With almost 250 million antibiotic prescriptions issued annually, outpatient antimicrobial prescribing accounts for the majority of antibiotic use within the United States. 1 Unfortunately, it is estimated that 30%–50% of these antibiotics are prescribed unnecessarily or inappropriately, meaning that even when antibiotics are needed, they are not optimized by drug chosen, dose, or duration of therapy. Reference Fleming-Dutra, Hersch and Shapiro2 The goal of the US National Action Plan was to reduce inappropriate antibiotic use by 50% in the outpatient setting between 2015 and 2020, but prescribing trends have remained relatively stagnant as of 2018. 3,Reference Durkin, Jafarzadeh and Hsueh4
Antimicrobial stewardship programs (ASPs) consisting of coordinated efforts to improve antimicrobial prescribing have consistently demonstrated improved antibiotic utilization and patient outcomes. Reference Barlam, Cosgrove and Abbo5 Although most of these programs have traditionally focused on inpatient prescribing, the Centers for Disease Control and Prevention (CDC) has recently developed guidelines for implementing outpatient ASPs, noting the critical importance of establishing these programs where most antibiotics are prescribed. More than 35% of oral antibiotics are prescribed by primary-care physicians. 1 These guidelines provide a framework for ASP implementation, including establishing program leadership, provider education, and interventions such as prescription audit with feedback. Reference Sanchez, Fleming-Dutra, Roberts and Hicks6
Despite guidance from the CDC to establish outpatient ASPs, literature examining successful ASP interventions within primary care sites is limited; most studies target upper respiratory tract infections (URIs). Reference Blanchette, Gauthier and Heil7–Reference Gerber, Prasad and Fiks12 Furthermore, few efforts have targeted medical residents, an important target for ASPs in establishing good habits for antibiotic prescribing. Reference McCormick, Cardwell and Wheelock13–Reference Van Langen, Dumkow and Axford15 Although infectious diseases (ID) practitioners typically serve as ASP leaders in inpatient settings, this resource may be scarce in the outpatient setting. Ambulatory care pharmacists (AMCPs) working within primary-care practice sites may serve a critical role as stewardship collaborators and leaders in the outpatient setting; they have established relationships with the provider team and skills needed to identify problematic trends in antimicrobial use and to provide active intervention. The purpose of this study was to determine whether a multifaceted ASP intervention using education, guideline-distribution, and AMCP-led audit with feedback improved antibiotic prescribing in a family medicine resident clinic for common infections, including URIs, skin and soft-tissue (SSTIs), and urinary tract infections (UTIs).
Methods
Study site and antimicrobial stewardship program
This study was conducted at a single primary-care office in Grand Rapids, Michigan. The practice site serves as an ACGME-accredited physician training and practice site for 30 family medicine resident physicians (10 per class) and 4 attending physicians. The office is also staffed with 1.0 full-time equivalent (FTE) split between 2 AMCPs. They spend ~0.5 FTE devoted to chronic disease state management and resident teaching within the primary-care office and the remaining 0.5 FTE teaching and precepting at the college of pharmacy. Beginning in October 2018, one of the AMCP’s time was restructured to devote 0.1 FTE (1 day every other week) to implementing antimicrobial audit and feedback within the family medicine resident clinic (FMRC). Two second-year medical residents served as physician champions to foster support and collaboration with the AMCP and to work with the prescriber team to assess preferred methods of feedback communication.
The FMRC offers comprehensive care for both adults and children and is affiliated with Mercy Health Saint Mary’s Hospital. Mercy Health Physicians Partners has 22 primary-care locations within the Grand Rapids metropolitan area; however, only 4 offices currently have an AMCP. The primary-care offices are supported by the health system’s ASP, which is directed by an ID pharmacist (1.0 FTE) and supported by an ID physician (0.1 FTE). The stewardship program publishes an outpatient antibiogram annually as well as empiric therapy guidelines that provide treatment recommendations for antimicrobial therapy based on national guidelines, current best practices, and local susceptibility patterns. Prior to the initiation of the intervention in the FMRC, the ASP team had piloted an audit-and-feedback program within a single, nonteaching, family medicine clinic staffed with a full-time AMCP (1 FTE). Reference Burns, Johnson and Pham16 The FMRC intervention was modeled after this pilot-site program that had targeted both URI and UTI prescribing. The ID pharmacist and physician mentored the AMCP to provide baseline education regarding local treatment recommendations and a model for provider feedback.
