INTRODUCTION
Schistosomes are long-lived parasitic flat worms inhabiting host vasculature. Five species out of 20 are able to infect man, establishing a chronic infection (Standley et al. Reference Standley, Mugisha, Dobson and Stothard2012). One immune evasive strategy employed by the parasite and presumed to prolong its survival is the acquisition of host molecules. Several host molecules have been reported on the surface of Schistosoma mansoni adult worms, including blood group antigens (Smithers et al. Reference Smithers, Terry and Hockley1969), major histocompatibility complex antigens (Simpson et al. Reference Simpson, Hackett, Kelly, Knight, Payares, Omer-Ali, Lillywhite, Fleck and Smithers1986), immunoglobulins (Kemp et al. Reference Kemp, Damian, Greene and Lushbaugh1976), host lipids (Furlong et al. Reference Furlong, Thibault and Rogers1992), lipoproteins (Dinguirard and Yoshino, Reference Dinguirard and Yoshino2006), α-2-macroglobulin (Damian et al. Reference Damian, Greene and Hubbard1973), contrapsin (Modha et al. Reference Modha, Parikh, Gauldie and Doenhoff1988) and components of the complement system (Skelly, Reference Skelly2004).
A relationship between a host-derived chymotrypsin-like serine protease present in detergent extracts of S. mansoni worms that had been perfused from infected mice and also in mouse blood was previously reported by Darani and Doenhoff (Reference Darani and Doenhoff2008). The enzyme was found in a detergent (deoxycholic acid – DOC) extract of worms with relatively little being found in non-detergent extracts and it was seemingly identical antigenically and enzymatically to the molecule in mouse serum. The antigen in the worm extract and normal mouse serum were both immunoprecipitated in immunoelectrophoresis by a rabbit antiserum raised against whole mouse serum [anti-normal mouse serum (anti-NMS)] (Darani and Doenhoff, Reference Darani and Doenhoff2008). The enzyme activity was visualized using N-acetyl-DL-phenylalanine β-naphthyl–ester (NAPBNE), a chromogenic substrate of chymotrypsin-like enzymes. The molecule in both the DOC worm extracts and NMS is of interest because it is unusual for an enzymatically active form of a protease to be present in blood – most are there as inactive pro-enzymes or in zymogen form. Furthermore, while the active enzyme has been found in mouse and rat blood, it was not found in the blood of several other mammalian species including sheep, cattle and humans (Darani and Doenhoff, Reference Darani and Doenhoff2008).
This study reports the purification and characterization of the mouse plasma-derived chymotrypsin-like enzyme, which we believe to be identical to that found on the surface of S. mansoni worms recovered from the infected mice. Mass spectrometry (MS) helped determine the amino acid sequence of the enzyme which in turn enabled it to be identified and further characterized. Information so derived gave insights into its possible role in the host–parasite relationship.
MATERIALS AND METHODS
All chemicals and buffers were of analytical grade and bought from Sigma-Aldrich Company limited, UK, except when otherwise stated. These included absolute ethanol, sodium acetate, the salt constituents of phosphate buffered saline, Tris, triton X-100, NAPBNE, fast blue B salt (FBB), β-naphthyl acetate, α-naphthyl acetate, fast red TR, dimethyl sulfoxide (DMSO) and glycine.
Parasite extract and antigens
A Puerto Rican isolate of S. mansoni was routinely used for infection of mice for experimental work and production of adult worm antigens. The isolate has been maintained by continuous passage in random-bred mice and Biomphalaria glabrata snails, the intermediate host for the generation of cercaria. Adult schistosomes were recovered by portal perfusion of infected mice 42 days after infection, as described originally by Smithers and Terry (Reference Smithers and Terry1965) and modified by Doenhoff et al. (Reference Doenhoff, Bickle, Long, Bain and McGregor1978).
Detergent extracts of S. mansoni worms (WM) were prepared by re-suspending freshly perfused, gravity-sedimented worms which had had all visible traces of erythrocytes removed from the suspending fluid, in twice their volume of 2% DOC (detergent) solution in isotonic saline as previously described by Doenhoff et al. (Reference Doenhoff, Modha, Curtis and Adeoye1988). The suspension was gently agitated for 4 h at room temperature and then centrifuged. The supernatant was removed and stored at −80 °C until used for rabbit immunization or in immunoprecipitation studies.
NMS and normal rat serum (NRS)
NMS and NRS were prepared by exsanguinating healthy uninfected animals of the respective species. Collected blood was put into universal tubes and stored in a fridge at 4 °C for 4 h to clot. The blood clots were ringed with a pipette to aid separation of the clot from serum and the serum removed, centrifuged at 2500 × g for 6 min at 4 °C and the clear supernatant removed and stored at −80 °C until use.
Rabbit anti-NMS
A polyspecific rabbit anti-NMS antiserum was prepared as described by Darani and Doenhoff (Reference Darani and Doenhoff2008) by repeated weekly injections of 1 mL emulsion containing equal volumes of normal mouse serum and Freund's adjuvant. The response was assessed qualitatively in terms of the intensity of immuno-precipitation lines yielded by the serum and the homologous antigen extract. The rabbit was serially bled weekly from alternate ears until enough serum was collected. The serum pool was divided into 5 mL aliquots and stored at −20 °C.
Rabbit anti-complete Freund's adjuvant (anti-CFA)
Rabbit anti-CFA was prepared as described for anti-NMS except that rabbits were injected only with repeated weekly doses of 1 mL emulsion of CFA.
