparticles, to ballistic phonon transport, to the presence of solvate layers at the boundary of the solid and liquid phases, to the interaction of particles with liquid molecules and to the Brownian motion of nanoparticles (Keblinski et al., Reference Keblinski, Phillpot, Choi and Eastman2002). In addition, it is assumed that the suspended particles increase the heat-exchange surface area and heat capacity of basic fluids. Nevertheless, the noted increase in thermal conductivity cannot explain the anomalously high (>200%) heat transfer at the boiling of aqueous NFs as compared with water. Previous studies have shown that the increased values of CHF (q cr) and HTC (α) are determined mainly by the state of the heat-dissipating surface and its topological and chemical properties as a result of deposition of a nanoparticle layer on it (Kim et al., Reference Kim, Bang, Buongiorno and Hu2006, Reference Kim, Kim and Kim2007a,Reference Kim and Kimb; Wen, Reference Wen2008a,Reference Wenb; Pham et al., Reference Pham, Kim, Lee and Chang2012; Bondarenko et al., Reference Bondarenko, Moraru, Sydorenko, Komysh and Khovavko2012, Reference Bondarenko, Moraru, Ilyenko, Khovavko, Komysh, Panov, Sydorenko and Snigur2013, Reference Bondarenko, Moraru, Sydorenko and Komysh2015, Reference Bondarenko, Moraru, Sydorenko and Komysh2016a,Reference Bondarenko, Moraru, Sydorenko, Komysh and Khovavkob).
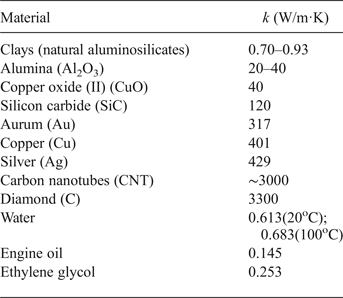
There are three means of heat transfer: conduction, convection and radiation (Rumyantsev, Reference Rumyantsev2006). The heat-transfer mechanism that will prevail in the various NFs might be predicted from the thermal conductivity (cf. Table 1). Hence, nanofluids obtained from highly conducting materials might have greater thermal conductivity (Ding et al., Reference Ding, Chen, Wang, Yang, He, Yang, Lee, Zhang and Huo2007). However, this rule might not always be satisfied with respect to heat transfer during boiling of the NFs, especially in NFs consisting of a mixture of anisometric particles, boiling of which may be dominated by the convective heat-transfer mechanism, independently of the thermal-conductivity value.
Table 1. Thermal сonductivity (k) of various materials.
The wide use of boiling processes is explained by the significantly higher HTC (2000–50,000 W/m2K) compared to the heat-transfer rate for convective heat transfer without changing the aggregate state (Kutateladze, Reference Kutateladze1979). Boiling and heat-transfer increase with increasing specific heat load, but heat removal is limited by the value of CHF, which corresponds to the boiling crisis, characterized by a sharp decrease in HTC due to the formation of a steam coat (film) on the heating surface. This is accompanied by a sudden increase in the temperature of the external heating surface which damages the integrity of the equipment. Therefore, an extremely important task for heat-power engineering is the development of effective NFs, which not only allow the device to work at high CHF, but also to avoid the sudden onset of a boiling crisis. The boiling crisis arises from the expansion and merging of dry/hot spots on the heating surfaces, the existence of which is a characteristic feature of boiling (Yagov, Reference Yagov2003). The hydrophilicity of NFs (or NPs), therefore, is very important in attempting to avoid the crisis of boiling.
Previous studies of heat transfer during boiling of NFs (Kim, H.D. Reference Kim2011; Kim, H.D. et al., Reference Kim, Kim and Kim2007a,Reference Kim and Kimb, Reference Kim, Kim and Kim2014; Kim, S.J. et al., Reference Kim, Bang, Buongiorno and Hu2007a; Wen, Reference Wen2008a; You et al., Reference You, Kim and Kim2003; Bondarenko et al., Reference Bondarenko, Moraru, Sydorenko, Komysh and Khovavko2012, Reference Bondarenko, Moraru, Ilyenko, Khovavko, Komysh, Panov, Sydorenko and Snigur2013) showed that even very small concentrations of NPs in the basic fluids (0.001–0.1 vol.%) led to significant increases in CHF. Also, the thermophysical properties of water with NPs (surface tension, viscosity, heat of vapourization, boiling point), appear to not differ noticeably from the thermophysical properties of distilled water (Kim et al., Reference Kim, Bang, Buongiorno and Hu2007a,Reference Kim, Bang, Buongiorno and Hub). On this basis, the authors explained the mechanism of CHF growth by deposition and sintering of nanoparticles on the boiling surface, which increase the area and wettability of the heat-exchange surface. The CHF values have also been studied thoroughly and evaluated for the boiling of NFs based on metal oxides (Vassallo, Reference Vassallo2004; Bang & Chang, Reference Bang and Chang2005; Wen & Ding, Reference Wen and Ding2005; Wen et al., Reference Wen, Ding and Williams2006; Kim et al., Reference Kim, Bang, Buongiorno and Hu2006, Reference Kim, Kim and Kim2007a,Reference Kim and Kimb; Fokin et al., Reference Fokin, Belen'kii, Al'myashev, Khabenskii, Al'myasheva and Gusarov2009; Pham et al., Reference Pham, Kim, Lee and Chang2012). Nevertheless, the optimal NFs, which meet all the technical requirements of modern energetics (e.g. high sedimentation stability, stability of NFs against radiation and multiple boiling-cooling cycles in combination with high technological characteristics, ecological compatibility and accessibility), have not yet been developed.
The ability to increase the intensity of heat transfer, especially during boiling, is one of the most remarkable properties of NFs. Nevertheless, the thermal properties of clay-mineral nanodispersions have not received much attention by researchers. However, the clay-water-based NFs exhibit remarkable heat-transfer capacity, particularly during boiling and thus they may be more promising as coolants for energetics than many other types of NFs such as oxides, carbides, etc., due to their superior heat-engineering parameters. For example, the specific heat flux (SHF) at boiling of water-aluminosilicate NFs may increase to 300–400% compared to ordinary water and reach values of q max = 3–4×106 W/m2; the heat-transfer coefficient (α) may exceed 40,000 W/m2K (Bondarenko et al., Reference Bondarenko, Moraru, Sydorenko, Komysh and Khovavko2012, Reference Bondarenko, Moraru, Ilyenko, Khovavko, Komysh, Panov, Sydorenko and Snigur2013, Reference Bondarenko, Moraru, Sydorenko and Komysh2015, Reference Bondarenko, Moraru, Sydorenko and Komysh2016a,Reference Bondarenko, Moraru, Sydorenko, Komysh and Khovavkob).
To prepare nanofluids for energetics purposes, we first proposed natural aluminosilicates (clay minerals) and performed thermophysical studies and thermal-engineering tests (Bondarenko et al. Reference Bondarenko, Moraru, Sydorenko, Komysh and Khovavko2012, Reference Bondarenko, Moraru, Sydorenko and Komysh2015, Reference Bondarenko, Moraru, Sydorenko and Komysh2016a,Reference Bondarenko, Moraru, Sydorenko, Komysh and Khovavkob). The nano-size, diverse shape and anisometry of their particles, the significant hydrophilicity of the surface and the ability to disperse spontaneously in aqueous solutions predetermine the high thermal parameters of such NFs when used as coolants, especially at boiling.
In the present study, for water nanodispersions of clay minerals with various crystalline structures, shape and anisometry of nanoparticles, the boiling curves of the corresponding NFs under conditions of free convection are studied, and their thermal parameters characterizing heat transfer are determined. The aims of this work were: (1) to study heat transfer at the boiling of aluminosilicate NFs and the possible relationship with the physicochemical parameters of the nanoparticles; (2) to determine the NFs with maximum thermal parameters of boiling – the specific heat flux q (W/m2) and the heat-transfer coefficient α (W/m2K) for their subsequent use in heat-power engineering; and (3) to highlight the intensification of heat transfer in the NF boiling based on clay minerals.