Study design
This investigation was a retrospective quasi-experimental study conducted to evaluate the outcomes of a multifaceted outpatient ASP intervention including education, guideline dissemination, and pharmacist-led audit and feedback. Two time periods were designated for comparison. Antibiotic prescriptions issued between November 1, 2017, and April 31, 2018, were eligible for inclusion in the preintervention period (pre-ASP group), while the postintervention period included all antibiotic prescriptions issued between October 1, 2018, and March 30, 2019 (ASP group). Prescribing data were generated electronically from the Athenahealth electronic health record (EHR) system. The EHR required each antibiotic prescription to be linked to a diagnosis code, and categorization of the antibiotics was consistent through the entire study period.
Baseline prescribing habits
To identify baseline prescribing practices, a report was generated from the EHR of all antibiotics prescribed during the 6-month preintervention period. This report was reviewed by the AMCP, and antibiotics were assigned to one of several indication categories, based on the diagnosis code, to determine the 3 most common indications for which the providers prescribed antibiotics. The 3 most common indications identified were URIs, SSTIs, and UTIs. Using this same data set and the Excel random-number generator (Microsoft, Redmond, WA), the AMCP performed an audit of a random sampling of 30 prescriptions from these 3 indications to determine baseline guideline-concordant prescribing, educational needs, and targets for improved prescribing during the intervention period.
Provider and staff education
The ID pharmacist, AMCP, and ID physician provided a live education session to all resident clinic providers prior to the intervention period. The session reviewed baseline prescribing data, including areas for improvement, local resistance trends, and a review of the institutional guidelines. This presentation also emphasized the importance of not prescribing antibiotics for viral infections based on symptom persistence and severity. Providers were given pocket cards of the institutional guidelines for URIs, SSTIs, and UTIs as a quick, abbreviated reference tool (Fig. 1).
