Introduction
For a long time, the most important means by which swine herds were becoming infected with pathogens was considered to be the introduction of subclinically infected pigs. Thus, the chain of transmission was believed to be primarily through direct contact. Over the years, despite considerable effort to introduce only animals that came from herds free of the various pathogens of importance, refinement in diagnostic tests available to detect the presence of these micro-organisms, the use of quarantine barns and adhesion to strict biosecurity protocols, herds continued to become infected with significant pathogens, sometimes at a rate that made these efforts look almost questionable.
The evidence that gradually increased over the years is that there appeared to be two main types of pathogens as far as their epidemiology is concerned. First, there are those which under field conditions seem to be transmitted mainly by direct contact, which means that animals subclinically infected with a given pathogen are unknowingly introduced into a previously uninfected herd. The second type is pathogens that frequently find their way into uninfected herds by means other than direct introduction of infected animals. This could include means such as people; contaminated fomites such as boots, clothes, material or equipment; transport vehicles; semen and aerosol. This review will discuss three pathogens that can be considered as being of the first type, namely Sarcoptes scabiei, toxigenic Pasteurella multocida and Brachyspira hyodysenteriae, and three that belong to the second type, foot and mouth disease virus, Mycoplasma hyopneumoniae and porcine reproductive and respiratory syndrome (PRRS) virus.
Pathogens mainly transmitted by direct contact
S. scabiei
The mange mite S. scabiei var suis is present in almost all countries of the world where swine are raised and is considered the most important ectoparasite of that animal species. It does not, however, have a lot of attributes that would make it difficult to prevent. Clinical evidence of infection could not be demonstrated when non-infested pigs were exposed on repeated occasions to contaminated bedding vacated 3 days previously (Cargill and Dobson, Reference Cargill and Dobson1977). This was supported by laboratory experiments conducted by the same authors, which showed that mites did not survive 96 h at temperatures of less than 25°C, longer than 24 h from 25 to 30°C, and less than 1 h at temperature above 30°C. Other species do not appear to play a role in porcine scabies and transmission from one herd to another usually occurs when pigs with subclinical infestations are introduced in a herd (Cargill and Davies, Reference Cargill, Davies, Straw, Zimmerman, D'Allaire and Taylor2006).
Sarcoptic mange was widely distributed in North American herds 10 to 20 years ago. In a study conducted in Quebec in 1989 (Caissie et al., Reference Caissie, Klopfenstein, D'Allaire, Bigras-Poulin, Villeneuve and Piché1992), the authors reported that 67% of the 61 finishing units tested were positive for S. scabiei. In a US study conducted in 50 US herds, 56% were found to have mite infestations (Davies et al., Reference Davies, Bahnson, Grass, Marsh, Garcia, Melançon and Dial1996). Today a majority of the herds are free of that parasite and this is not only due to the fact that eradication programs have made it relatively easy to get rid of the arthropod (Smets et al., Reference Smets, Neirynck and Vercruysse1999; Jacobson et al., Reference Jacobson, Bornstein, Palmér and Wallgren2000), but also because once negative, it has not been difficult to maintain herds that way. This is true even for herds which are located in hog dense areas, as long as a simple biosecurity program is followed and that introduced animals are not infested with S. scabiei.
Toxigenic P. multocida
Progressive atrophic rhinitis (PAR) is associated with toxigenic strains of P. multocida. It does have more potential than S. scabiei to be transmitted indirectly as it has been identified in other animal species such as calves, cats, dogs, rabbits and turkeys (Nielsen et al., Reference Nielsen, Bisgaard and Pedersen1986), goats and rats (Frandsen et al., Reference Frandsen, Foged and Petersen1990) and sheep (Frymus et al., Reference Frymus, Leśniewski and Żurawski1996). Toxigenic strains of P. multocida have also been found in people, and Danish researchers (Nielsen and Frederiksen, Reference Nielsen and Frederiksen1990) were able to produce atrophic rhinitis in pigs using a strain that came from the blood of a human patient. So people could seemingly serve as a source of infection as well. Furthermore, toxigenic P. multocida was isolated from the air of pig barns (Baekbo and Nielsen, Reference Baekbo and Nielsen1988), and viability of the organism in the air was still at 8% after 45 min (Thomson et al., Reference Thomson, Chanter and Wathes1992). This suggests that aerosol transmission would have the potential to play a role in the transmission of toxigenic strains of P. multocida. Finally, the organism could be detected for more than 49 days in nasal lavages kept at 4°C, suggesting that contaminated fomites would also have to be taken into consideration in the epidemiology of atrophic rhinitis (Thomson et al., Reference Thomson, Chanter and Wathes1992).
PAR was very common in the 1970s and 1980s. In a US study atrophic rhinitis was diagnosed in 27% of necropsies in both 1984 and 1986 (Turk et al., Reference Turk, Turk, Fales, Kintner, Nelson, Shaw, Brown and Morehouse1989). Nevertheless, while it is still possible to see pigs affected with this condition nowadays, it is becoming a rare event. As for S. scabiei, once a herd is negative to that organism it does not appear to be a problem to maintain freedom from infection. In a study conducted in the UK, Goodwin and Whittlestone (Reference Goodwin and Whittlestone1983) reported the results of a control scheme for atrophic rhinitis that was initiated in 1977. During the first 5 years 45 herds qualified at some stage and at the end of 1982, 34 herds, comprising about 7200 sows, were still listed. During these years, atrophic rhinitis had not appeared within the herds of the scheme or in herds established entirely from listed herds, despite the fact that 31 of the 45 qualifying herds had imported stock from 15 other qualifying herds. As for S. scabiei, introducing only negative animals in non-infected herds is usually enough to remain non-infected.