MATERIALS AND METHODS
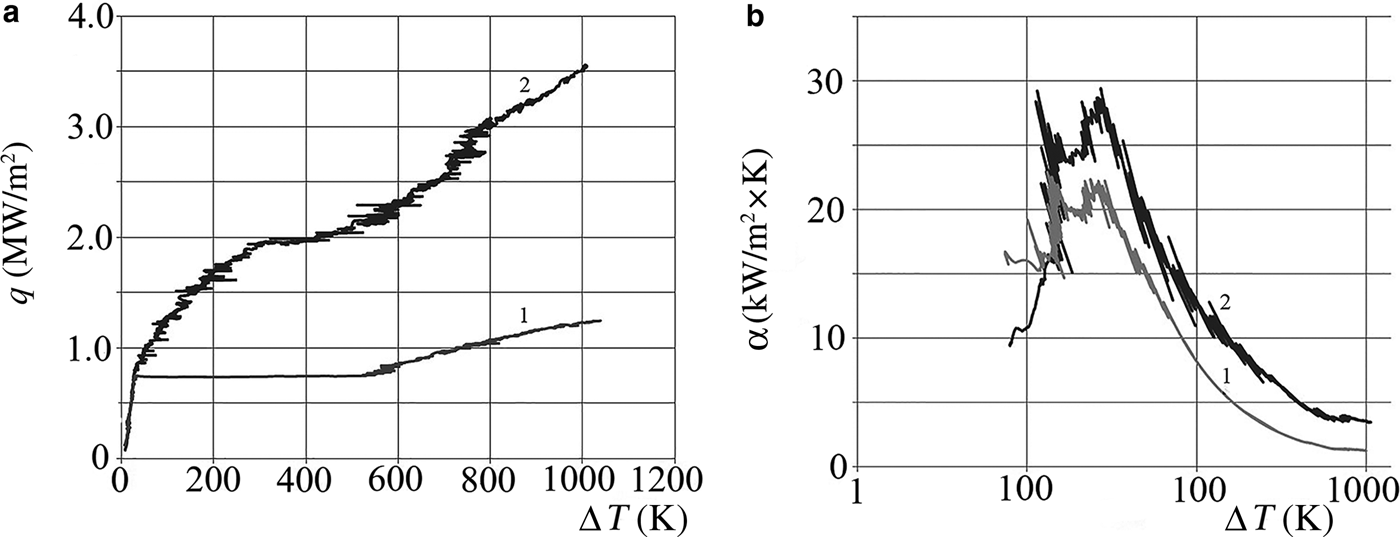
The samples studied were aqueous nanofluids prepared from natural aluminosilicates from the Cherkassky deposit, Ukraine: montmorillonite (AlSi-1), palygorskite (AlSi-5), illite (AlSi-6) and a 70:30 mixture of montmorillonite and palygorskite (AlSi-7) (Table 2). A mixture of AlSi-7 and carbon nanotubes (AlSi-7 + 0.1% CNTs) was also studied. The nanofluid NF-8 with rutile isometric particles (k = l/d = 1–3) was used for comparison (Table 2). Nanofluids were prepared on installation of UZDN-2 T (f = 22 kHz, P = 350 W) by a short-term (5 min) ultrasonic dispersion of purified natural clay powders in distilled water. The clay powders were purified after settling in distilled water, centrifugation and drying. The Supplementary Tables (ST 1 and ST 2, available from 10.1180/clm.2018.17) list the chemical composition (determined using conventional analytical methods for analysis of silicate rocks (Hillebrand et al., Reference Hillebrand, Lundell, Bright and Hoffman1966), and also taken from previous analyses performed by Ovcharenko et al. (Reference Ovcharenko, Kirichenko, Ostrovskaya and Dovgy1966)) and X-ray diffraction (XRD) analytical data of the clays studied; the calculated structural formulae of the clay minerals used are listed in Table 3. The structural formulae are indicative only, due to the presence of impurities. The chemical analysis (ST1) and the XRD analysis (DRON-UM1 diffractometer, Co-Kα radiation) (ST2) of the AlSi-1 show typical compositions for the Fe,Al-montmorillonite (MM). Major impurities are quartz, palygorskite and illite and minor impurities are opal-CT, calcite, dolomite and manganese hydroxide. After purification, the impurities did not exceed 10 wt.%. Minerals which have been purified to this extent are suitable for use as NFs.
Table 2. Physicochemical characteristics, q max and αmax values, of some aqueous nanofluids based on clay minerals.
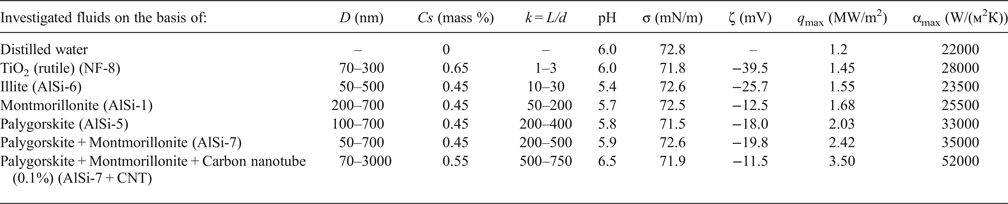
D – average particle size; Cs – particle concentration; k – anisometry coefficient (where L is the long axis and d the minor axis of the ellipsoid); σ – surface tension at 20°С; ζ – electrokinetic potential; q max – maximal specific heat flux; αmax – maximal heat transfer coefficient.
Table 3. Structural formulae of the samples studied.

The AlSi-5 sample (palygorskite) contains minor montmorillonite, illite, quartz and manganese hydroxide impurities. In the mixture of montmorillonite and palygorskite (AlSi-7), in addition to those of the MM lines, are reflections (d = 10.5 and d = 3.23 Å) typical of palygorskite (ST2). Finally, the illite sample contains minor montmorillonite and quartz (diffraction lines at d = 14–15 Å and 3.33 Å, respectively).
The compositions of the dispersions and the ζ-potential of the NFs were determined using a laser correlation spectrometer ZetaSizer NANO-ZS (Malvern Instruments, UK) and by surface tension, using the Wilhelmy plate method (modified tensometer K6 (KRŰSS GmbH, Germany). For the study of nanostructured deposits on the heating surfaces after boiling of the NFs, a porosimeter (QuantaChrome® AsiQwinTM) and scanning electron microscopy (SEM, cold field emission JEOL JSM6700 F) were used. Samples of Ni/Cr wires (heaters) with nanoparticle deposits after the boiling of nanofluids and after drying at room temperature were transferred to the SEM without additional heat treatment, in order to avoid changes in the sample surface.
The thermophysical studies of the NFs were carried out using a specially created automated experimental stand (Bondarenko et al., Reference Bondarenko, Moraru, Ilyenko, Khovavko, Komysh, Panov, Sydorenko and Snigur2013), operating on direct current (DC) and controlled by a flexible computer program which allowed the authors to fix the necessary parameters (current, voltage, heat fluxes, heat-transfer coefficient, etc.) and adjust them over a wide range. The surface temperature of the heater was determined using the dependence of the resistivity of the Ni-Cr wire on the temperature. The computer produced graphical plots between the main, above-mentioned values and parameters in real time.
The experimental vessel was filled with water or with one of the selected NFs and the working fluid was preheated using an auxiliary heater (500 W). Then, the main heater was turned on and input power was increased automatically at a constant rate until maximum SHF. The power supply was then stopped after heater burnout. SHF and CHF measurement error did not exceed 5%. Fluid samples were collected for measurement of surface tension, ζ-potential and pH in order to control the stability of NF before and after the experiment.
The main heat-transfer parameters, HTC (α) and SHF (q), were obtained using the following equations:
where t w (°С) is the outer-surface temperature of the heater, and t f (°С) is the liquid boiling temperature (see Bondarenko et al., Reference Bondarenko, Moraru, Ilyenko, Khovavko, Komysh, Panov, Sydorenko and Snigur2013 for calculation of t w):
where Q is the full heat load on the main heater, I is current, U is voltage, and F is outer surface area of the heater (Ni-Cr wire). F = πdl, where d and l are the diameter and length of the Ni-Cr wire, respectively.
RESULTS AND DISCUSSION
Stability of the NFs
The stability of the nanodispersions investigated was evaluated by the ζ-potential and the sedimentation methods and was quite satisfactory even after multiple boilings and measurements of the thermal parameters. After prolonged boiling for >60 min, the stability of aluminosilicate NFs increased due to further dispersion of NPs, while other types of NFs (e.g. carbon-based) coagulated completely or partially and precipitated (Fig. 1). This important feature of aluminosilicate NFs is probably related to the crystal-chemical structure of the clay minerals, as well as to their significant hydrophilicity (Grim, Reference Grim1959; Tarasevich, Reference Tarasevich1988).

Fig. 1. Comparison of the stability of aluminosilicate NF, AlSi-7 (1,2) and carbon-based NF (3,4) before (1,3) and after (2,4) 60 min of boiling.
The low ζ-potential values of the aluminosilicate NFs (Table 2), varied from –25.7 mV to –11.5 mV in a weakly acid medium (pH 5.4–6.5). This might indicate instability in the NFs in the case of hydrophobic colloids. In hydrophilic systems such as clay minerals, the stability of nanodispersions does not correlate with their ζ-potential as their stability is mainly due to the structural component of the disjoining pressure (this pressure occurs when overlapping double electrical and boundary solvate layers at the moment of approach of neighbouring particles at a distance of the action of electrostatic and structural forces generates a barrier of repulsion between them) (Deryaguin et al., Reference Deryaguin, Churaev and Muller1985). Small values of the ζ-potential for hydrophilic colloids are due to the formation on the particle surface of the hydrodynamically stationary boundary hydrate layer and the displacement of the slip boundary deep into the liquid phase (Kruit, Reference Kruit1955; Moraru et al., Reference Moraru, Ovcharenko and Moraru1999).