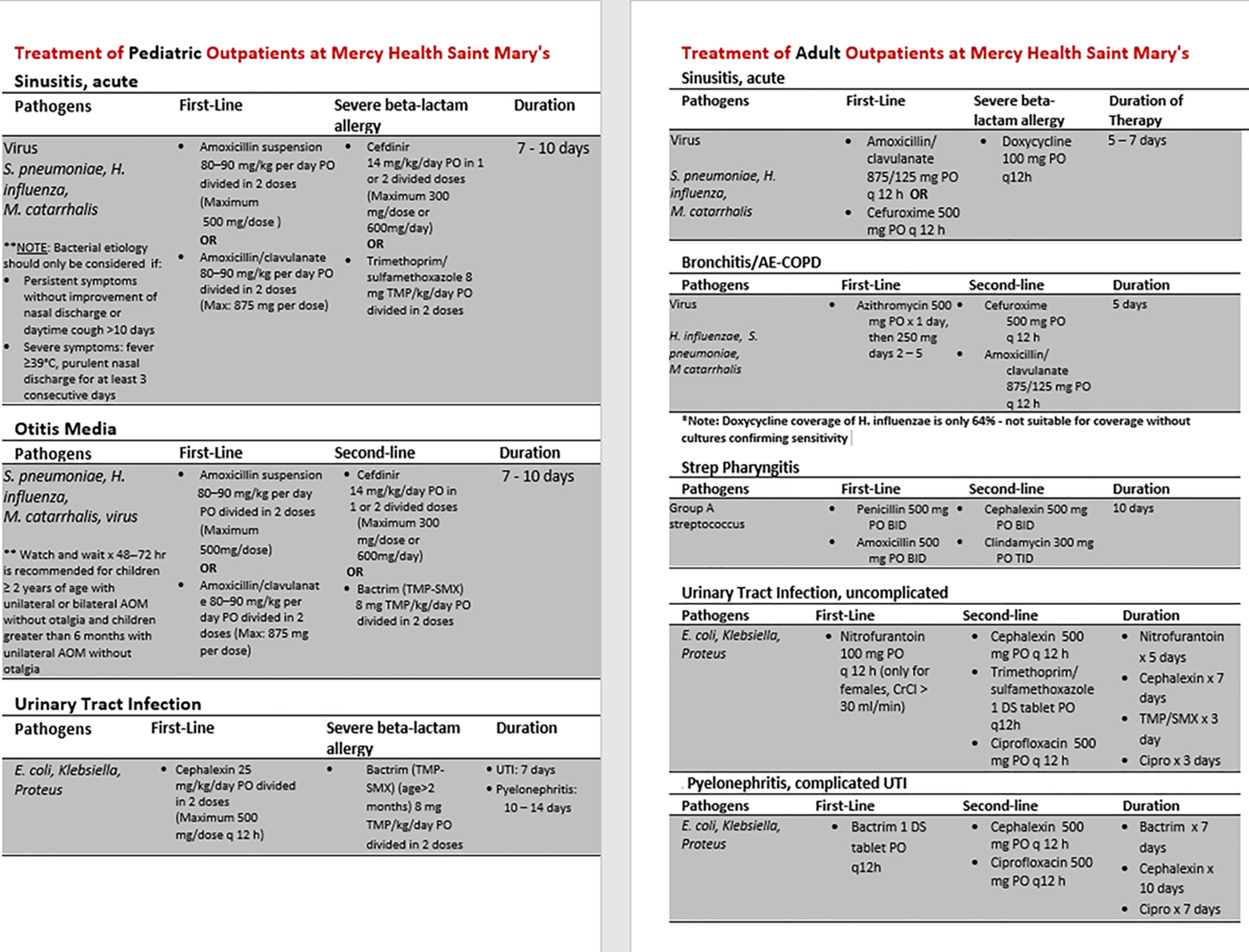
Fig. 1. Example pocket reference cards. Note. MRSA, methicillin-resistant Staphylococcus aureus; UTI, urinary tract infection; AE-COPD, acute exacerbation of chronic obstructive pulmonary disease; TMP, trimethoprim.
Audit and feedback of prescribing practices
The AMCP was responsible for every-other-week audit and feedback for all prescribed antibiotics for URIs, SSTIs, and UTIs, beginning October 1, 2018, and continuing for 6 months through March 31, 2019. The AMCP accessed the EHR antibiotic report and performed indication categorization in the same way as the baseline data. Prescriptions for both adult and pediatric patients were eligible. Feedback was provided electronically through an EHR messaging system that was not part of the patient record. Feedback included recommendations regarding treatment indication and antibiotic drug selection, dose (including both strength and frequency of administration), and duration of therapy based on the institutional guidelines. Providers were also given positive feedback for other habits such as utilizing watch-and-wait prior to prescribing an antibiotic or prescribing a topical dosage form over an oral antibiotic when indicated. Feedback was not provided in any quantitative way or with peer comparison. The electronic message condensed feedback from all antibiotics prescribed during a 2-week window and utilized language that was supportive and encouraging and referenced the particular case using a patient medical record number (MRN). An example of written feedback is provided in Figure 2.

Fig. 2. Example of provider feedback. Note. ID, infectious disease; FM, family medicine; MRN, medical record number; UTI, urinary tract infection.