Rabbit anti-mouse serum albumin (anti-MSA)
A rabbit anti-MSA antiserum was raised by immunization with replicate immunoprecipitin arcs produced by immunoelectrophoresis of mouse serum albumin and anti-NMS as described by Goudie et al. (Reference Goudie, Horne and Wilkinson1966), adapted as in Dunne et al. (Reference Dunne, Agnew, Modha and Doenhoff1986). Immunoprecipitin arcs were excised, homogenized and injected into rabbits at weekly intervals. Antibody responses of Immunized rabbits were monitored and finally bled as described for the rabbit anti-NMS.
Rabbit anti-worm protease antiserum
A rabbit antiserum specific for the chymotrypsin-like enzyme in NMS was prepared as described by Darani and Doenhoff (Reference Darani and Doenhoff2008).
Reduction of albumin in NMS and NRS
Serum albumin was depleted from both NMS and NRS as described by Colantonio et al. (Reference Colantonio, Dunkinson, Bovenkamp and Van Eyk2005). NaCl was added to a known volume of each serum in a micro-tube to give a final concentration of 0·1 m and the mixture incubated with gentle rotation for 60 min at 4 °C. Cold ethanol was added to the mixture to yield a final concentration of 42% v/v and incubated for a further 60 min at 4 °C. The mixture was centrifuged at 16 000 × g for 45 min at 4 °C and the resultant supernatant transferred into a sterile micro tube ‘B’ for further processing while retaining the pelleted precipitate in the first tube ‘A’. The pH of the supernatant was lowered to 5·7 by adjusting with cold 0·8 m sodium acetate, pH 4·0 and incubated at 4 °C for 60 min. The mixture was centrifuged as above and supernatant containing mainly albumin transferred into sterile micro-tube ‘C’ while retaining the pelleted precipitate. The first and second precipitates were separately reconstituted with 0·1 m PBS, pH 7·4 (w/v) and stored at −20 °C. The protein concentration in NMS was 23·70 mg mL−1, while concentrations in solutions of the two precipitates A and B and the supernatant were 5·50, 5·90 and 10·90 mg mL−1, respectively.
Immunochemistry for the detection and purification of the chymotrypsin-like enzyme
Single radial immunodiffusion (RID) (Mancini et al. Reference Mancini, Carbonara and Heremans1965) was carried out as described by Darani et al. (Reference Darani, Curtis, McNeice, Price, Sayers and Doenhoff1997), on microscopic glass slides using 1% molten agarose in 0·06 m barbitone buffer, pH 8·6. The immuno-precipitate was washed in several changes of 0·9% saline to remove non-immunoprecipitated material.
Purification of the chymotrypsin-like enzyme in mouse and rat sera was achieved in one-dimensional sodium dodecyl 12% polyacrylamide gel electrophoresis (1-D SDS–PAGE) (Laemmli, Reference Laemmli1970) as modified by Studier (Reference Studier1973). 18 µg of each serum (mouse or rat) were loaded into separate gels with broad wells (6·2 cm long) in replicates and electrophoresed. Bands with enzymatic activity were excised from replicate thin strips of the polyacrylamide gel, put into a 1·5 mL Eppendorf container and covered with a minimum volume of elution buffer (0·06 m Tris–HCl, 10% SDS, pH 7·0) (Beyer et al. Reference Beyer, Schou, Houen and Heegaard2008). The tube was incubated at 37 °C for 24 h, centrifuged at 14 000 × g for 30 min at the same temperature and the resultant eluate was removed. Further purification of the enzyme was achieved by re-electrophoresing the eluate in a fresh SDS–PAGE gel. The whole sequence of (i) SDS–PAGE, (ii) elution of enzyme activity from gel strips, (iii) re-electrophoresing in PAGE was repeated three times in an effort to obtain a sufficiently pure sample of the enzyme suitable for analysis by MS. PAGE gels carrying proteins for analysis in MS were stained using SimplyBlue SafeStain (Invitrogen, Carlsbad, CA).
Ouchterlony double immunodiffusion was adapted from Bailey (Reference Bailey and Walker John1996). Glass microscope slides were sterilized by spraying 70% alcohol on both sides and wiping with a sterile tissue. They were placed on a levelled table and 4 mL, 1·2% molten agarose dissolved in sodium barbitone solution (pH 8·6) was spread on the glass slide and allowed to set. Circular wells were cut in the gel and these were loaded with desired antigen solutions and antisera. The slides were incubated in a humid chamber for 16 h to allow immunoprecipitation and then washed in 0·9% saline solution for 48 h. Enzymatic activity was assayed using both chymotrypsin-like (NAPBNE + FBB) and carboxylesterase (CES) (α-naphthyl acetate + fast red TR) substrates as described below.
Immunofluorescence on the surface of adult worms
Immunofluorescence on adult worms was done as described in Doenhoff et al. (Reference Doenhoff, Modha, Curtis and Adeoye1988). Briefly, adult worms perfused from infected mice were washed several times in perfusion fluid and fixed in 4% paraformaldehyde in isotonic PBS solution pH 7·4, overnight. Thereafter, the worms were washed thrice in isotonic PBS solution. The fixed worms were incubated in blocking buffer [1% BSA in PBST (PBS + 0·2% Tween-20] for an hour at room temperature and washed in PBST solution.
Primary incubation of the blocked adult worms was done in a 1:40 dilution of a rabbit antiserum raised against the host-derived protease/CES) in 1 mL PBST and incubated overnight at 4 °C. Worms were washed three times in PBST as above and incubated in 1 mL 1:80 diluted FITC-labelled secondary goat anti-rabbit IgG (Abcam, Cambridge, UK) in PBST for 30 min at room temperature in the dark. The labelled worms were washed three times in PBST and examined with the aid of a GX fluorescence microscope (GMXL3201LED) using the 10 × objective lens under blue light attached to a GXCAMFLUOMAX camera (GT Vision Ltd www.gxoptical.com).