The problem of NF stabilization with the convective mechanism of heat transfer is associated with two mutually exclusive effects: the improved stability and simultaneous deterioration of the heat-transfer properties of the NFs. The use of dispersants, stabilizers and especially surfactants adversely affects the stability of the NFs to boiling and to the coagulating effect of the electric field, and also deteriorates sharply the intensity of heat exchange, leading to a profuse foaming at boiling. This might be due to the appearance of electrostatic and steric barriers of repulsion between nanoparticles and the heat-exchange surface because of the simultaneous adsorption of molecules/ions of the stabilizer or dispersant onto them. This prevents the deposition of nanoparticles on the heating surface during boiling and the formation of a porous layer, which is a powerful generator for formation of bubbles which are amplifiers of convections. The roughness of the porous layer determines the increase in critical heat flux (Pham et al., Reference Pham, Kim, Lee and Chang2012).
Stable nanofluids based on clay minerals may be obtained without the addition of surfactants or dispersants. The most effective method for this is ion-exchange substitution for Li+ or Na+ cations. In the present study the clay dispersions with intrinsic hydrophilicity are resistant to boiling and are stable in DC unlike the nanodispersion of carbon particles with acquired hydrophilicity and charge, after stabilization with surfactants (Fig. 1).
The average size of the primary particles in the nanodispersions of the clays studied was 50–500 nm, i.e. the nano-suspensions had a polydisperse character of particle-size distribution. The corresponding coefficients of particle anisometry (k = L/d) varied from ~10 to 700 (Table 2).
Changing the heater temperature and heat-transfer parameters during boiling of NFs and water at a constant heat-load increase rate (HLIR)
The increase in thermal conductivity when dispersing solids in liquids in NFs occurs because solid particles conduct heat much better than liquids (Das et al., Reference Das, Choi, Yu and Pradeep2007, Table 1). However, the role of NPs in improving the heat transfer is not limited by this fact (Kim et al., 2007; Kim Reference Kim2011; Bondarenko et al., Reference Bondarenko, Moraru, Ilyenko, Khovavko, Komysh, Panov, Sydorenko and Snigur2013, Reference Bondarenko, Moraru, Sydorenko and Komysh2016a,Reference Bondarenko, Moraru, Sydorenko, Komysh and Khovavkob).
During boiling, porosity and roughness develop on the heating surface due to the spontaneous formation of nanostructures, causing sharp intensification of heat exchange with the surrounding medium (Bondarenko et al., Reference Bondarenko, Moraru, Sydorenko, Komysh and Khovavko2016b). The porous layer formed on the heater surface may suppress or eliminate the boiling crisis caused by overheating and equipment failure (Bondarenko et al., Reference Bondarenko, Moraru, Sydorenko, Komysh and Khovavko2012, Reference Bondarenko, Moraru, Ilyenko, Khovavko, Komysh, Panov, Sydorenko and Snigur2013, Reference Bondarenko, Moraru, Sydorenko and Komysh2016a). These positive effects may be related to the change in the size, the wettability and roughness of the heat-exchange surface and to the fact that the porous structure and properties of the deposited layer may control the heat transfer during boiling of the NFs.
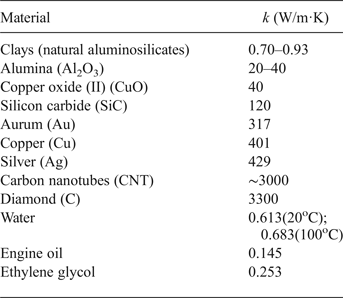
On the abscissa in Fig. 2 the device registers not the time, but the number of measurements of heater temperature in time, at a speed equal to eight measurements per second. Accordingly, the experiment time, expressed in seconds, is equal to the number of measurements divided by 8, i.e. 1730/8 = 216.2 s = 3.6 min. The slow increase in the temperature of the heater over time is observed with the boiling of the AlSi-7 nanofluid (curve 1) in comparison with the sharp increase in t h during boiling of DW (curve 2) at the same HLIR. This indicates high heat transfer upon boiling of the NFs compared to the base liquid. While the boiling of distilled water expires (1730/8) s The time dependences of the heater temperature (t h) at aluminosilicate NF boiling (1) in comparison with distilled water (2) at constant heat-load increase rate (HLIR) are shown in Fig. 2, a boiling crisis occurs on the surface of the heater, followed by a transition to a film boiling mode and a sharp increase in t h. A bubbling boiling regime with a high heat transfer is maintained in the medium of the NFs. This boiling mode with maximum heat dissipation is established on the porous surface of the heater, formed by deposition of a layer of clay nanoparticles (Fig. 3).
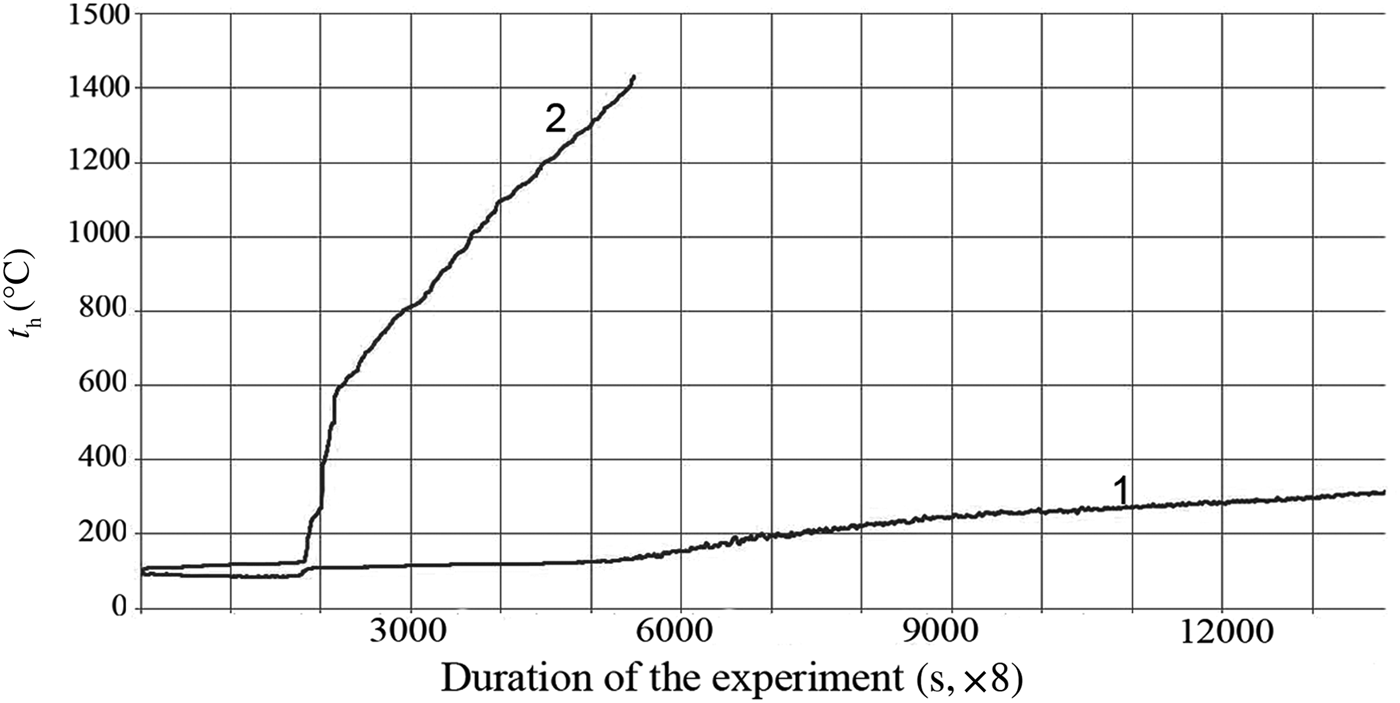
Fig. 2. Kinetics of temperature increase of the heater (t h) upon boiling of AlSi-7 (1) and distilled water (2) at the same rate of heat-load rise (1.1 kW/m2s).