Study end points
The primary end point of the study was total guideline-concordant antibiotic prescribing. Guideline concordance was determined by the health system’s local outpatient ASP guidelines. The prescription regimen was considered guideline concordant if all of the following criteria were met: treatment was indicated and the drug choice, dose, and duration of therapy were each appropriate. Treatment was indicated for UTI only if the patient was symptomatic or was pregnant with bacteriuria regardless of symptoms. Definitions of treatment indicated for both URIs and SSTIs followed national guideline recommendations and were outlined in the institution’s guidelines and summarized on pocket cards utilized for provider baseline education. Antibiotics prescribed for URI where a likely viral diagnosis was considered or noted (eg, bronchitis, viral URI) were considered not indicated. Antibiotics prescribed to patients not meeting the CDC diagnostic criteria for bacterial sinusitis were also considered not indicated as well. Antibiotics prescribed for patients with SSTI with noted well-drained abscesses without surrounding cellulitis or documented “infection unlikely” were considered not indicated.
Statistical analysis
All statistical analyses were performed using SPPS version 22 software (IBM, Armonk, NY). Descriptive statistics were used to present demographic information. Categorical variables are presented as numbers and percentages. To compare the primary end point of total guideline-concordant antibiotic prescribing between the pre-ASP and post-ASP groups, the χ2 test was performed. Secondary end points collected as categorical data were analyzed using the χ2 test or the Fisher exact test as appropriate. The Student t test was performed to compare interval data, including duration of therapy prescribed in the pre-ASP and ASP groups.
Results
Patient and prescribing demographics
In total, 1,397 antibiotic prescriptions were issued over the 12-month study period (Fig. 3). Patient demographics were similar between groups including sex (pre-ASP 75.6% female vs ASP 73.3% female; P = .663) and average age (pre-ASP 40.8 years ± 20.1 vs ASP 39.8 years ± 23.1; P = .719). Most antibiotic prescriptions (64%) were ordered by third-year medical residents, followed by attending physicians (15%), second-year medical residents (13%), and first-year medical residents (8%).

Fig. 3. Study flowchart. Note. ASP, antimicrobial stewardship program; URI, upper respiratory infection; UTI, urinary tract infection; SSTI, skin and soft-tissue infection.
Baseline prescribing characteristics
In total, 698 antibiotic prescriptions were issued during the 6-month pre-ASP period. The most common indications were URIs (24%), SSTIs (20%), and UTIs (19%). A sample of prescriptions from a randomly generated list of each indication was assessed by the AMCP to establish guideline-concordant antibiotic prescribing at baseline (URIs, n = 30; SSTIs, n = 30; UTIs, n = 30). Total guideline-concordant antibiotic prescribing at baseline was 38.9% for URIs, SSTIs, and UTIs. Of the 30 prescriptions evaluated for URIs, 16 (53.3%) met the criteria for total guideline-concordant prescribing: indicated, 90%; appropriate drug, 76.7%; appropriate dose, 73.3%; and appropriate duration, 80%. The median duration of therapy was 7 days (interquartile range [IQR], 5–10). Of the 30 prescriptions evaluated for SSTI, 5 (16.7%) met the criteria for total guideline-concordant prescribing: indicated, 86.7%; appropriate drug, 56.7%; appropriate dose, 63.3%; and appropriate duration, 73.3%. The median duration of therapy for SSTI was 7 days (IQR, 7–10). Of the 30 prescriptions audited at baseline for UTI, 14 (46.7%) met the criteria for total guideline-concordant prescribing: appropriate drug, 73.3%; appropriate dose, 93.3%; appropriate duration, 66.7%. The median duration of therapy was 7 days (IQR, 5–7). One patient (3.3%) had no recorded urinary symptoms and therefore did not meet the criteria for UTI treatment.
Postintervention guideline-concordant prescribing
During the 6-month intervention period, 699 antibiotic prescriptions were issued with 435 prescriptions eligible for audit and feedback by the AMCP: URI, 183; SSTI, 125; and UTI, 127. Total guideline-concordant prescribing improved to 57.9% (P = .002). Additionally, significant improvement was observed across the total population in guideline-concordant antibiotic selection (pre-ASP 68.9% vs ASP 80.2%, P = .018), dose (pre-ASP 76.7% vs ASP 86.2%; P = .023), and duration of therapy (pre-ASP 73.3% vs ASP 86.2%; P = .02) (Fig. 4). Changes in guideline-concordant prescribing for individual disease states are shown in Figure 5. There were no significant differences in guideline-concordant prescribing based on provider type. Data were also analyzed by monthly intervals during the 6-month intervention period, demonstrating significant improvement in total guideline-concordant prescribing over time (P = .001) (Fig. 6).