B. hyodysenteriae
Swine dysentery (SD) is caused by B. hyodysenteriae. As for toxigenic strains of P. multocida, B. hyodysenteriae does have some attributes that should make it a potential threat for indirect transmission between farms. It can survive 112 days in pig feces (Boye et al., Reference Boye, Baloda, Leser and Møller2001); has been found in feral pigs (Philips et al., Reference Philips, La, Adams, Harland, Fenwick and Hampson2009), laying chickens (Feberwee et al., Reference Feberwee, Hampson, Philips, La, van der heijden, Wellenberg, Dwars and Landman2008), mallards (Jansson et al., Reference Jansson, Johansson, Olofsson, Råsbäck, Vågsholm, Petterson, Gunnarsson and Fellström2004), rheas (Jensen et al., Reference Jensen, Stanton and Swayne1996), seagulls (Hampson et al., Reference Hampson, Fellström, Thomson, Straw, Zimmerman, D'Allaire and Taylor2006), mice (Fellström et al., Reference Fellström, Landén, Karlsson, Gunnarsson and Holmgren2004), rats (Hampson et al., Reference Hampson, Combs, Harders, Connaughton and Fahy1991) and dogs (Songer et al., Reference Songer, Glock, Schwartz and Harris1978); and has recently been detected in insects such as cockroaches (Blunt and McOrist, Reference Blunt and McOrist2008) and flies (Gallie et al., Reference Gallie, Chesworth, Blunt and McOrist2009). So, again it appears that there are ample opportunities for the organism to be introduced into swine barns by means other than infected pigs.
As for mange and PAR, SD was a common disease in North American herds in the past, but has become much less of a threat today. In Quebec, where it was one of the most frequent conditions observed in finishing units, it has virtually disappeared. There is a system (RAIZO, Réseau d'alerte et d'information zoosanitaire) in that province that compiles at the end of each year the number of diagnoses of the more relevant diseases that have been made in the various veterinary diagnostic laboratories. In 1997 and 1998, 11 and 12 cases of SD were diagnosed respectively, and this does not include the many cases that may have been diagnosed clinically without submission of samples for laboratory confirmation. Then from 1999 to 2008, no cases of that disease were reported in any of the laboratories (RAIZO annual reports, 1997–2008). The situation is slightly different in the US where the incidence of the disease is clearly much less frequent than it was in the past, but not to the point where it is in Quebec and the rest of Canada (Rovira and Torrison, Reference Rovira and Torrison2009). In 1978, a control scheme for SD was initiated in the UK (Goodwin and Whittlestone, Reference Goodwin and Whittlestone1984). In order to qualify, herds had to be free of clinical signs in the absence of any pharmaceutical compounds that could mask the disease, and laboratory tests had to be negative for the causal organism. During the first 6 years, 91 herds qualified at some stage and at the end of 1983, 56 herds (average size 200 sows) were still listed. During this time, 72 herds had imported stock from 36 other qualifying herds; despite this degree of inter-herd connection, there is no evidence that SD occurred within the scheme since its inception, nor has this disease appeared in herds established from listed herds. These findings suggested to the authors that freedom from SD could be readily maintained in a controlled group of pig herds identified by these monitoring methods.
Reasons for the reduction in prevalence of the selected diseases
The reason for which it has been relatively easy to maintain swine herds negative for sarcoptic mange, PAR and SD is most likely due to the fact that the causal organisms are mainly transmitted between farms by direct contact. Hence, a simple biosecurity program is often all that is needed as long as introduced animals are negative for S. scabiei, toxigenic P. multocida and B. hyodysenteriae. This could explain why the prevalence of these conditions has not increased, but it does not provide a satisfactory explanation as to why it has decreased so much over time.
Other factors have likely contributed to make these conditions less of a problem today than they were in the past. First, most pigs today are raised in confinement buildings that can be washed and disinfected, which was not necessarily the case for pigs that were raised outside. Disinfection strategies have progressed so that effective elimination of pathogens from pig barns and transport vehicles is more likely to occur than in the past. Sources of negative-breeding animals have become readily available, and so previously uninfected herds were having easier access to animals free of many common pathogens. In fact, the availability of high-health animals of superior genetics has incited many to depopulate and repopulate their herds so that not only would they eliminate the drugs and performance costs associated with unhealthy pigs, but they would also benefit from the improved growth performance and carcass characteristics of genetically superior animals. This increased availability of negative animals has forced suppliers of infected animals to either stop selling pigs or breeding stock, or to get rid of these pathogens in their herds. Eradication programs for mange and SD have been proposed and found to be successful. Raising pigs using an ‘all in – all out’ system has allowed producers to introduce pigs into uncontaminated premises, which was not the case in times when continuous flow systems were the norm. Early weaning and medicated early weaning have reduced the likelihood that sows would infect their piglets in the farrowing crates, and three-site as well as multiple-site systems have made it easier to eliminate pathogens from the sow herd, since the nursery and finishing units are not on site, as they were in traditional one-site farrow-to-finish operations. Instead of introducing pigs from many different sources, for example, in nurseries and finishing units, modern raising systems are often limited to one or just a few compatible sources, which obviously reduces the risk of introducing pathogens from the outside. Diagnostic tools and techniques have improved, providing better knowledge of which sources of breeding animals, nursery or finishing pigs are free or not of undesired pathogens. Finally, increasingly complex and scrutinized biosecurity programs have been put in place.