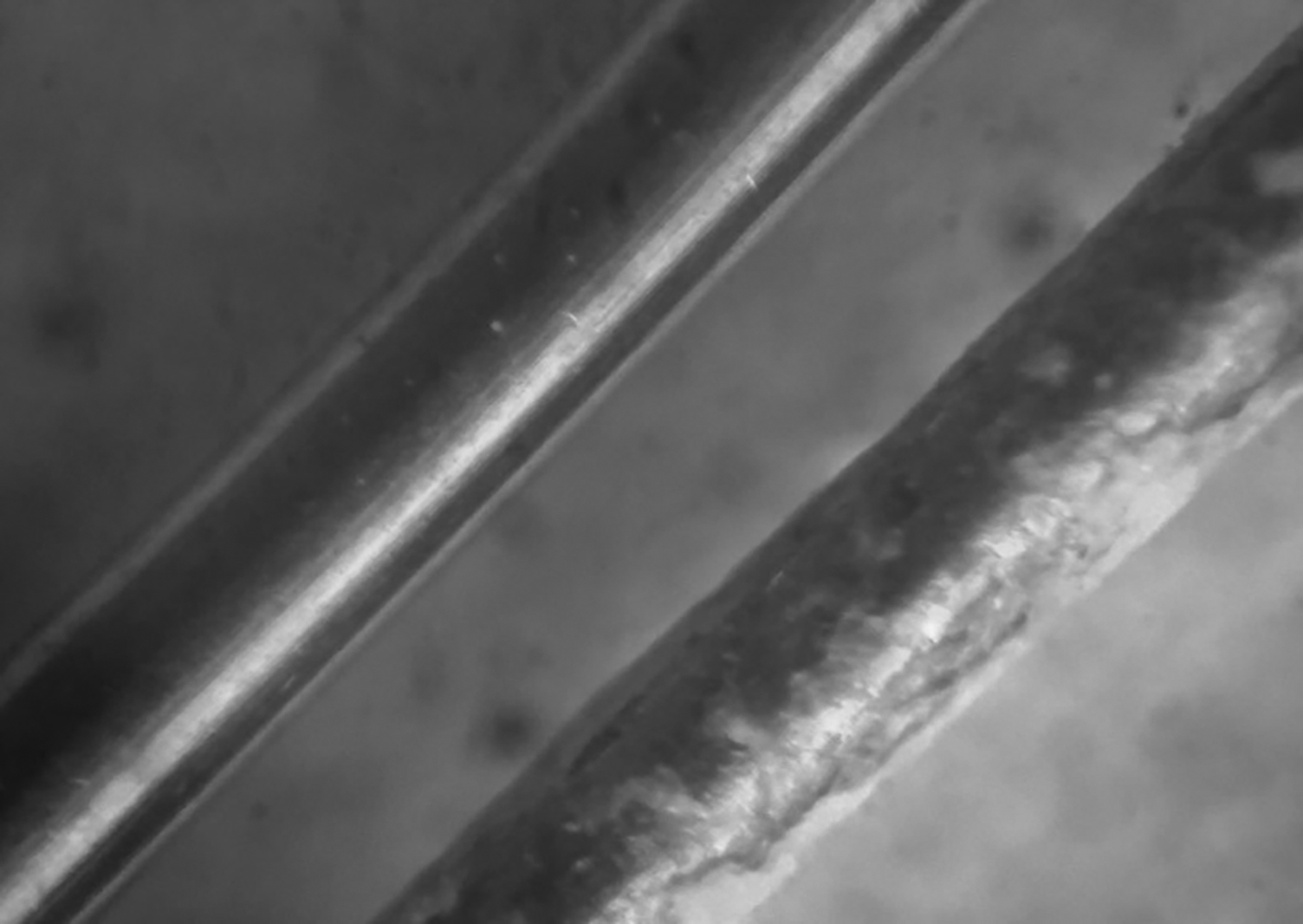
Fig. 3. Image of the heater after boiling of distilled water (left) and NF AlSi-7 on the basis of a genetic mixture of montmorillonite and palygorskite (right).
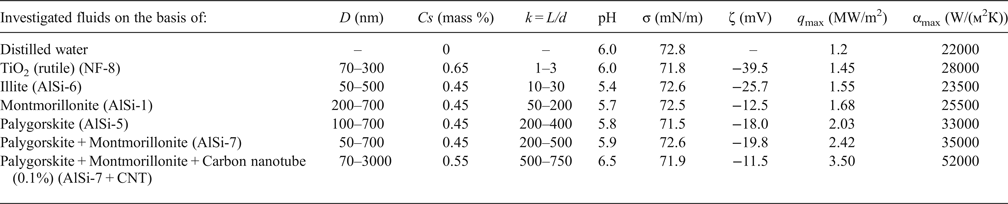
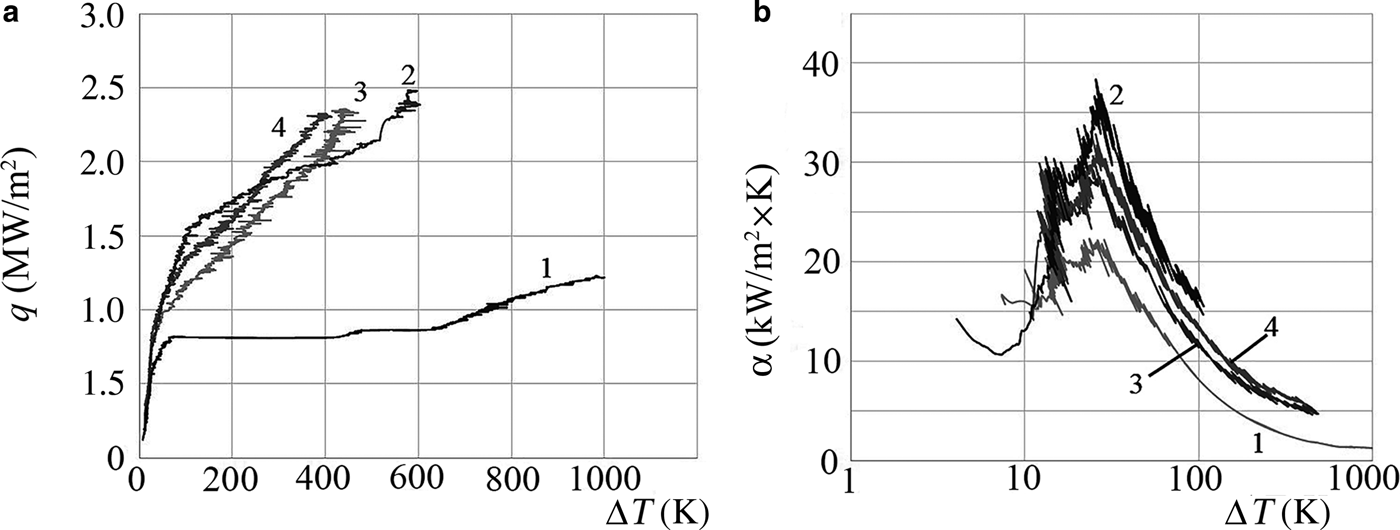
The numerical values of SHF and HTC for the NFs are 1.5–3 times greater than those for distilled water in the range of superheat values ΔT = 50–300 K (Fig. 4, Table 1)
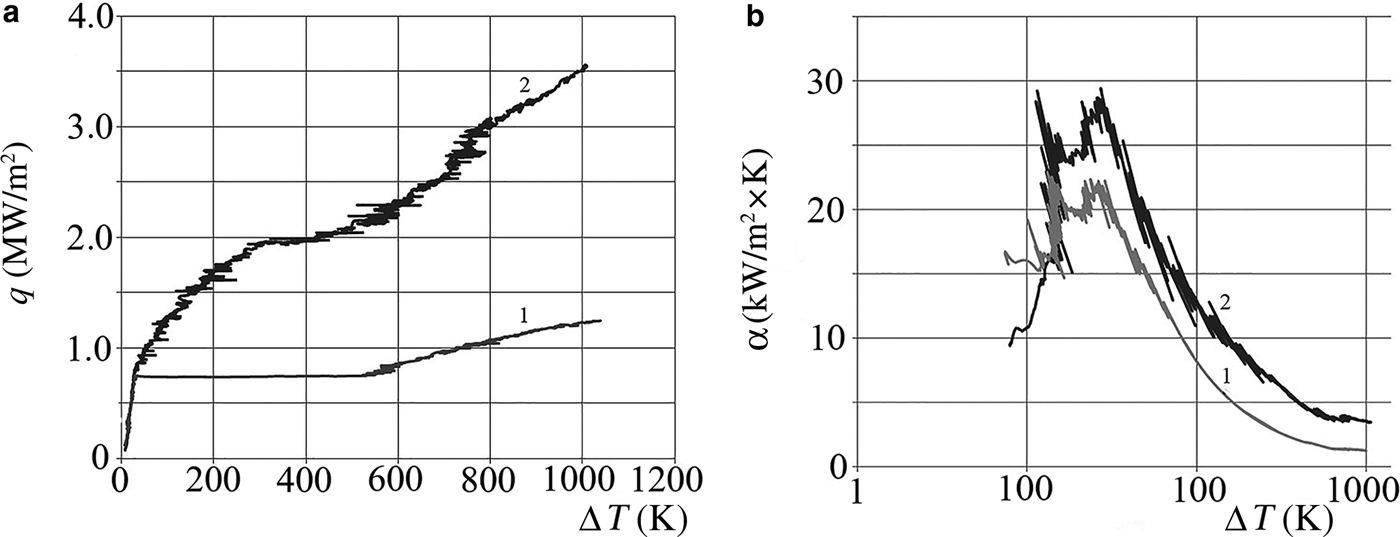
Fig. 4. Dependences of the specific heat flux (a) and the heat-transfer coefficient (b) on the superheat value (ΔT) at the boiling of distilled water (1) and of the NF AlSi-7 (2).
During the boiling of the NFs, when a layer of sediment has already formed on the heating surface, both the first and the second boiling crises are absent. During an intensive heat exchange (at a heat-flux value of up to 1.5 MW/m2) on the boiling surface, there is an unstable layer with a highly developed differentiated porous structure that ensures the supply of non-superheated liquid to and the removal of superheated steam from the heater. The intensity of heat exchange is maintained as long as this hydrodynamic system exists and this is dictated by system instability. This allows the boiling centres to move along the surface of the heater and avoid the expansion of ‘hot/dry’ spots on the boiling surface, which pushes back the boiling crisis characteristic for distilled water.