Fig. 4. Appropriateness of all antibiotics. Note. ASP, antimicrobial stewardship program.

Fig. 5. Appropriateness of antibiotics by infection type. Note. ASP, antimicrobial stewardship program; URI, upper respiratory infection; UTI, urinary tract infection; SSTI, skin and soft-tissue infection. Indication: Whether or not antibiotic treatment was indicated. Dose: strength and frequency of antibiotic administration. Regimen: total guideline-concordance (indication, drug, dose, and duration are all appropriate).

Fig. 6. Percentage of total guideline-concordant regimen prescribing by month. Indication: Whether or not antibiotic treatment was indicated. Dose: strength and frequency of antibiotic administration. Regimen: total guideline-concordance (indication, drug, dose, and duration are all appropriate).
Changes in antibiotic agent prescribed
Significant changes in the agents prescribed were observed across all 3 infection types. For URI indications, amoxicillin moved to the primary agent prescribed (pre-ASP 25.9% vs ASP 41.8%), and azithromycin prescribing decreased from 25.9% to 18.5% (P = .044). For SSTI indications, cephalexin moved to the primary agent prescribed between groups (pre-ASP 10% vs ASP 31.2%), and the use of sulfamethoxazole/trimethoprim (30% vs 19.2%) and clindamycin (10% vs 1.6%) decreased (P = .01). The use of ciprofloxacin for UTI decreased between study groups (pre-ASP 16.7% vs ASP 3.1%) as well as sulfamethoxazole/trimethoprim (pre-ASP 23.3% vs ASP 14.2%), while nitrofurantoin use increased from 33.3% to 53.5% (P = .020). The median duration of therapy for URI was similar to baseline at 7 days (IQR, 5–7 days, P = .372), while median duration of therapy for UTI decreased to 5 days (IQR, 5–7; P = .098). Prescribed duration of therapy was significantly shorter in the ASP group for SSTI (median, 7 days; IQR, 5–7 days; P = .008).
Discussion
Our findings demonstrate significant improvement in guideline-concordant antibiotic prescribing for the treatment of URIs, SSTIs, and UTIs following the implementation of an AMCP-led, multifaceted, ASP intervention within an FMRC. This study also adds to the growing body of evidence showing the importance of ASP interventions involving physician residents to establish good prescribing habits early in practice. Reference Van Langen, Dumkow and Axford15,Reference Davey and Garner17,18 Furthermore, these results support the finding that syndrome-specific interventions are successful in improving antibiotic prescribing in the outpatient setting. Reference Pollack and Srinivasan19 Previous studies evaluating multifaceted approaches to implementing ASPs in outpatient settings have also demonstrated success when targeting syndrome-specific prescribing by combining educational interventions with EHR optimization and peer-to-peer comparison. Reference Buehrle, Shively and Wagener20–Reference Peterson, Fergus and Yost23
As of January 2020, The Joint Commission requires all accredited ambulatory health organizations to implement ASPs. 24 Our study findings demonstrate that the new elements of performance are measurable and achievable when given adequate resources. Marcelin et al Reference Marcelin, Chung and Van Schooneveld25 proposed framework for outpatient stewardship, called C-DIFF (ie, collaboration/communication, data, interventions, feedback, and follow-up) highlights the significant role of pharmacists in outpatient stewardship and provides a process that outpatient stewardship teams can utilize to implement a successful ASP. A study by Craddock et al 9 demonstrated the impact of pharmacist-led, education-only, interventions that targeted both prescribers and patients with viral acute URI in the ambulatory care setting. These researchers found a significant reduction in inappropriate antibiotic prescribing after implementation of the intervention (17.2% vs 13.1%; P = .02). Although their stewardship team included AMCPs and trainees, the interventions were led by an ID-trained pharmacist. Wattengel et al Reference Wattengel, Sellick and Mergenhagen10 also found that an ID pharmacist performing off-site postprescription audit and feedback in the outpatient setting reduced 30-day treatment failure (5% vs 28%; P < .001) and readmission rates (07% vs 11%; P = .001) when pharmacist interventions were accepted. Reference Wattengel, Sellick and Mergenhagen10 Our study furthers the evidence that a clinical pharmacist can serve as an ASP