MS analysis of purified enzymes
Analyses of purified gel bands were carried out using tandem MS (Papayannopoulos, Reference Papayannopoulos1995; Steen and Mann, Reference Steen and Mann2004). Purified protein was digested by trypsin and fragmented peptides ionized and accelerated in a mass analyser where ion fragments were separated on the basis of mass-to-charge to produce spectra. Data from the resulting mass spectra were searched using the MASCOT software for peptide matching and protein identification. Amino acid sequence searches used the protein basic local alignment search tool (pBLAST) at the National Centre for Biotechnology Information (NCBI) against the non-redundant protein sequences database (nr) (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to identify homologous proteins, while the protein sequence alignment was achieved using ClustalW software (http://www.ebi.ac.uk/Tools/msa/clustalw/).
Detection of chymotrypsin-like enzyme activity
Zymography for the detection of chymotrypsin-like enzyme activity in agarose films in RID and SDS–PAGE gels was performed as described by Pearse (Reference Pearse1968), adapted as in Darani and Doenhoff (Reference Darani and Doenhoff2008), using 5 mg NAPBNE as the chromogenic substrate and 5 mg FBB as the coupling agent. The substrate mixture was dissolved in 2 mL DMSO and diluted to 40 mL by adding 10 mL, 0·1 m PBS solution, pH 7·4 and 30 mL deionized water. Prior to zymography in SDS–PAGE, the gel was incubated for an hour in 2·5% triton X-100 solution.
Detection of esterase activity
Esterase activity of purified mouse and rat enzymes was assayed using two substrates: β-naphthyl acetate and α-naphthyl acetate. The chromogenic substrate solution for β-naphthyl acetate was adapted from Bahar et al. (Reference Bahar, Ohura, Ogihara and Imai2012), using FBB as coupling agent dissolved in DMSO and diluted in 50 mm sodium acetate buffer, pH 7·4. The substrate for α-naphthyl acetate was adapted from Duysen et al. (Reference Duysen, Koentgen, Wiliams, Timperey, Schopfer, Cerasoli and Lockridge2011); Otto et al. (Reference Otto, Ronai and von Deimling1981) using fast red TR (BDH chemicals Ltd., Poole, England), as coupling agent, dissolved in DMSO and diluted in 50 mm sodium acetate buffer, pH 7·4.
Inhibition of enzymatic activities
The inhibition of the purified chymotrypsin-like enzyme from the sera of mice and rats was carried out using phenylmethanesulphonyl fluoride (PMSF) (Darani and Doenhoff, Reference Darani and Doenhoff2008). The inhibition of the carboxylesterase activity of the purified enzyme from the sera of mouse and rat using bis-p-nitrophenyl phosphate (BNPP) was adapted from Xie et al. (Reference Xie, Yang, Liu, Xue and Yan2002). Briefly, the purified mouse and rat enzymes were electrophoresed in replicate SDS–PAGE gels. After electrophoresis, the gels were first incubated at room temperature in 2·5% triton X-100 solution for an hour (to allow the enzymes to refold), rinsed thrice in deionized water, followed by a second incubation in 0·1 m PBS solution, pH 7·4 for 10 min. Thereafter, the gels were divided into three groups with each of the groups containing three replicates of each of the purified mouse and rat enzymes. The first group was treated for 3 h by incubation in 10 mm PMSF dissolved in DMSO and diluted in PBS at 37 °C. A second was incubated at 37 °C in a solution containing 5 mm BNPP dissolved in PBS for 2 h, while the third group was incubated only in PBS under the same conditions. The reactions were stopped by washing five times in PBS and the gels immersed in chromogenic substrate mixtures for detection of enzymatic activity. A gel piece was taken from each of the three groups and incubated in three substrate solutions containing the NAPBNE (for the detection of chymotrypsin-like enzyme activity), β-naphthyl acetate and α-naphthyl acetate (both esterase substrates).
RESULTS
Partial purification of the chymotrypsin-like enzyme from NMS
Each of the three fractions from albumin-depleted mouse serum (precipitates A and B and the supernatant) was investigated using rabbit anti-MSA and anti-NMS in RID for the presence and concentration of albumin and the chymotrypsin-like enzyme (Fig. 1).
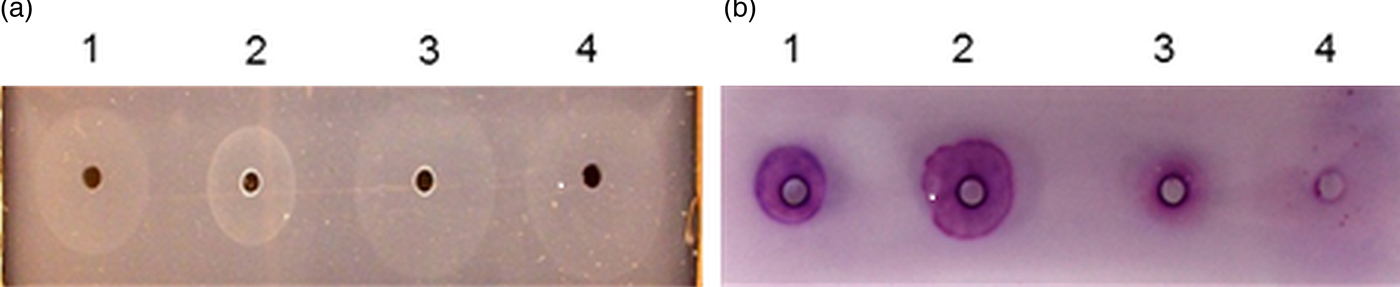
Fig. 1. RID of diluted NMS and fractions from albumin-depleted NMS. Immunoprecipitation was carried out with: (a) rabbit anti-whole mouse serum albumin antiserum (anti-MSA) and (b) anti-NMS and stained with chromogenic substrate NAPBNE + FBB. Wells (1) NMS, (2) Precipitate A of albumin-depleted NMS, (3) Precipitate B of albumin-depleted NMS, (4) supernatant from albumin-depleted NMS. (2 µg of each eluate was loaded into each of the wells).