The effect of so-called capillary wicking on boiling on porous surfaces has been observed in previous studies (Kim, H. D. et al., Reference Kim, Bang, Buongiorno and Hu2007a,Reference Kim, Bang, Buongiorno and Hub, Reference Kim, Kim and Kim2014; Fokin, Reference Fokin, Belen'kii, Al'myashev, Khabenskii, Al'myasheva and Gusarov2009). This phenomenon is based on the penetration (capillary adsorption) of liquid along micro- and nanopores under the surface of vapour bubbles that appear on the surface of the heater during boiling. Capillary humidification together with the wetting effect promotes more efficient washing of dry spots on the surface of the heater, which leads to an increase in the CHF value and delay in the boiling crisis.
Effect of pre-modification of a heating surface on heat-transfer parameters at boiling of distilled water
Because the surface of the heater remained clean during boiling of distilled water (Fig. 3), nanoparticles were deposited on it at the boiling of NFs (Figs 3, 5).
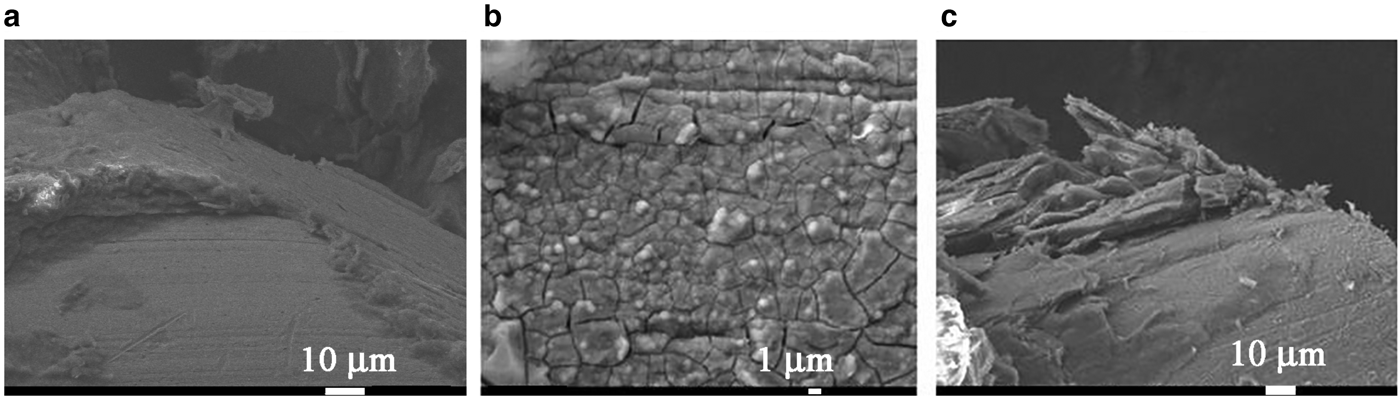
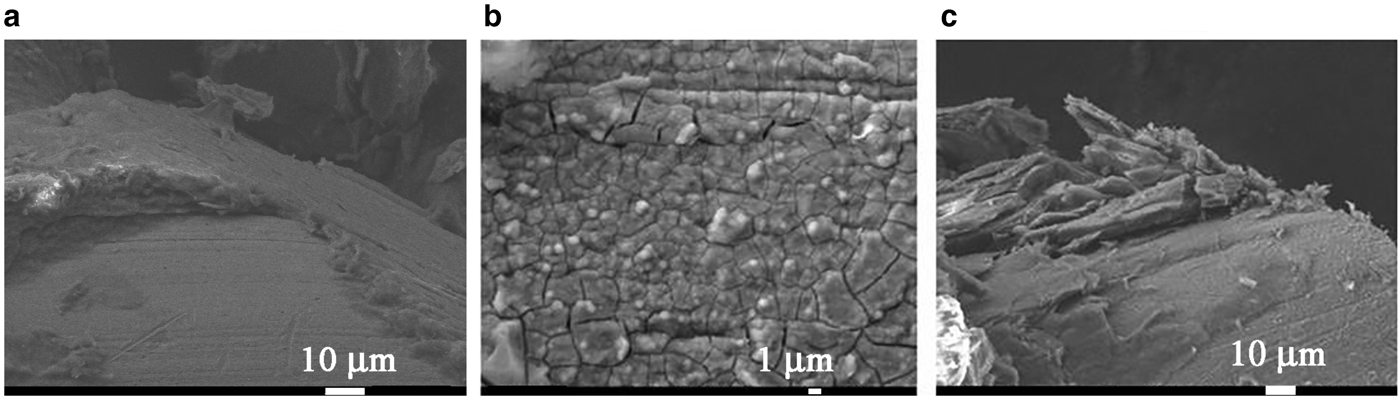
Fig. 5. SEM images of the heating surface after the boiling of NFs AlSi-5, AlSi-6 and AlSi-7 on the basis of palygorskite (a), illite (b) and the genetic mixture of montmorillonite and palygorskite (c).
In order to interpret the mechanism of increasing CHF, we performed an analysis of the microrelief of the heater surface after boiling of the NFs. As the viscosity and surface tension of the NFs are comparable to those for water, their effect on the increase of CHF upon boiling of the NFs may be ignored. The SEM images of the heating surface obtained (Fig. 5) showed that after boiling of the AlSi-7 nanofluid with the maximum CHF (Fig. 5c), the nanoparticle agglomerates were deposited on the heating surface yielding significant surface roughness and porosity. At the same time, after boiling of AlSi-6 (Fig. 5b), the surface of the heater at even greater magnification had a smoother texture, lacking porosity, which explains the smaller CHF values. The value of CHF may depend on the change in the topography of the heating surface as a result of the deposition of the porous layer of nanoparticles upon boiling of the NFs.
To prove this assumption, the following experiment was performed. In a DC-powered installation, the boiling curve of the AlSi-7 nanofluid was recorded automatically before the onset of the crisis, and the Ni-Cr wire with deposited sediment was not heated, but kept for subsequent experiments. Then, through the lower tap, the entire NF was poured gently from the unit and was replaced with distilled water. At the same time, the deposit on the Ni-Cr wire remained intact. The boiling curve of distilled water was recorded, whereupon the unit was unloaded and again filled with distilled water so that the structure of the deposit on the wire remained intact, and again the boiling curve of water was recorded.
The curves obtained are shown in Fig. 6. The boiling curves of water with a modified heater (curves 3,4) resemble the boiling curve of the NFs, rather than those of distilled water, both in terms of shape and magnitude of the CHF. On these curves there is no horizontal section corresponding to the boiling crisis of water. The bending observed at 450–500°C on the boiling curve of the initial AlSi-7 is related to the onset of recrystallization of aluminosilicate particles deposited on the surface of the heater, as this temperature corresponds to the dehydroxylation of palygorskite (Grim, Reference Grim1959; Tarasevich, Reference Tarasevich1988). It follows that the preliminary coating of the heater surface by the layer of nanoparticles at NF boiling greatly increases the SHF and HTC at boiling of distilled water with the same heater, delaying the onset of boiling crisis. Thus, the increase in SHF and HTC is not related directly to the use of the NF as a coolant. Instead, the porous layer of the nanoparticles generates bubble formation. Rapid release of bubbles increased dramatically heat transfer due to convection, even for such weakly heat-conducting NFs as the clay minerals.
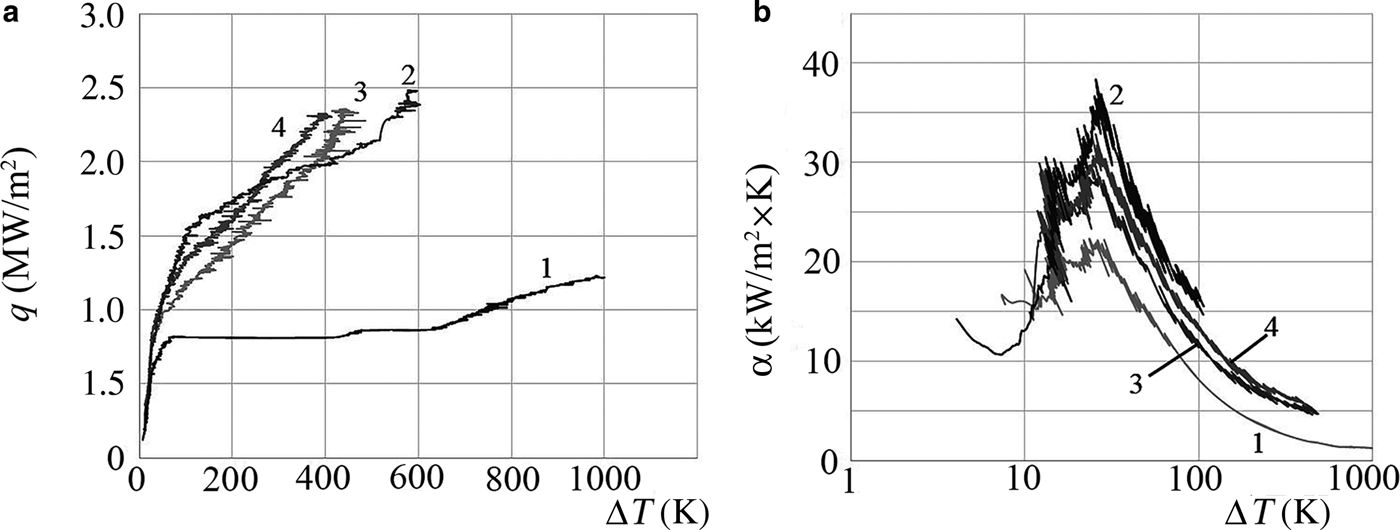
Fig. 6. The boiling curves (a) and the heat-transfer coefficient as a function of the superheat value (ΔT) (b) for distilled water (1) and AlSi-7 NF (2), as well as distilled water after preliminary modification of the heater surface by boiling AlSi-7 (3,4).