Pathogens often transmitted by indirect means
Foot and mouth disease virus (FMDv)
FMDv is one of the most important pathogens worldwide. Countries into which the virus is introduced usually go through costly efforts to get rid of it, since having the virus within its borders means not only direct losses for the producers within their own herds, but also indirect losses associated with reduced market and export opportunities. Gibbens et al. (Reference Gibbens, Sharpe, Wilesmith, Mansley, Michalopoulou, Ryan and Hudson2001) evaluated the most likely method of spread in 1847 cases during the epidemic that hit UK in 2001. Table 1 shows the results that were obtained.
Table 1. Most likely method of spread of foot-and-mouth disease virus in 1847 premises evaluated during the UK epidemic of 2001
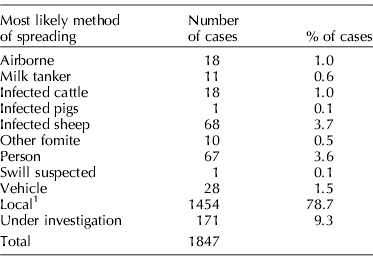
1 Local: new infected premises within 3 km of a previously infected premises and more than one possible conveyor identified.
As shown in Table 1, less than 5% of the cases were thought to have been associated with introduction of infected animals. This means that the vast majority of investigated cases were thought to have been due to indirect transmission. The authors reported that the exact mechanisms of ‘local’ spread were not fully determined, but it was believed that the majority would be associated with local aerosol spread between animals, or contamination in the area near an infected premise, resulting in infected material on roads or other common facilities. Airborne spread by plumes over greater distances was not found to have played a significant role. Two reasons could possibly explain, at least partially, why this was the case. First, there were very few pigs in the area where the epidemic occurred, and it has been shown that pigs are the strongest emitters of aerosols of FMDv (Donaldson and Alexandersen, Reference Donaldson and Alexandersen2002). Secondly, there are large variations in the capacity of various strains to be shed in the air, and adult pigs infected with the strain involved in the 2001 UK epidemic released 300 times less virus in the atmosphere than was observed previously with a different FMDv strain (Gloster et al., Reference Gloster, Champion, Sørensen, Mikkelsen, Ryall, Astrup, Alexandersen and Donaldson2003). Similarly, in a study conducted in The Netherlands by Bouma et al. (Reference Bouma, Dekker and de Jong2004), a strain of the virus was not transmitted by aerosol between calves kept only 1 m apart, or even in direct contact with experimentally infected animals. In the UK epidemic there were nevertheless some cases that were thought to have been associated with aerosol transmission and Gloster et al. (Reference Gloster, Champion, Sørensen, Mikkelsen, Ryall, Astrup, Alexandersen and Donaldson2003) suggested that distances involved ranged from less than 1, to 9 km. Numerous studies have concluded that aerosol transmission of FMDv was possible, and in some epidemics it was believed to be responsible for a majority of the cases (Sellers and Gloster, Reference Sellers and Gloster1980; Sellers et al., Reference Sellers, Garland, Donaldson and Gloster1981). However, one of the most important features as far as FMDv is concerned is the distance over which some strains have been suggested to be transmitted by aerosol. Distances of up to 100 and even 300 km have been reported (Donaldson et al., Reference Donaldson, Gloster, Harvey and Deans1982; Gloster et al., Reference Gloster, Sellers and Donaldson1982; Alexandersen et al., Reference Alexandersen, Kitching, Mansley and Donaldson2003).
M. hyopneumoniae
M. hyopneumoniae is the cause of enzootic pneumonia, a very common disease of swine not only in North America, but throughout the world. This organism does not appear to be particularly well suited for indirect transmission. Evidence of its presence in wild boars (Sibila et al.,in press; Vengust et al., Reference Vengust, Valencak and Bidovec2006) and in feral pigs (Baker et al., Reference Baker, Gramer and Dee2009) has been documented in different countries, but there seems to be no reports in the literature of species other than swine that have become infected with this organism. In four trials conducted by Goodwin (Reference Goodwin1972), negative pigs placed in a room that had contained M. hyopneumoniae-infected and coughing pigs only 5–47 min before remained negative, even though no efforts were made to clean the room in any way or to change clothes between the infected and negative pigs. The author concluded that M. hyopneumoniae was not highly infectious by indirect contact. Friis (Reference Friis1973) reported that M. hyopneumoniae could remain viable for about 4–8 days after being left to dry in air at room temperature, indicating that the organism may not be that fragile in the environment.
The difficulty in maintaining herds negative to M. hyopneumoniae in hog dense areas does not, however, fit well with the idea that it is mainly transmitted by direct contact. Goodwin and Whittlestone (Reference Goodwin and Whittlestone1984) reported many years ago that while control schemes to maintain herds negative to atrophic rhinitis and SD were very successful in the UK, this was not the case for enzootic pneumonia. The authors stated that the original concept of high-health herds had suffered from the extent to which enzootic pneumonia had appeared in them, despite elaborate precautions. In a study conducted in Quebec, Desrosiers (Reference Desrosiers2002) reported that of 37 herds that were populated from Mycoplasma hyopneumoniae-negative sources, 18 became infected over the years. None were infected through direct contact since the multipliers that supplied the replacement animals remained negative. All farms that became infected were 1.5 km or less from infected premises, while all farms that were 2 km or more remained negative. The author suggested that aerosol transmission was likely the main cause for these cases of contamination. Desrosiers (Reference Desrosiers2004, Reference Desrosiers2005) reviewed some of the overwhelming evidence supporting aerosol transmission of this pathogen between farms.