The presence of the chymotrypsin-like enzyme was indicated by a purplish colour after chromogenic staining using NAPBNE + FBB (Fig. 1b). The results indicated that precipitate ‘A’ (well 2) had relatively less albumin (Fig. 1a), while retaining more of the enzyme activity than the other fractions and it was therefore used for further purification of the enzyme by means of SDS–PAGE.
Purification of the chymotrypsin-like enzyme from precipitate A using SDS–PAGE
Further purification of the enzyme from precipitate A was followed by its isolation from three successive SDS–PAGE gels, the band with enzymatic activity being excised from replicate thin strips of the polyacrylamide gel, eluting the enzyme therefrom and re-electrophoresing the product in a further PAGE. The procedure of (i) SDS–PAGE, (ii) elution of enzyme activity from gel strips and (iii) re-electrophoresing in PAGE was repeated two times (Fig. 2). The Coomassie-stained band in lane 5 showing the purified enzyme (blue arrow) was subjected to tandem MS and the results are shown in Table 1. Tandem MS analysis and a Mascot search of the Swiss-Prot database revealed significant peptide matches with mouse (Mus musculus) enzyme CES 1C (EST1C_MOUSE) and mouse α-1B-glycoprotein (A1BG_MOUSE). The same proteins with identical amino acid sequences are present also in the NCBI database (Mouse CES 1C: GI:247269929 and NP:031980·2; Mouse A1BG: GI:124486702 and NP: 001074536·1).

Fig. 2. Steps towards the purification of the chymotrypsin-like enzyme in NMS using an albumin-depletion method and repeated SDS–PAGEs. (M) Molecular weight markers, (1) NMS, (2 & 3) Precipitate A from albumin-depleted NMS, (4 & 5) Purified enzyme after two successive SDS–PAGEs. Lanes 1, 3 & 5 were stained with SimplyBlue SafeStain. Lanes 2 & 4 reveal the presence of the enzyme by zymography. Blue arrow shows the gel position of the enzyme.
Table 1. MASCOT search output of tandem MS data from the purified ~85 kDa gel band from mouse serum. Peptides with significant scores are shown in italics.

Mascot Protein score, the sum of all the peptide ion scores matching a protein; Mass, predicted protein mass in Daltons (Da); Coverage, percentage of sequence covered by MS-matched peptides; Peptide Ion score, a score assigned to individual matching peptide by Mascot based on the probability of best match; Expect, frequency of chance of obtaining an equal or higher score for a peptide.
Purification of the chymotrypsin-like enzyme from rat serum
A seemingly analogous chymotrypsin-like enzyme was also found after SDS–PAGE and zymography of NRS using the same chromogenic substrate NAPBNE and its coupling FBB (Darani and Doenhoff, Reference Darani and Doenhoff2008). The purification of the enzyme in NRS was undertaken to help confirm the identity of the mouse-derived enzyme and help in the characterization of the enzyme. Extracts of NRS and NMS sera were loaded into adjacent wells and electrophoresed in SDS–PAGE. The result of zymography to detect enzymatic activity in NMS and NRS in SDS–PAGE is shown in Fig. 3. The result revealed that the enzyme in NRS has a slightly smaller size in SDS–PAGE compared with that in NMS.

Fig. 3. Zymography of SDS of NRS and NMS to display the enzyme using the chromogenic substrate mixture of NAPBNE + FBB. M: Molecular weight marker (1) NRS, (2) NMS (2 µg was loaded in each well).
The same method that had been adopted for the purification of the enzyme in NMS was applied to NRS; i.e. albumin depletion followed by SDS–PAGE, elution from the gel and re-electrophoresing the contents of the eluate. The result is shown in Fig. 4.

Fig. 4. Purified chymotrypsin-like enzyme in rat serum. (M) Molecular weight marker, (a) zymography of purified rat enzyme, (b) purified rat enzyme stained in SimplyBlue SafeStain and investigated in MS.
The partially purified Coomassie Brilliant blue-stained band indicated by the arrow in Fig. 4b was subjected to tandem MS and derived peptides were searched in MASCOT for protein identification. Significant matches for the peptides identified by MS were given by two protein entries in the Swiss-Prot database, namely rat carboxylesterase 1C (RCES 1C) (Table 2) and rat α 1B-glycoprotein (RA1BG) (result not shown). The same proteins with identical peptide sequences are present in the NCBI database (RCES 1C: GI: 2506388 and NP: 10959·3; RA1BG: GI: 25453392 and NP: 071594·2).
Table 2. MASCOT search output of tandem MS data from the purified ~85 kDa gel band from rat serum. Other details as in the legend to Table 1.

The MS result for RCES 1C indicated it was marginally smaller than MCES 1C, as had been indicated by the results of zymography after PAGE (Fig. 3).