The connection of the main mechanism of heat transfer with convection in the heat-exchange zone through intensification of the nucleate boiling (also known as bubble boiling), is confirmed by the fact that the thermal properties of some NFs based on clay minerals at boiling are superior to those for other types of NFs with more heat-conducting NPs (e.g. TiO2, SiC). Therefore, in order to obtain NFs with high heat-transfer parameters during boiling, it is desirable to use NPs with increased thermal conductivity. It is important that the NFs show an enhanced convective heat-transfer mechanism at boiling, along with thermal conductivity, which requires fine control of shape, anisometry and hydrophilicity of NPs.
Thus, the sudden intensification of heat transfer and increase in the specific heat flux (q sp) at boiling of NFs compared with DW is associated with the change in microrelief (roughness, porosity) and the properties of the heating surface because of the deposition of the structured layer of NPs.
Influence of the shape and anisometry of clay nanoparticles on heat transfer at pool boiling
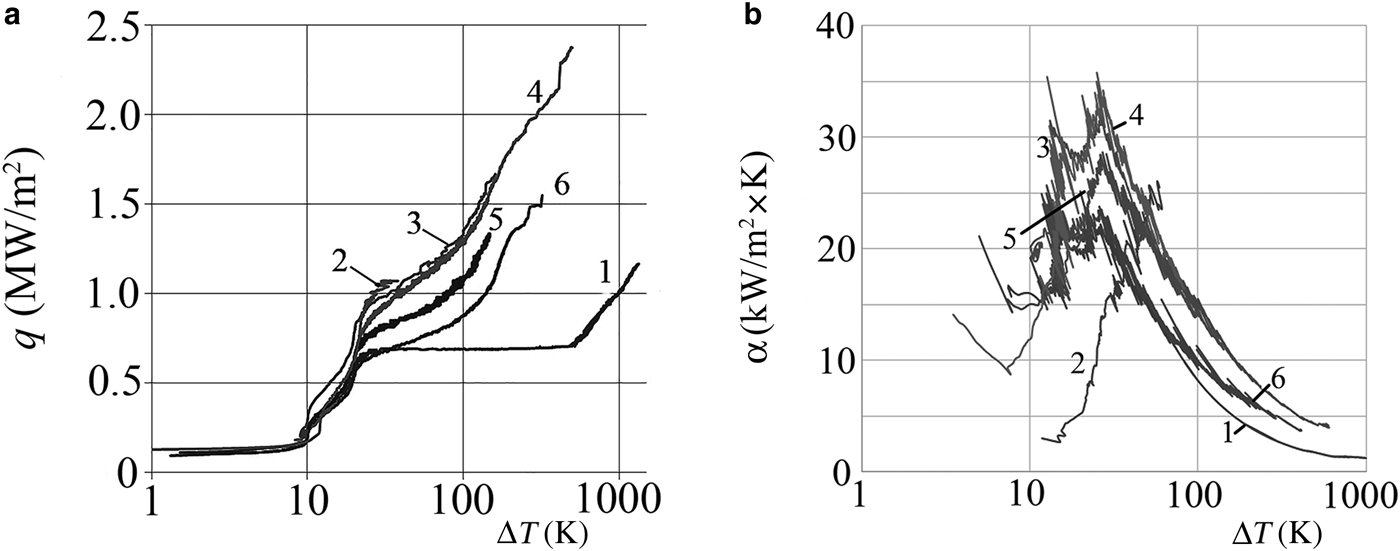
The results of thermal-engineering tests of water as the base fluid and the NFs are given in Table 2 and Fig. 7. The boiling curve of distilled water (curve 1) has a characteristic appearance with a horizontal section, which corresponds to the onset of a boiling crisis and a sudden increase in the temperature of the heater. The introduction of nanoparticles into water (Fig. 7, curves 2–6) changes sharply the character of the boiling curve of the water, and increases significantly the critical heat flux from 0.7 to 1.8–2.3 MW/m2, depending on the concentration of NPs.
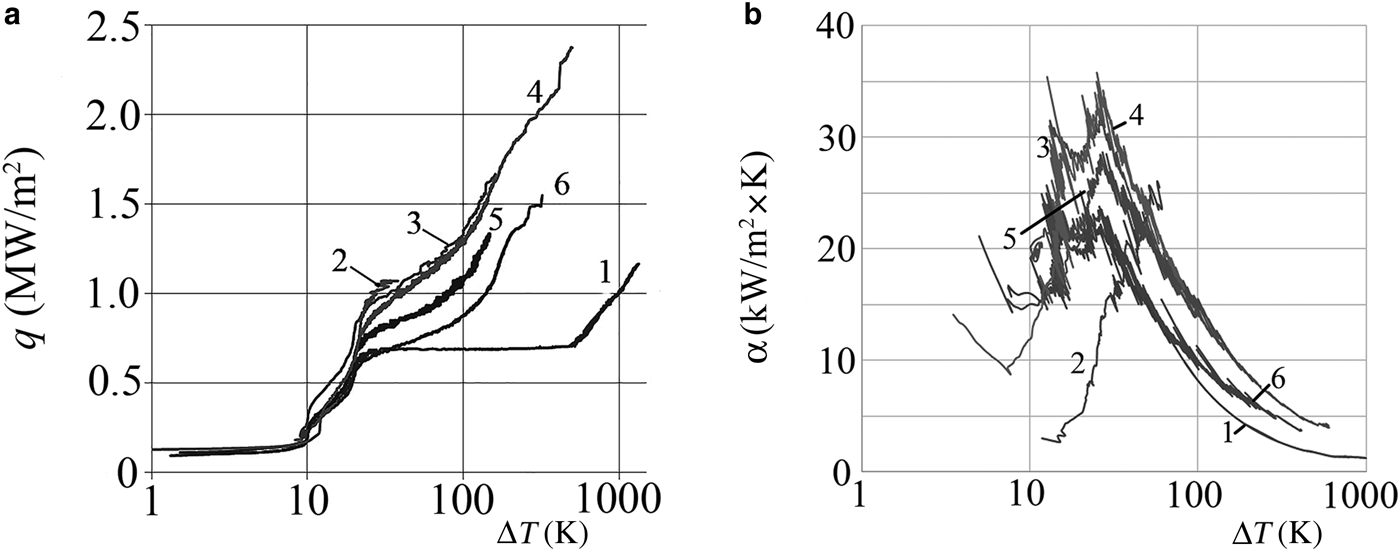
Fig. 7. Boiling curves (a) and the dependence of the heat-transfer coefficient (b) on the superheat value (ΔT) for distilled water (1) and NFs AlSi-1 (2), AlSi-5 (3), AlSi-7 (4), NF-8 (5) and AlSi-6 (6).
On the other hand, the shape and anisometry of particles affects the thermal parameters of the NFs significantly upon boiling (Fig. 7). The larger the q and α values the larger the coefficient of particle anisometry, e.g. the NFs of palygorskite (AlSi-5) and its mixture with montmorillonite (AlSi-7) with particles of the same size, but with greater anisometry, lead to greater increases in SHF and HTC than the montmorillonite NF (AlSi-1), the illite NF (AlSi-6) and NF-8 at the same particle concentration. Under boiling of NFs with a mixture of strongly anisometric particles, the surface of the heater became rougher than at boiling of the NFs with particles of isometric (TiO2) or platy form. This was confirmed by the SEM images of the heating surface after boiling of the NFs (Fig. 5). The roughness also determined the density of the evaporation centres on the boiling surface (Kutateladze, Reference Kutateladze1979; Fokin et al., Reference Fokin, Belen'kii, Al'myashev, Khabenskii, Al'myasheva and Gusarov2009). Although the coefficient of anisometry is high, the particles are flat; e.g. for illite (NF-6) and montmorillonite (AlSi-1) the porosity and roughness of deposits are low and the thermal parameters at boiling of such NFs are low.
As mentioned previously, the CHF growth mechanism might be attributed to the deposition of nanoparticles and the formation of a specific structure on the boiling surface, as a result of which the heat-exchange surface area, porosity and roughness of this surface increased sharply. These particles affect the state of the heating surface, changing its roughness and wettability, which ultimately leads to a change in the internal characteristics of the boiling process of liquids, rather than change the thermophysical and physicochemical properties of the NFs (Table 2).