More recently, Dee et al. (Reference Dee, Otake, Oliveira and Deen2009a) showed that during a 3-year study, frequent aerosol transmission of both M. hyopneumoniae and PRRS virus occurred over a distance of 120 m in a small unit not protected by air filtration, while contamination never occurred in air-filtered units located only a few meters away from the non-filtered unit. These results confirmed the possibility that M. hyopneumoniae and PRRS virus were transmitted by aerosol. The next question was to know how far the organism could be transmitted through the air. In a study involving 55 herds that developed enzootic pneumonia without a simple explanation and 57 herds that remained negative, Goodwin (Reference Goodwin1985) suggested that the airborne route was the most probable manner of infection, and that the crucial distance from infected pig barns for maximum survival appeared to be 3.2 km. However, Dee et al. (Reference Dee, Otake and Deen2009b) showed that both M. hyopneumoniae and PRRS virus could be identified in air samples collected at 4.7 km from their source, in the direction of prevailing winds, and recently, not only was this distance increased to 9.1 km for PRRS virus and 9.2 km for M. hyopneumoniae, but both organisms recovered in the air samples were found to be infectious by bioassays (Otake et al., Reference Otake, Dee, Corzo, Oliveira and Deen2010).
PRRS virus
PRRS is the main reason why biosecurity programs have become increasingly sophisticated and scrutinized over the last 10 to 15 years. Since the disease can be so costly and since regular biosecurity programs frequently failed to prevent outbreaks in herds introducing animals known to be PRRS-negative, indirect sources of contamination had to be involved. Table 2 shows the percentage of PRRS cases in various studies that were reported to be associated with indirect transmission of the organism.
Table 2. Percentage of PRRS cases reported to be associated with indirect transmission of the virus in various studies
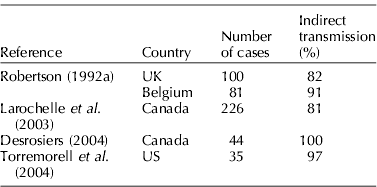
As is evident in Table 2, in the vast majority of cases, disease was spread by some means other than simple introduction of infected animals in the herd. In one study (Desrosiers, Reference Desrosiers2004), 44 PRRS cases were diagnosed in commercial sow herds of a production company during a period of 4 years. During these years, not only did the multiplier herds supplying breeding animals to these commercial herds remain PRRS-negative, confirming that all cases had been associated with indirect transmission, but also semen going to these herds came from boar studs that remained PRRS-negative. This type of situation, where the source of infection could not be easily identified, has led researchers to dig deeper into the various possibilities.
The virus was isolated from water kept at 25–27°C for up to 11 days (Pirtle and Beran, Reference Pirtle and Beran1996), and in swine lagoon effluent kept at 4°C for 8 days (Dee et al., Reference Dee, Martinez and Clanton2005). Viable virus could also be detected in meat kept at 4°C for 7 days and obtained from pigs that had been experimentally infected and euthanized 7 days later (Cano et al., Reference Cano, Murtaugh and Dee2007).
Kim et al. (Reference Kim, Luo, Yang, Moon, Park, Lee, Kim and Park2007) reported the detection of PRRS viral RNA in 9 of 49 complete feed samples from South Korea, but viability of the virus was not determined. Under North American conditions, however, feed is not considered to be a significant source of infection. PRRS virus has been detected in wild boar (Reiner et al., Reference Reiner, Fresen, Bronnert and Willems2009) as well as in feral pig populations (Baker et al., Reference Baker, Gramer and Dee2009), but the prevalence of positive animals is usually low. Wills et al. (Reference Wills, Osorio and Doster2000) found no evidence of PRRS virus replication in cats, dogs, mice, rats, opossums, raccoons, skunks, sparrows and starlings. Following experimental infection, the virus was found to be passed in the feces of Mallard ducks, Guinea fowls and Cornish cross chickens in one study (Zimmerman et al., Reference Zimmerman, Yoon, Pirtle, Wills, Sanderson and McGinley1997), but subsequent researchers could not reproduce these results (Trincado et al., Reference Trincado, Dee, Jacobson, Otake, Rossow and Pijoan2004a). Until proven otherwise, animal species other than pigs are not considered to play an important role in PRRS virus epidemiology. It is clear both from field (Robertson, Reference Robertson1992b) and experimental (Yaeger et al., Reference Yaeger, Prieve, Collins, Christopher-Hennings, Nelson and Benfield1993) data that the virus can be transmitted by semen, and this is a very significant factor that needs to be borne in mind in the control of this condition. It has also been shown that fomites (Otake et al., Reference Otake, Dee, Rossow, Deen, Joo, Molitor and Pijoan2002a), needles (Otake et al., Reference Otake, Dee, Rossow, Deen, Joo, Molitor and Pijoan2002b), personnel (Pitkin et al., Reference Pitkin, Deen and Dee2009a), insects such as mosquitoes (Otake et al., Reference Otake, Dee, Rossow and Pijoan2002c) and flies (Otake et al., Reference Otake, Dee, Rossow, Moon, Trincado and Pijoan2003) and vehicles (Dee et al., Reference Dee, Deen, Rossow, Mahlum, Otake, Joo and Pijoan2002, Reference Dee, Deen, Otake and Pijoan2004) are potential sources of infection. However, biosecurity programs that have tried to properly address all of these different contamination means have frequently not been successful at preventing introduction of the virus in swine barns.