leader in the outpatient setting. Importantly, our intervention was led by an AMCP working directly with the primary-care medical staff. Although the institution’s antimicrobial stewardship pharmacist and physician collaborated with the AMCP to develop the outpatient program plan, assess baseline data, provide live education, and be available for questions, the biweekly feedback intervention relied solely on the AMCP. Only 1 other study has been published that highlights the impact of a non–ID-trained AMCP in antibiotic stewardship. Our organization previously piloted a similar audit-and-feedback intervention within a single family medicine primary care office led by the site’s AMCP with support of the local stewardship team. Reference Burns, Johnson and Pham16 Similar to our findings, an improvement in prescribed guideline-appropriate agent and duration of therapy were seen for UTIs and URIs. We chose to build on this intervention within the FMRC by further including SSTI evaluation as well as evaluating appropriate indication and dosing for all disease states.
This study has several limitations. Ambulatory care practice sites will require a certain amount of resources to meet these updated standards, including a leader with adequate time that can be dedicated to ASP activities. These activities include initial provider education, audit-and-feedback interventions, and data analysis. Although this study included a pharmacist with that protected time and the opportunity to audit and provide feedback for every antibiotic prescribed within a 6-month period, it may be unrealistic to expect that other practice sites can perform these activities to the same level. We recommend that an AMCP partner with the local antimicrobial stewardship team to evaluate opportunities to optimize the level of outpatient antimicrobial stewardship that is manageable for the institution’s available resources. We did not provide feedback with a quantitative score or peer comparison because we felt that this approach would not be the most conducive to the teaching and learning style or culture of the FMRC. Our study demonstrated that an AMCP can still significantly impact stewardship outcomes without quantitative feedback. The current study included typical limitations of a retrospective study; we relied on appropriate documentation of patient assessment and plan in the EHR to determine appropriateness during audit. We were unable to capture when providers utilized a watch-and-wait strategy throughout this study because cases eligible for audit and feedback were identified via a report of antibiotics prescribed and not a report of diagnoses during the study periods. Thus, we may have underestimated the impact of the intervention. Finally, we did not determine whether there were sustained outcomes (ie, a 6-month postintervention audit); however, ongoing education will always be needed with new residents entering the FMRC annually.
Despite these limitations, this study provides important evidence demonstrating the impact of AMCPs as antimicrobial stewardship leaders in primary care. Further studies are needed to determine whether alternate methods of audit and feedback can decrease workload and still achieve success with guideline-concordance prescribing habits. Further studies could also evaluate methods of real-time recommendations by an AMCP or EHR order sets developed from the institution’s guidelines that are maintained and updated by an AMCP.
In summary, AMCP-led audit-and-feedback within a FMRC significantly improved guideline-concordant antibiotic prescribing for common infection types. AMCPs can serve as key leaders in implementing successful outpatient antimicrobial stewardship interventions. As resources for outpatient efforts are often scarce, ASPs looking to expand to outpatient settings should evaluate opportunities to collaborate with AMCPs to implement sustainable interventions promoting optimal prescribing.
Acknowledgments
1) Lindsey Westerhof was affiliated with Ferris State University College of Pharmacy in Grand Rapids, MI during the time of study protocol development and data collection. Data analysis, presentation and manuscript preparation were completed while affiliated with Mercy Health Saint Mary’s.
2) We’d like to thank the Wege Family Medicine Residency Clinic for graciously collaborating on this quality improvement project.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.