Influence of the type of electric heating current on the heat-transfer parameters at the boiling of clay NFs
The type of electric heating current affects the structure of the deposits and the thermal parameters during boiling of the NFs. Thus, when boiling in a DC-fed plant, an intensive deposition of NPs is observed on all copper-free positive poles (anode) for all clay NFs without addition of dispersants. The deposits formed from the NPs around the wire Ni/Cr heater have a gel-like structure because of their hydrophilicity. This is a great advantage of these NFs compared to others which form solid deposits (cathode) (Fig. 8a). If the heater is fed with a direct current, the deposits that form around the heater are not uniform in thickness but have the shape of a cone directed with its base towards the cathode (–) (see Fig. 8). This might be due to the fact that some of the negatively charged clay particles are discharged and deposited densely on the anode, whereas the remaining particles, which are deposited on the surface of the heater, experience increasing repulsion as they approach the cathode. At the cathode, either the precipitate does not form or the aggregates of the particles are eroded by the turbulence caused by boiling because the electric field is weak (Bondarenko et al., Reference Bondarenko, Moraru, Sydorenko, Komysh and Khovavko2016b).
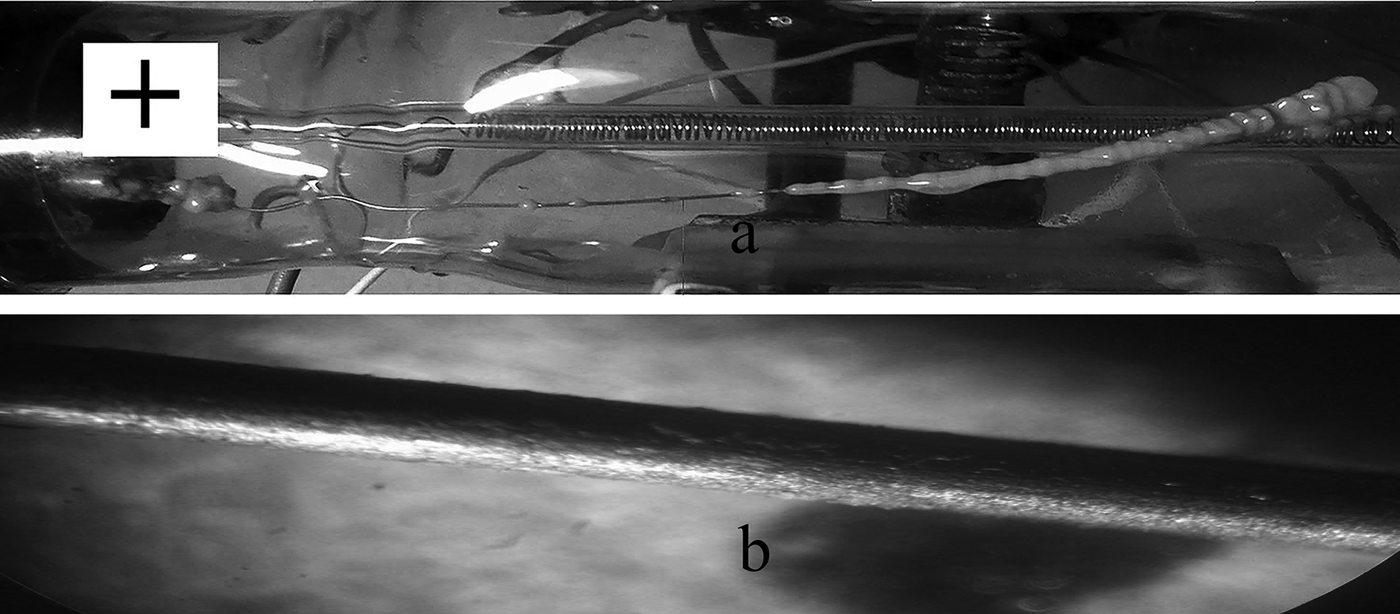
Fig. 8. Images of the heater with sediment deposited after 50 min of boiling of the AlSi-7 NF and burns during operation of the installation using direct (a) and alternating current (b).
At the same time, when the heater is operated with alternating current (AC), the deposits have more or less uniform thickness and are distributed along the entire length of the Ni/Cr wire, although they do not form a continuous coating (Fig. 8b). In this case, the thickness of the sediment is much less. Accordingly, the CHF value at AC is much greater than at constant current (cf. Bondarenko et al., Reference Bondarenko, Moraru, Sydorenko, Komysh and Khovavko2012, Table 2). Nevertheless, the ratio of CHF values for NFs and distilled water remains constant (2.1–3.0). The observed difference in the structure of the deposits on the heater surface when operating at a constant current and using AC is explained by the superposition of the electrophoresis and electrocoagulation of the NPs during boiling of NFs as a result of the direct action of the electric field on the suspension, which is stronger in a constant field.
Emergency cooling of overheated heat-transfer surfaces
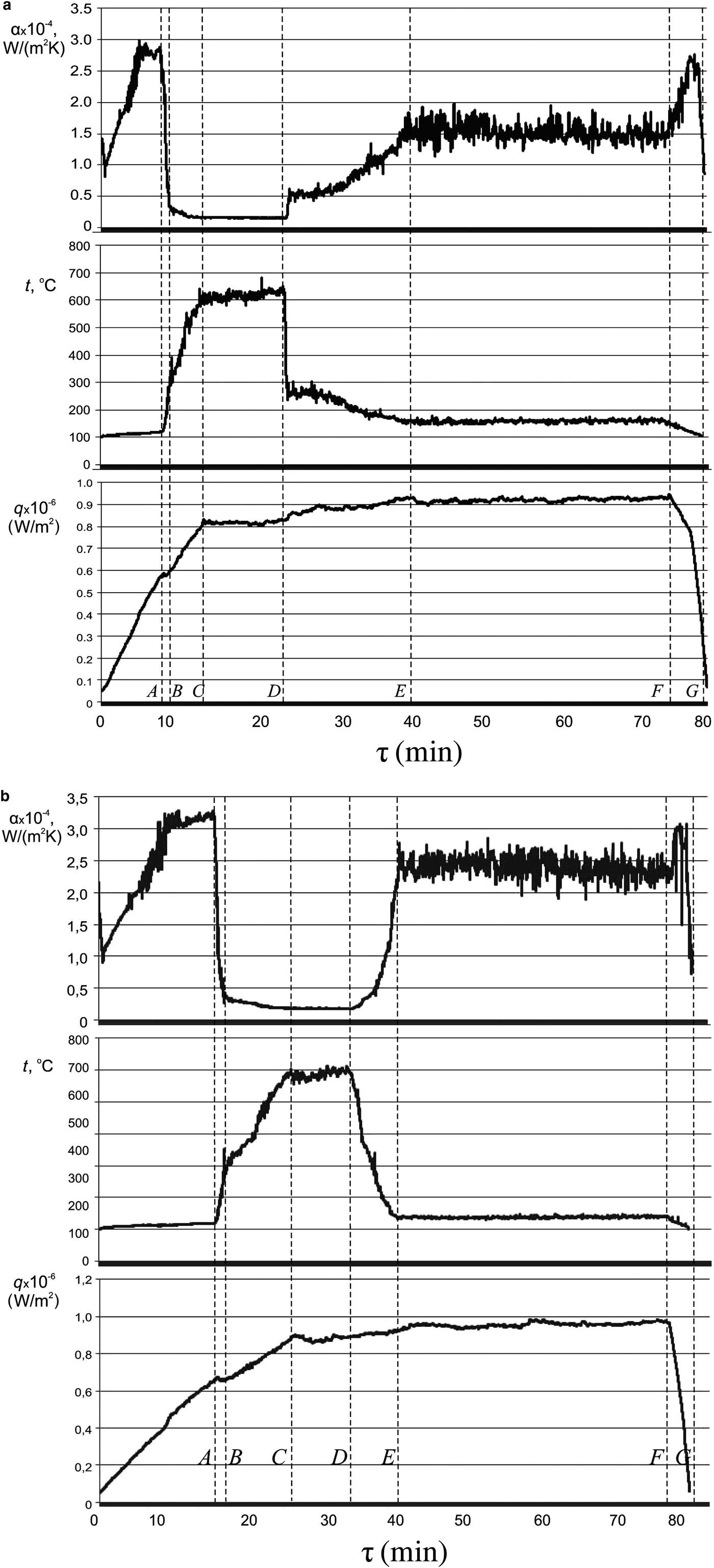
Unlike previous studies (Pham et al., Reference Pham, Kim, Lee and Chang2012; Bhogare & Kothawale, Reference Bhogare Rahul and Kothawale2013, Reference Bhogare and Kothawale2014; Chougule & Sahu, Reference Chougule and Sahu2014; De Risi et al., Reference De Risi, Milanese, Colangelo and Laforgia2014), distilled water was a heat-transfer medium and nanofluid additives were used as coolants. In order to clarify the possibility of emergency cooling of the superheated equipment surface in an emergency situation, the state of the film boiling regime developed was chosen to introduce a portion of the cooling NF. This crisis state corresponds to the section C–D on the curves of synchronous recordings of the thermal boiling parameters in time and is characterized by the maximum temperature of the heating surface (t = 600–700 °C) and the minimum heat transfer (αmin) at q > q cr (Fig. 9a,b). Comparative analysis of the synchronous recordings of the main parameters of heat exchange in time during the boiling of distilled water and the subsequent introduction of cooling NFs (Fig. 9a,b) made it possible to identify all parameters and heat-exchange regimes (Bondarenko et al., Reference Bondarenko, Moraru, Sydorenko and Komysh2016a). Obviously, the way to avoid the boiling crisis (section D–E) is to add a small portion (a fifth) of the NF to the total working volume of the coolant. Depending on the nature and concentration of NPs, particles contained in NFs, as well as on their shape and anisometry, a partial or total output of the heat exchange surface occurs on the pre-crisis temperature regime. When comparing the curves for the temperature change of the heater (t = f(τ)) and the heat-transfer coefficient (α = f(τ)) after the introduction of the NFs AlSi-7 and NF-8 (TiO2) (Fig. 9a,b), it follows that the cooling of the superheated heat-exchange surface is quicker and more efficient in the case of AlSi-7.