Desrosiers (Reference Desrosiers2004, Reference Desrosiers2005) reviewed some of the field and experimental information suggesting that PRRS virus and some other infectious organisms could be transmitted by aerosol between farms. The possibility for this particular virus to be transmitted in this manner has now been confirmed (Dee et al., Reference Dee, Otake, Oliveira and Deen2009a, Reference Dee, Otake, Oliveira and Deenb; Pitkin et al., Reference Pitkin, Deen and Dee2009b; Otake et al., Reference Otake, Dee, Corzo, Oliveira and Deen2010), and as mentioned above, viable virus has been detected in air samples collected 9.1 km away from their source (Otake et al., Reference Otake, Dee, Corzo, Oliveira and Deen2010). The quantity of viable virus that was detected at that distance was 1 log TCID50, and it could be asked if this is enough to infect a pig by aerosol. In a recent experiment conducted by Cutler et al. (Reference Cutler, Kittawomrat, Hoff, Wang and Zimmerman2009), that quantity of virus was found to be enough to infect pigs by aerosol infection. Taken together, these two studies suggest that aerosol contamination with the PRRS virus could possibly occur over distances of at least 9.1 km, and possibly more. In Denmark, the presence of the virus was first detected in 1992 on an island, and was suspected to have been introduced from Northern Germany by the airborne route (Mortensen and Madsen, Reference Mortensen and Madsen1992). If it is confirmed that this is the way by which the virus was introduced into Denmark, the distance involved would be at least 15 km (S. Mortensen, personal communication, 2002). This distance would have been mainly over water though, which is known to favor greater distances of aerosol transmission. Finally, more recently, a boar stud became infected about a week after the closest pig barn, a sow herd located 28 km away, broke with PRRS. The strains from both farms were compared and, based on the sequence of open reading frame 5, were considered to be the same strain. The direction of the wind between the two incidents as well as the epidemiological investigation that was conducted to determine whether other causes could have been involved suggested that aerosol transmission had to be considered as the leading hypothesis to explain contamination of the boar stud (L. Dufresne, personal communication, 2009).
Proof of concept: the Danish specific pathogen free (SPF) system
Results obtained in Denmark in their SPF system are in accord with the idea that some pathogens are relatively easy to keep out of pig barns, even in pig dense areas, while it is very difficult for others. For example, in 2009 there were 3219 commercial herds in that country within their SPF system. Of these only 7 and 61 were infected with B. hyodysenteriae and toxigenic P. multocida, respectively, but 2125 were infected with M. hyopneumoniae and 1668 with PRRS virus. Similarly, of 253 multiplier SPF herds in which biosecurity programs are stricter than in commercial herds, none were infected with B. hyodysenteriae or toxigenic P. multocida, but 108 were infected with M. hyopneumoniae and 39 with PRRS virus (P. Baekbo, personal communication, 2009). Finally, Table 3 shows the number of herds within the Danish SPF system that became infected with these four pathogens for each year starting in 2004–2005 (P. Baekbo, personal communication, 2009).
Table 3. Number of herds within the Danish SPF system that became infected with Brachyspira hyodysenteriae (Bh), toxigenic Pasteurella multocida (TPm), Mycoplasma hyopneumoniae (Mh) and porcine reproductive and respiratory syndrome virus (PRRSv) each year between 2004–2005 and 2008–2009
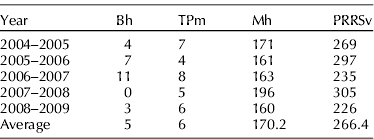
These numbers demonstrate that within the Danish SPF system, where the level of biosecurity applied is greater on average than in non-SPF herds of that country, the risk of becoming infected with M. hyopneumoniae and PRRSv is much greater than it is for B. hyodysenteriae and toxigenic P. multocida. Table 3 shows that the number of herds that annually became infected with M. hyopneumoniae and PRRSv was more than 25 and 40 times, respectively, what it was for B. hyodysenteriae or toxigenic P. multocida.
One could rightly argue that today there are more herds in Denmark that are infected with M. hyopneumoniae and PRRSv than with B. hyodysenteriae or toxigenic P. multocida, and that the potential for infection is thus increased. However, this has not always been the case and in the early days of the Danish SPF system, the danger of becoming infected with B. hyodysenteriae and toxigenic P. multocida because of non-SPF surrounding herds was evidently greater than with PRRSv, since that virus had not been introduced in Denmark at the time. Once it was introduced, however, the same principles of biosecurity that had been successfully applied for organisms such as B. hyodysenteriae and toxigenic P. multocida were simply not good enough for that pathogen, which could easily be transmitted between barns by means other than direct contact with infected pigs.
Determining and quantifying indirect transmission risks
It should be obvious from what was discussed above that to be coherent and successful, biosecurity programs and strategies will have to consider the main means by which the targeted pathogens are transmitted. For pathogens such as S. scabiei, toxigenic P. multocida and B. hyodysenteriae, a simple program that provides assurance that the animals introduced are not carriers of these organisms is frequently enough to maintain herds free of them. For others, such as FMDv, M. hyopneumoniae and PRRSv, which on some occasions can be mainly introduced into swine herds by indirect means, such programs are frequently not enough and have to be adapted according to the main ways by which they are transmitted. The first step should thus be to determine by what means a given pathogen can be introduced into a swine herd. While this may seem to be relatively simple, the reality is often different. An example is the possibility for PRRSv to be transmitted by aerosol. While the disease was first diagnosed in 1987, it took about 20 years of debate for scientists and researchers to agree that it can be transmitted by aerosol. The problem arose from the fact that while epidemiological investigations strongly suggested that aerosol transmission could take place, it was very difficult to reproduce experimentally (Otake et al., Reference Otake, Dee, Jacobson, Torremorell and Pijoan2002d; Trincado et al., Reference Trincado, Dee, Jacobson, Otake, Rossow and Pijoan2004b; Fano et al., Reference Fano, Pijoan and Dee2005). Clarifications on that apparent discrepancy were obtained when it was realized that, as for FMDv, there could be significant differences in the ability of various PRRSv strains to be transmitted by aerosol (Cho et al., Reference Cho, Deen and Dee2007; Cutler et al., Reference Cutler, Kittawomrat, Hoff, Wang and Zimmerman2009).