Investigation of the purified chymotrypsin-like enzyme for esterase activity
Following the indications from MS that the purified host-derived chymotrypsin-like enzyme from mouse and rat plasmas may be carboxylesterases, samples of the enzymes purified from mouse and rat sera were subjected to zymography with two esterase substrates: β-naphthyl acetate and α-naphthyl acetate and NAPBNE as a positive control. The purified enzymes from both mouse and rat were observed to hydrolyse both esterase substrates at the same position in the gel as the activity against NAPBNE, providing additional evidence for the enzyme in question to be an esterase (Fig. 5).
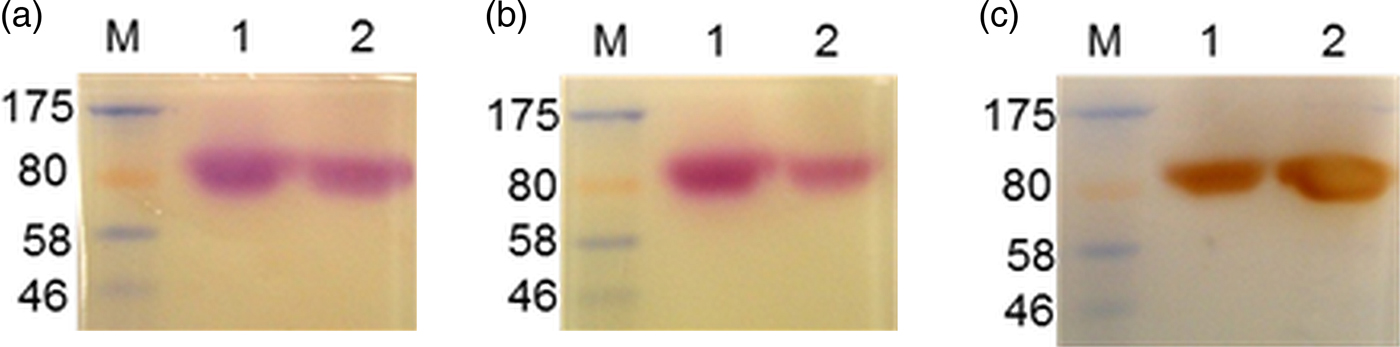
Fig. 5. SDS–PAGE and zymography investigating the purified CES enzymes from mouse and rat using two esterase substrates. (a) Stained with chymotrypsin-like enzyme substrate: NAPBNE + FBB, (b) stained with carboxylesterase enzyme substrate: β-naphthyl acetate + FBB, (c) stained with carboxylesterase enzyme substrate: α-naphthyl acetate + fast red TR. (M) Molecular weight markers, (1) purified enzyme from NMS, (2) purified enzyme from NRS.
Inhibition of the chymotrypsin-like enzyme and esterase activities in samples purified from NMS and NRS
The enzyme activities purified respectively from mouse and rat sera and visualized by zymography with the two esterase substrates were further characterized using a carboxylesterase substrate inhibitor: BNPP and the protease inhibitor PMSF. Both inhibitors were observed to have inhibited the ability of both the purified enzymes to hydrolyse the two esterase substrates as well as the substrate of chymotrypsin-like enzymes (Fig. 6b, c, e, f, h and i), as compared with the control groups which were incubated in the absence of any of the two inhibitors (Fig. 6a, d and g).

Fig. 6. Investigating the effect of CES inhibitor BNPP and the protease inhibitor PMSF on purified CES. (a, b & c) was assayed with chymotrypsin-like substrate: NAPBNE + FBB. (d, e & f) was assayed with CES substrate: β-naphthyl acetate + FBB. (g, h & i) was assayed with CES substrate: α-naphthyl acetate + fast red TR. (a, d & g) were controls. (b, e & h) were treated with 5 mm BNPP inhibitor. (c, f & i) were treated with 10 mm PMSF inhibitor. (M) Molecular weight marker, (1) Purified enzyme from mouse, (2) purified enzyme from rat.
Enzyme detection in adult worm membrane and purified CES from NMS
The purified CES was investigated in Ouchterlony double immunodiffusion to ascertain its immunological identity with an enzyme in a detergent extract of mouse-derived adult worms and the enzyme in NMS. A rabbit anti-NMS antiserum was loaded in one well while the purified CES and unfractionated WM or NMS were loaded in wells adjacent to each other and opposite the well containing the antiserum. A pattern of identity of precipitin lines formed between the purified CES enzyme and an antigen in WM and NMS indicated that antibodies in the anti-NMS were immunoprecipitating the same molecule in all three antigen solutions (Fig. 7).

Fig. 7. Ouchterlony (Immunodiffusion) and zymography showing that the purified CES from NMS and an antigen in WM are immunoprecipitated similarly by antibodies in a rabbit anti-NMS. Gels ‘a’ and ‘b’ (1) rabbit anti-NMS, (2) NMS, (3) WM. Gels ‘c’ and ‘d’ (1) rabbit anti-NMS, (2) purified CES, (3) WM. Gels ‘a’ and ‘c’ were stained with the chromogenic substrates for chymotrypsin-like enzyme (NAPBNE and FBB), ‘b’ and ‘d’ were stained with an esterase substrate (α-napththyl acetate and fast red TR). All stained gels were photographed over direct light.
Immunofluorescent detection of the host-derived enzyme on adult worms
The presence of the enzyme on the surface of mouse-derived adult worms was investigated. An immunofluorescent test was done by probing the surface of freshly perfused adult worms with rabbit antibodies raised against the chymotrypsin-like enzyme derived from a detergent extract of adult worms (WM), while control worms were probed with rabbit antibodies raised against complete Freund's adjuvant (anti-CFA). Results showed that the surfaces of worms probed with antibodies raised against the chymotrypsin-like enzyme present in a detergent extract of adult worms were immunofluorescent while control worms were not (Fig. 8).