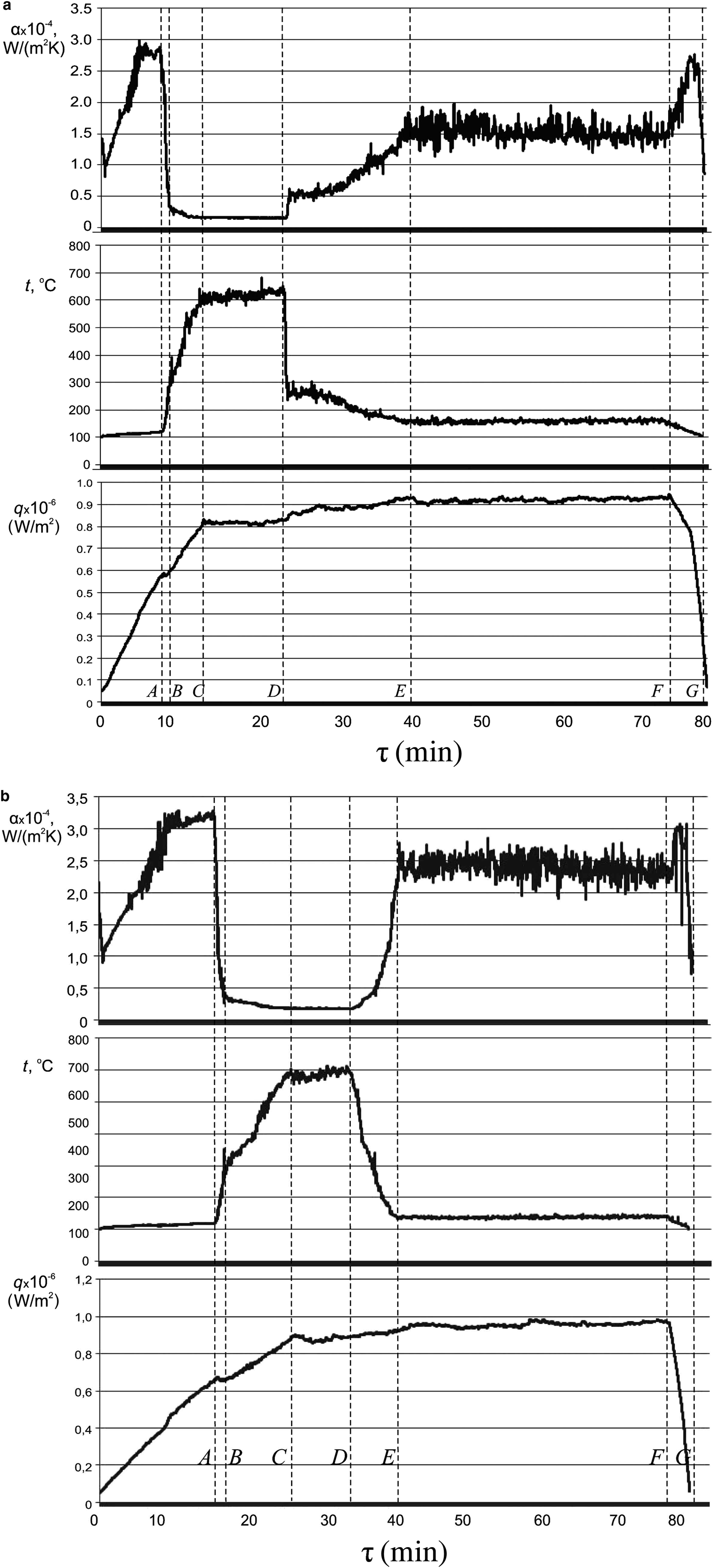
Fig. 9. Synchronous recording of the change in the main heat-exchange parameters (q, t h and α) in time (τ) at the boiling of distilled water, followed by the addition of the NFs NF-8 (a) and AlSi-7 (b) at point D.
Most importantly, the introduction of a hot NF to a boiling coolant in a state of crisis leads to a sharp decrease in the temperature of the heating surface and a return to a stationary and safe bubble-boiling regime with an SHF value q > q crH2O. Here it is important that this boiling mode (section D–F in Fig. 9) is preserved for an arbitrarily long time at q > q cr and T = 125–130°С, which prevents equipment failure immediately. For emergency cooling, instead of introducing an equivalent amount of distilled water into the reactor, after a slight decrease in the temperature of the heating surface, the system returns immediately to film mode, i.e. to the boiling crisis. This indicates that an emergency exit from the crisis after the introduction of the NF is associated with the instantaneous deposition of NPs on the heating surface and with the formation of a porous layer that intensifies heat transfer sharply.
It follows that, depending on the type of NF added, when returning to the bubble-boiling regime, the temperature of the heating surface may decrease to a different extent. This is explained by the different ‘nanoarchitectures’ that appear on the surface of the heating element during the boiling of different NFs (Bondarenko et al., Reference Bondarenko, Moraru, Sydorenko, Komysh and Khovavko2012, Reference Bondarenko, Moraru, Ilyenko, Khovavko, Komysh, Panov, Sydorenko and Snigur2013, Reference Bondarenko, Moraru, Sydorenko and Komysh2016a,Reference Bondarenko, Moraru, Sydorenko, Komysh and Khovavkob). The microrelief, porosity and heat-exchange specific surface area may vary widely, depending on the dispersity, shape and anisometry of the particles deposited (Bondarenko et al., Reference Bondarenko, Moraru, Sydorenko and Komysh2016a,Reference Bondarenko, Moraru, Sydorenko, Komysh and Khovavkob). Obviously, different degrees of increase in heat transfer and cooling efficiency when using different nanofluids are related to these factors.
The advantage of the clay NFs developed is that they allow an immediate transition from a crisis water-boiling mode (with a heat-transfer surface temperature of >600°C) to a normal safe bubble boiling regime with a wall temperature of <150°C without reducing the specific heat load. This opens up a new application area in heat-exchange technology – emergency cooling of overheated equipment surfaces. Moreover, the reduction in the temperature of the heater occurs even at a residual concentration of NPs in a liquid of the order of 0.1% by mass.
Another important advantage of clay NFs is that the porous layer deposited on their boiling surface on the heat-exchange surface has a gel-like mobile structure, so that, if regeneration is necessary, it can be removed easily from the surface by a strong flow of the base fluid. With a similar reversibility of the precipitate, the clay NFs display better behaviour than most oxide, carbide or other types of NFs that form hard deposits on the boiling surface.
SUMMARY AND CONCLUSIONS
Aqueous NFs were developed for power engineering, based on Ukrainian illite, montmorillonite, palygorskite and a mixture of the latter two clays, using colloid-chemistry methods and ultrasonic dispersion. The NFs based on natural clays have excellent colloidal stability and stability under multiple boiling-cooling cycles, due to the high hydrophilicity of nanoparticles; as a rule, they do not need a stabilizer.
A strong influence of the shape and anisotropy of NPs on the heat-transfer parameters of NFs was detected. The NFs at pool boiling increase the CHF and HTC in comparison with the base fluid (water) by more than a factor of two. The maximum values of CHF and HTC were shown by NFs, which consist of NP mixtures with various shapes and high anisometry and which form, at the boiling surface, delicate nanostructures with the greatest specific surface area, porosity and roughness. High heat transfer is probably due to the maximum density of vapourization centres and the area of heat-exchange surface formed in these nanostructures. The preliminary deposition of a NP layer on the heater surface increases significantly the value of the SHF and HTC at the boiling of distilled water, greatly reducing the onset of the boiling crisis. This suggests that the increase in the heat transfer is associated with the deposition of the porous layer upon boiling, which generates bubble formation. The rapid bubbling of the latter increases sharply the heat transfer due to convection, even for weakly heat-conducting NFs.
The nanofluids are very efficient coolants for emergency cooling of superheated heat-exchange surfaces and are suitable for use in heat-power engineering. The advantages of the NFs developed are the high cooling efficiency and stability to multiple boiling-cooling cycles, as well as the low cost and availability of the natural raw materials for their production.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1180/clm.2018.17.