Demonstrating the possible means by which a given pathogen can be introduced into swine herds is obviously a necessary step in understanding its epidemiology, but being able to weigh the significance of each of these means is also crucial. For example, if aerosol transmission of PRRSv was possible, but occurs in only 1% of the cases, the installation of expensive air filtration systems would make little sense. But if it was possible to prove that in swine dense areas, this percentage can be 50% or more, it would clearly then be something that would be worth considering. Unfortunately not enough time, money and effort have been placed in quantifying transmission risks for some of our important pathogens. While grading these risks is by no means an easy task, this should not be an excuse to not at least try to do so. Many different ways can be considered to assess how frequently a given pathogen can be transmitted by a specific means. While all of them seem to have weaknesses, some obviously more than others, they may, when put together, allow us to at least have an idea as to whether or not a transmission mode can be viewed as minor, or important. Taking aerosol transmission of the PRRSv as an example, the following section will consider some of the ways that have been used so far to get some type of quantification of that risk.
Opinions of health specialists
While the opinions of health specialists cannot be considered as strong scientific evidence, they may have some value depending on the number of people involved and their level of expertise. In a survey conducted in 2006, 10 veterinarians had to first indicate what percentage of PRRS breaks in commercial sow herds they believed were associated with direct contact (Desrosiers, Reference Desrosiers2007a). Since this percentage was 10%, it left 90% of cases associated with indirect contact. They then had to give, for 12 different indirect transmission means, what they felt were the lowest and highest percentages of cases that were associated with each of these means in cases where commercial sow herds were breaking with new strains of PRRS virus. Table 4 shows the results that were obtained (Desrosiers, Reference Desrosiers2007b).
Table 4. Lowest and highest estimated percentages of new PRRS cases in commercial sow herds which, according to the opinion and personal experience of ten veterinarians, were associated with each of 12 different potential causes of indirect transmission

1 Veterinarians gave what they felt were the lowest and highest percentages of cases in which a contamination source was involved in the cases they dealt with.
While this survey suffered several weaknesses that were openly admitted by the author, it involved veterinarians who cumulatively had more than 125 person-years of experience dealing with PRRS, and the questionnaires were independently filled, so that each participant could not know what other participants had answered. In this respect, it was of interest to note that all veterinarians placed, by far, aerosol as their single most important cause of contamination. Baekbo and Mortensen (Reference Baekbo and Mortensen2001) reported that 80–85% of PRRS cases in Denmark were thought to be associated with area spread, and only 15–20% to introduction of infected animals. This area spread was in most cases believed to be associated with aerosol transmission (P. Baekbo, personal communication, 2004).
Epidemiological investigations
Some epidemiological investigations or studies have reported the most likely sources of contamination for PRRS breaks. Robertson (Reference Robertson1992a) suggested that of the first 100 cases of PRRS that were diagnosed in the UK, 63% were believed to be associated with aerosol transmission. Similarly, of 81 cases investigated in Belgium, 69% were thought to have been associated with neighborhood infection (Robertson, Reference Robertson1992a). Mortensen et al. (2002) conducted an epidemiological study that included 73 case herds and 146 control herds. In their conclusions, the authors suggested that the cases did not seem to be associated with the level of biosecurity and that spread from neighboring herds by aerosol was frequent. The same year Zhuang et al. (Reference Zhuang, Barfod, Wachmann, Mortensen and Willeberg2002) reported on the findings of a study that started in 1994, ended in 1998 and involved 344 genetic herds. The authors concluded that there was a predominant feature of local spread of PRRS virus in Danish pig herds, probably mainly via airborne transmission. Torremorell et al. (Reference Torremorell, Geiger, Thompson and Christianson2004) looked at the most likely sources of infection for 35 farms that were PRRS negative, but became infected. Area spread was considered to have taken place in 15 cases and location was considered the most important risk factor for lateral infections to occur. Seventy percent of the cases occurred during the cold season and infection caused by insects was considered likely in only one case. In a Quebec study involving 226 field cases and 174 herds, strains were sequenced and a questionnaire filled trying to establish epidemiological links between these cases. The authors (Larochelle et al., Reference Larochelle, D'Allaire and Magar2003) reported that the main relationship found within a grouping of similar strains was introduction of infected animals (19%) and area spread (33%).
Many field results suggest that proximity to other pig farms greatly increases the risk of becoming infected with PRRS strains. From 1999 to 2002, 44 cases of PRRS were diagnosed in sow herds of a Quebec integration company (Desrosiers, Reference Desrosiers2005). Both the multipliers supplying gilts to these herds as well as the boar studs from which semen came remained negative during these years. About 65% of the cases occurred during the period from September to December. Also of interest is the fact that the strains of virus involved in the outbreaks were virtually always different from those that had been previously detected in farms of the company, suggesting an outside source of infection. A comparison was made between the five sow herds that had the most outbreaks (about 1 every 12 to 15 months) and the three herds with the least outbreaks (no outbreaks in the last 5 years). All herds with no outbreaks were 4 km or more from swine farms, while all herds that had the most outbreaks were within 1 km of swine farms. The feed, trucks, people (including technicians, veterinarians and maintenance personnel) and biosecurity rules observed were considered to be similar for most of these herds. It seems logical to think that if this type of contamination had played a major role in the outbreaks, the same strains would likely have been identified in different herds of the company, which was rarely the case. Within the same company, results obtained with negative commercial pigs as well as with gilt developer units were going in the same direction, in the sense that negative animals placed on sites close to other pig barns were much more likely to break with PRRS than pigs placed on sites further away from other pigs (Desrosiers, Reference Desrosiers2005).