Fig. 8. Immunofluorescence probing the surface of mouse-derived S. mansoni adult worms. (a) Worm was probed with a rabbit antiserum raised against adult worm-derived mouse chymotrypsin-like enzyme; (b) was probed with a rabbit anti-complete Freund's adjuvant antiserum (anti-CFA).
Investigating peptide homology between mouse and rat CES 1C
The homology of the host-derived mouse CES 1C (GI: 247269929, NP: 031980·2) to other mammalian proteins was investigated by a pBlast search of its amino acid sequence on the NCBI database. The search identified RCES 1C (GI: 2506388, NP: 10959·3) with a score of 913, an E value of 00 and identity of 83%, as the mammalian protein most closely similar to that of mouse CES 1C.
Furthermore, the amino acid sequences of mouse and rat enzymes were investigated for homology with any human protein. The result revealed that both enzymes were homologous with human carboxylesterases, one of the most similar being human CES 1 (monocyte/macrophage serine esterase 1) (GI: 15214585, NP: AAH12418·1) with peptide identity of 65·52%, a score of 759, and an E value of 00 (result not shown). An alignment of the amino acid sequences of the mouse, rat and human CES using the ClustalW software revealed a peptide identity of 83·06% between mouse and rat CES 1C, 65·52% identity between mouse CES 1C and human CES 1 and 71·22% identity between rat CES 1C and human CES 1. MS-derived peptides which matched each of the mouse and rat sequences are highlighted by underlining in Fig. 9.

Fig. 9. An alignment of mouse and rat CES 1C and human CES. MS-derived peptides that match the aligned sequences of mouse and rat CES 1C in MASCOT are underlined. Residues forming the catalytic triad for CES and chymotrypsin-like enzymes are shown in bold font and italicized.
DISCUSSION
The uptake of host molecules by schistosomes is considered to play a crucial role in immune evasion and enhancement of parasite survival (Smithers et al. Reference Smithers, Terry and Hockley1969; McLaren et al. Reference McLaren, Clegg and Smithers1975; Skelly, Reference Skelly2004; Dinguirard and Yoshino, Reference Dinguirard and Yoshino2006). Here, the purification and characterization of an enzyme, apparently with chymotrypsin-like activity, and present in a DOC detergent extract of S. mansoni adult worms (WM) recovered from infected mice, was undertaken to determine its identity and perhaps thus provide insight into its role(s) in the parasite–host relationship.
The enzyme in DOC extracts of the parasite (WM) was observed to be similar antigenically and enzymatically to that in NMS and little or none was present in aqueous extracts of adult worms without DOC (Darani and Doenhoff, Reference Darani and Doenhoff2008). It has here been confirmed (Fig. 7) that the enzyme in mouse blood was identical to that in the detergent extracts of worms and purification from NMS was necessitated because of the limited availability of worm-derived material. The enzyme was previously reported to have a molecular weight of ~70 KDa (Darani and Doenhoff, Reference Darani and Doenhoff2008), and thus quite similar to that of serum albumin. The high concentration of albumin in serum made it difficult to isolate the enzyme and methods to reduce the concentration of the former were therefore employed (Chen et al. Reference Chen, Lin, Yeh, Hsiao, Wu, Chen and Wang2005; Colantonio et al. Reference Colantonio, Dunkinson, Bovenkamp and Van Eyk2005).
The method of albumin depletion resulted in fractionating NMS into two precipitates (first and second) after two successive centrifugations and a supernatant containing mainly albumin (Fig. 1). The first precipitate, which had a reduced concentration of albumin, but had contained more of the enzyme, was purified further (Fig. 2). Tandem MS-derived peptides showed a significant match for mouse CES 1C (Table 1), although some MS-derived peptides also matched that of mouse α-1B-glycoprotein (result not shown). The host-derived enzyme was considered most likely to be carboxylesterase as the MS-derived peptides gave a lower match with α-1B-glycoprotein and the latter is not known to exhibit any enzymatic activity. The low molecular weight recorded for the enzyme in MS compared with the results from SDS–PAGE could be due to post-translational modification as these enzymes have been reported to be glycosylated (Otto et al. Reference Otto, Ronai and von Deimling1981; Ghesquiere et al. Reference Ghesquiere, Van Damme, Martens, Vandekerckhove and Gevaert2006; Bernhard et al. Reference Bernhard, Kapp and Simpson2007)
Further evidence of the identity of the chymotrypsin-like enzyme was obtained from examination of a seemingly analogous enzyme in normal rat serum (NRS) which in SDS–PAGE and zymography had a stronger staining intensity and a slightly lower molecular weight than the enzyme in mouse serum (Fig. 3). Tandem MS analysis of the partially purified enzyme from rat serum revealed peptides, the sequence of which significantly matched those of rat CES 1C and RA1BG (Table 2). Interestingly, the MS-derived peptides matching the aligned amino acid sequences of the mouse and rat enzyme were present in only one or the other sequence, with the exception of two (Fig. 9). A study of the properties and characteristics of the two MS-identified molecules showed similar characteristics/properties between mouse CES 1C and the chymotrypsin-like enzyme. Thus CESs, similarly to the serine protease chymotrypsin, possess a serine residue in their catalytic triad (Fig. 9) (Stoops et al. Reference Stoops, Horgan, Runnegar, De Jersey, Webb and Zerner1969; Satoh and Hosokawa, Reference Satoh and Hosokawa2006).