In Quebec again, all herds of a genetic company had been PRRS negatives for a long time, and almost all were run within a multiple site system where breeding herds, nurseries and finishing units were on different sites. These sites, except for finishing units raising barrows, are located in areas of low pig density. Between May 2001 and September 2004, the company introduced feeder pigs 268 times in finishing sites in Quebec and New Brunswick located more than 3 km from other swine farms. None (0%) of these farms broke with PRRS. Of these introductions, about 90 were composed of barrows, the rest being future breeding animals (gilts and boars). Barrows were also introduced 77 times during that period in finishing sites located 1 to 3 km from swine farms of unknown status. On three occasions (4%) pigs placed in these finishing units became clinically affected with PRRSV. Finally, barrows were introduced 36 times on finishing sites located less than 1 km from farms of unknown status, but with many, if not most, considered PRRS positive. On 12 occasions (33%), pigs placed in these sites became clinically affected with PRRSV. Biosecurity rules, trucks and personnel were thought to be fairly similar for all farms where barrows were raised, whether they were close to or far from other swine farms. Outbreaks occurred year round, including some during the heart of the winter (Desrosiers, Reference Desrosiers2005).
A recent study conducted in the US, involved 180 PRRS-negative sites investigated between May 2006 and August 2006 (Holtcamp et al., Reference Holtcamp, Polson, Wang and Melody2010). Of the 180 sites, 137 (76%) remained negative as of August 2009. Relatively few of the sites are located in pig dense areas of Iowa and North Carolina, because it is difficult to find PRRS-negative herds in those areas. Furthermore, many of the enrolled sites, which are in or surrounding these areas, have become positive. The survival probability was about 85% after 100 weeks if there were no pig sites within 1–3 miles, but only about 30% if there were four or more sites within that radius. Of the 43 farms that became positive, 89% did so between November and April; again this was a period during which insects are not likely to be an issue.
Finally, in the Mortensen et al. (Reference Mortensen, Stryhn, Søgaard, Boklund, Stärk, Christensen and Willeberg2002) study reported above the authors used a formula to quantify the risk of neighborhood exposure. In their model, a herd located 300 m away from an infected farm was 45 times more likely to become infected compared to the same size farm with no contaminated farm within 3 km.
Results obtained when a specific source of contamination is removed or reduced
Another way in which the importance of aerosol transmission may be assessed is to examine what happens in situations where farms become equipped with air filtration systems. If aerosol is an important source of contamination in field conditions, eliminating or greatly reducing that risk would normally have a significant impact on the number of cases associated with introduction of new PRRS strains. The French were seemingly the first to use air filtration in some of their swine herds of greater value (e.g. nucleus herds, boar studs). Biosecurity rules observed for these units are considered similar to the ones that would be observed for herds of high genetic and health value in North America. Desrosiers (Reference Desrosiers2005) reported the results obtained with air filtration in 11 PRRS-negative farms of a French company, which were equipped with filtration between 1996 and 2002. As of 2004, none of them had broken with PRRS, even though ten of them are located in Brittany, the area in France where swine density is the highest. One of the herds where filtration was installed in 1998 has 23 pig sites in a radius of 3 km around the farm, most or all of which were PRRS-positive, as well as a large hog slaughter house about 2.5 km away. This herd is still PRRS-negative in 2010. Today it is estimated that at least 30 swine farms are equipped with air filtration in France, and a rough estimate is that these farms have been equipped with their system for an average of at least 8 years. Several individuals directly or indirectly involved with air filtration in this country were contacted to determine how many farms with filtration systems had become infected with PRRSv over the years (Desrosiers, unpublished information). Most of the persons contacted thought that none had become infected, but one reported that two farms had become contaminated, including one that became contaminated while the filtration system was out of order after a storm. This means that even when taking into consideration the worst scenario of two contaminations, this represents less than 1% of contamination per farm per year (two contaminations for 30 farms for 8 years, or 240 farm-years). It should be noted, however, that the vast majority of farms in France are equipped with a positive pressure filtration system which, while much more expensive than the negative pressure systems generally used in North America, is also thought to allow a more complete protection. This is because in such a system there is no danger of air getting in the barn by non-filtered openings like non-functioning fans or opened doors. The first farm that became equipped with this type of system, the Ploufragan Station, did so in 1979. Most other farms that have used that system became equipped in the mid-1990s and later. In Quebec, four farms (three boar studs, one multiplier) have been equipped with a positive air pressure system since 2004 and none has broken with PRRS. By contrast, four of six farms using a negative air pressure system have had PRRS contamination. The reasons for contamination are unknown but non-filtered air getting into the barns through non-functioning fans or other non-filtered air entries is considered as one of the main possibilities.
A recent pilot study (Spronk et al., Reference Spronk, Otake and Dee2010) was conducted in seven large breeding herds of more than 3000 sows located in swine-dense regions of the US. All seven herds had a history of annual PRRSV infections secondary to the introduction of new PRRS variants over the past 4 years, despite the use of industry standard biosecurity practices. Two of the herds were equipped with a negative pressure air filtration system, and five remained as non-filtered controls. Over the 12-month study period, none of the two filtered herds broke with PRRS, while all five non-filtered herds did. In another comparison, the number of PRRS breaks was compared for 38 farms before and after they installed air filtration (Reicks, Reference Reicks2010). In the previous 5 years before filtration, the rate of new PRRS breaks was 34% per year. After filtration the rate of breaks dropped to 4% per year for farms where filtration was used all year long, and to 8% for those filtering air during the cool months of the year, but not during the warmer months. For the latter, three of the four breaks occurred during the period when filtration was not functioning. So farms filtering air 12 months a year were 8.5 times less likely to break than before installing air filtration. An improvement in biosecurity measures other than air filtration was not thought to have played a significant role in the results obtained, suggesting that previous breaks were mainly associated with aerosol contamination (D. Reicks, personal communication, Reference Reicks2010).
Results obtained when trying to remove or reduce all potential contamination sources except one
Another way of evaluating whether aerosol is important in the epidemiology of PRRS is determining what happens to the number of cases when efforts are made to control all other possible causes of contamination except aerosol. In other words, if all other means of transmission are addressed and the only way by which the virus can be transmitted is by aerosol, is the number of cases radically reduced, or are there still many cases occurring? This obviously is not something that is likely to occur often since it is very difficult to perfectly control all other possible sources of contamination in field situations, at all times. But the fact is that after 10 to 15 years of seemingly very significant efforts from producers and veterinarians to improve the biosecurity level of their herds, and to prevent introduction of the virus by means other than aerosol, the rewards often appear to have been very marginal for herds located in swine dense areas. For 1998 and 1999, the numbers of PRRS-associated cases diagnosed in Quebec laboratories of the RAIZO (Réseau d'Alerte et d'Information Zoosanitaire) system of Québec were 192 and 207, respectively. Ten years later, for 2008 and 2009, the numbers were 229 and 261. If there was any impact of the biosecurity efforts aimed at the control of PRRS during all these years, it did not seem to have an important effect on the number of cases reported in diagnostic laboratories. Apart from the few farms that became equipped with air filtration during these years, aerosol transmission was the main route which was not targeted on existing farms. For new farms, there was the possibility to establish them further away from swine dense areas.
However, not all information available suggests that the aerosol route is very important in the transmission of this pathogen. In a recent study conducted in Quebec, 36 herds located in a swine dense area were enrolled in an experimental control program. The strains present in these farms at the time of enrolment had been identified by nucleic acid sequencing. It was found that while some strains appeared to be closely related genetically, suggesting a possible contamination from neighbourhood exposure, the majority of those present in the area were different from each other (C. Klopfenstein and M. Bonneau, personal communications, 2010). It would seem logical to think that if aerosol transmission of the strains between herds of the study was frequent, there would be many strains with similar sequences (high percentage of homology) in these herds. Similar results were obtained by Goldberg et al. (Reference Goldberg, Hahn, Weigel and Scherba2000), where the authors did not detect a correlation between geographic proximity and genomic similarity. Preliminary results of another study conducted in Quebec (Lambert et al., Reference Lambert, Poljak, Arsenault and D'Allaire2010a) also suggested that there was no correlation between genetic and geographic distances. However, a more detailed analysis revealed that when specifically targeting shorter distances (e.g. 5 km or less), there was a correlation between genetic and geographic distances (M.E. Lambert, personal communication, 2010). This is in agreement with Mondaca-Fernandez et al. (Reference Mondaca-Fernandez, Murtaugh and Morrison2007) who concluded that the greater the distance between farms, the less genetic homology among PRRSv isolates, and seems to be in line with the results of another recent study conducted by Lambert et al. (Reference Lambert, Poljak and D'Allaire2010b), where farms located within 1500 m from neighboring pig operations were much more likely (odds ratios of 6.2 and 7.5 using two different models) to be PRRS-positive.
While some data may not fit well with this hypothesis, the global information currently available massively suggests that aerosol transmission of the PRRSv can be a significant source of infection and may, in some situations, be the most important cause of outbreaks in a given area. The importance of aerosol transmission in the epidemiology of this condition also means that in order to prevent introduction of this virus into swine farms located in hog dense areas collective efforts may be needed. This means that producers in a specific area may need to try together, as a group, to rid their farms of the virus. Alternatively, effective measures such as air filtration to avoid contamination through aerosol may be necessary to protect individual farms.
Conclusion
Different pathogens find their way into swine herds by different routes, therefore the means for efficiently preventing this from happening may also be different. For some pathogens transmission clearly occurs mainly by direct contacts and their epidemiology is thereby simplified. But for others, indirect transmission means are frequently involved, and for them it is not only important to quickly identify these possible sources of contamination, but perhaps just as important to quantify, or grade these sources. This seems to be the only way to make sure that time, efforts and resources to prevent the spread of these pathogens are placed where they are likely to have the most impact. Both identification and quantification of sources of contamination should be a priority for every important disease for which they have not been clarified, and for new, emerging diseases with a potential to produce significant losses. Not understanding the importance of these two basic epidemiological elements can result in decades of erring in terms of control efforts and in suffering losses that can not only jeopardize the survival of individual producers' businesses, but also inflict a severe toll on a whole industry. As an example, PRRS has been estimated to cost the US swine industry 560 million dollars per year, and has been by far the most costly pig disease for more than two decades (Neumann et al., Reference Neumann, Kliebenstein, Johnson, Mabry, Bush, Seitzinger, Green and Zimmerman2005). Yet, it took 20 years to identify and confirm one of the most important means by which the causal organism becomes transmitted from farm to farm. This should encourage government, private and other institutions to seriously consider epidemiological studies, particularly those focusing on identification and quantification of transmission means, when deliberating on funding for animal health research projects.