The chromogenic substrate for chymotrypsin-like enzymes (NAPBNE) has previously been reported to be hydrolysed by rat plasma CES (Choudhury, Reference Choudhury1974). The ability of the purified enzymes from both mouse and rat plasma to hydrolyse two esterase substrates (α- and β-naphthyl acetate) (Bahar et al. Reference Bahar, Ohura, Ogihara and Imai2012), is an indication of their esterolytic capability. Moreover, the protein bands with esterolytic activity were observed at the same molecular weight as the band which hydrolysed the chymotrypsin-like substrate (NAPBNE + FBB), a result consistent with the same molecule being active on the three different substrates.
In terms of mode of action of the chymotrypsin-like enzyme and CES, both share very similar active sites in possessing a catalytic triad composed of serine, histidine and either glutamic or aspartic acid (indicated in bold and italicized font in Fig. 9) (Bahar et al. Reference Bahar, Ohura, Ogihara and Imai2012; Brayer et al. Reference Brayer, Delbaere and James1979; Satoh and Hosokawa, Reference Satoh and Hosokawa2006; Stoops et al. Reference Stoops, Horgan, Runnegar, De Jersey, Webb and Zerner1969). Moreover, the observation that the esterase and chymotrypsin-like activities were both inhibited by PMSF, a chymotrypsin (serine protease) inhibitor and BNPP, a CES inhibitor, indicated similarities of hydrolytic action of the enzymes (Fig. 6). Previous findings on the inhibition of the chymotrypsin-like enzyme and CES in NMS using PMSF and BNPP respectively, further buttress this point (Xie et al. Reference Xie, Yang, Liu, Xue and Yan2002; Darani and Doenhoff, Reference Darani and Doenhoff2008).
The immunoprecipitation of the host-derived enzyme in detergent extracts of the parasite (WM), purified CES and NMS by Ouchterlony double immunodiffusion using a rabbit anti-NMS revealed patterns of immunological identity in both extracts (Fig. 7). This indicates that the purified CES is identical to an enzyme in WM. Moreover, the ability of the immunoprecipitates to hydrolyse substrates of both chymotrypsin-like enzymes (NAPBNE + FBB) and esterases (α-naphthyl acetate) helps substantiate the characterization of the enzyme on worms being host-derived and a CES. Furthermore, immunofluorescence detection on mouse-derived adult worms probed with antibodies specific for the chymotrypsin-like enzyme indicated the host-derived antigen was present on the surface of the worms (Fig. 8). The high intensity of immunofluorescence observed on adult worms, particularly on the female, could be an indication of the possible roles of the enzyme for easing the passage of the parasite in host's blood vessels, metabolism and in immune evasion.
Darani and Doenhoff (Reference Darani and Doenhoff2008) reported the chymotrypsin-like enzyme to be present with high staining intensity in both mouse and rat plasma, but absent among an array of other mammalian plasmas (hamster; guinea pig, rabbit, bovine or human) that were assayed. The same has been reported for the CES enzyme in the plasmas of mouse and rat among the mammalian plasmas investigated (Cerasoli et al. Reference Cerasoli, Maxwell and Lenz2000; Li et al. Reference Li, Sedlacek, Manoharan, Boopathy, Duysen, Masson and Lockridge2005; Bahar et al. Reference Bahar, Ohura, Ogihara and Imai2012). The reason for CES being present in mouse and rat blood has been explained in terms of their amino acid sequences. Thus, mouse and rat blood contain the secreted form of the CES enzyme. Secretion of CESs from cells in which they are synthesized is normally prevented by the presence of a retention signal tetra-peptide (namely: histidine, X, glutamic acid and a terminal leucine, with X representing any amino acid) at the carboxyl–terminal of the enzyme (Cerasoli et al. Reference Cerasoli, Maxwell and Lenz2000). In mice and rats, a disruption in the retention tetra-peptide sequence due to the replacement of the terminal leucine by either threonine or lysine results in the secretion of the CES from the liver into the blood of these animals (Satoh and Hosokawa, Reference Satoh and Hosokawa2006; Hosokawa, Reference Hosokawa2008). Twenty families of carboxylesterase are encoded for in the mouse genome and only one encoded by the ES-1 gene exhibits the disrupted retention signal, meaning that most CES activity in mouse serum is generated in the liver by expression of the ES-1 gene (Duysen et al. Reference Duysen, Koentgen, Wiliams, Timperey, Schopfer, Cerasoli and Lockridge2011).
A BLAST analysis of sequences in the NCBI database indicated the most similar homologue of mouse CES 1C was rat CES 1C and that human brain CES was the closest human CES to mouse CES 1C (results not shown). However, a human serine carboxylesterase expressed at high levels in the liver and less in lungs and heart was of particular interest as it has been shown to possess convertase activity in human alveolar lavages and to function as a lung detoxification enzyme (Munger et al. Reference Munger, Shi, Mark, Chin, Gerard and Chapman1991). Similarly to mouse CES 1C, inhibition of the human serine carboxylesterase by PMSF and BNPP has also been reported (Munger et al. Reference Munger, Shi, Mark, Chin, Gerard and Chapman1991). An alignment of amino acid sequences of the mouse, rat and human CESs using ClustalW software revealed an identity of 83·06% between mouse and rat CES 1C, 65·52% identity between mouse CES 1C and human CES and 71·22% identity between the rat and human molecules, reflecting homology between all three enzymes (Fig. 9).
A requirement for detergent (DOC) to extract the enzyme into solution suggests it is membrane-bound in the parasite, perhaps on the outer surface. If that is so, several roles could be suggested to explain the presence of CES on the surface membrane. Firstly, mouse CES 1C could be exploited by S. mansoni as an immunological disguise for masking surface antigens, thereby preventing the recognition and activation by antigen-presenting cells and activation of the complement system (Furlong et al. Reference Furlong, Thibault and Rogers1992).
Alternatively or additionally, the convertase potential of CES 1C (Krishnasamy et al. Reference Krishnasamy, Teng, Dhand., Schultz. and NJ1998), could be exploited by S. mansoni for inactivating the complement system of the host for the purpose of immune evasion. Another possible role of the CES on the surface of S. mansoni could be to neutralize harmful/foreign host molecules which pose a threat to the parasite's survival, specifically those stemming from anti-parasite immune activity. Members of the CES family from the liver microsome in the endoplasmic reticulum are known to hydrolyse and inactivate foreign substances such as toxins, although their physiological role in vivo is as yet unclear (Krishnasamy et al. Reference Krishnasamy, Teng, Dhand., Schultz. and NJ1998).
Mouse CES 1C has been shown to be capable of exhibiting convertase activity of lung surfactant subtypes (Genetta et al. Reference Genetta, Deustachio, Kadner and Finlay1988, Krishnasamy et al. Reference Krishnasamy, Teng, Dhand., Schultz. and NJ1998). Schistosoma mansoni could thus perhaps ‘use’ the CES 1C obtained from its mouse host's blood to ease its passage through the host blood vessels as it may help maintain blood vessel stability by inhibiting blood vessel constriction. Mouse blood CES is known to metabolize several pharmaceutical compounds such as temocapril: an angiotensin converting enzyme inhibitor (Takai et al. Reference Takai, Matsuda, Usami, Adachi, Sugiyama, Katagiri, Tatematsu and Hirano1997; Bahar et al. Reference Bahar, Ohura, Ogihara and Imai2012). Angiotensin converting enzyme plays an important physiological role, the outcome of which is a constriction of blood vessels, thereby raising blood pressure. Consistent with this pharmacological role, the enzyme has previously been found by indirect immunofluorescence to be present on the surface of mouse lung-derived schistosomula, but not on the surface of mechanically transformed larvae (Darani and Doenhoff, Reference Darani and Doenhoff2008).
Mammalian CESs are known to be involved in lipid metabolism (Meyer et al. Reference Meyer, Meyer and Bueding1970, Smith et al. Reference Smith, Brooks and Lockard1970; Holmes et al. Reference Holmes, Wright, Laulederkind, Cox, Hosokawa, Imai, Ishibashi, Lehner, Miyazaki, Perkins, Potter, Redinbo, Robert, Satoh, Yamashita, Yan, Yokoi, Zechner and Maltais2010). The metabolic potential of CES could be manipulated by S. mansoni in hydrolysing acquired host lipids and lipoproteins, as the parasite cannot synthesize all its needed fatty acids and steroids ab initio (Brouwers et al. Reference Brouwers, Smeenk, van Golde and Tielens1997; Berriman et al. Reference Berriman, Haas, LoVerde, Wilson, Dillon, Cerqueira, Mashiyama, Al-Lazikani, Andrade, Ashton, Aslett, Bartholomeu, Blandin, Caffrey, Coghlan, Coulson, Day, Delcher, DeMarco, Djikeng, Eyre, Gamble, Ghedin, Gu, Hertz-Fowler, Hirai, Hirai, Houston, Ivens, Johnston, Lacerda, Macedo, McVeigh, Ning, Oliveira, Overington, Parkhill, Pertea, Pierce, Protasio, Quail, Rajandream, Rogers, Sajid, Salzberg, Stanke, Tivey, White, Williams, Wortman, Wu, Zamanian, Zerlotini, Fraser-Liggett, Barrell and El-Sayed2009). These are important in the parasite's survival as they serve a crucial role in nutrition, membrane synthesis and maintenance (Furlong, Reference Furlong1991, Furlong et al. Reference Furlong, Thibault and Rogers1992; Dinguirard and Yoshino, Reference Dinguirard and Yoshino2006). The high intensity of immunofluorescence observed herein on mouse-derived adult worms, particularly on the female, could be directly proportional to the above metabolic roles which are crucial for their survival.
Paradoxically, none of the above possible roles of blood-borne CES enable S. mansoni to survive well in the rat, a host considered relatively non-permissive for this schistosome species (Cioli et al. Reference Cioli, Knopf and Senft1977), nor apparently is a presence of such an enzyme in the blood of humans a necessity for the S. mansoni-permissiveness of that host species. However, the successful characterization of a carboxylesterase seemingly present in extracts of S. mansoni is an addition to the list of host-derived molecules acquired by this schistosome species and is the first enzyme to be recorded to have this role. The means by which the molecule adheres to the schistosome remains to be determined. Moreover, the existence of a similar serine carboxylesterase in humans, released by alveolar macrophages, is noteworthy, but investigations to ascertain if it is exploited by the parasite in any of the ways suggested here for mouse CES 1C would of course be difficult.
ACKNOWLEDGEMENTS
We appreciate the intellectual and technical contributions of Dr Heidi Fuller, Katharine Wayland, Dr Wander de-Jesus Jeremias, Dr Alexandra Morassutti, Dr Mulugeta Aemero, Mrs Ann Lowe, Prof Jerzy Behnke and Dr Emily Dawson. Animals used in this study were maintained according to regulations set out by the UK government and permitted under legislation specified by the Animals (Scientific Procedures) Act 1986.
FINANCIAL SUPPORT
This research was partly funded by the University of Nottingham International Research Excellence scholarship 2011 (to J.I.).














