Introduction
A detailed understanding of the reproductive biology of native plant species (especially those that are endemic or threatened) is fundamental to our understanding of plant community dynamics (Thompson et al., Reference Thompson, Band and Hodgson1993; Lunt, Reference Lunt1995) and detailed knowledge of these processes will be essential for the conservation of populations directly affected by anthropogenic environmental degradation, observed in large areas designated as Brazilian high-altitude rocky complexes (Benites et al., Reference Benites, Caiafa, Mendonça, Schaeffer and Ker2003).
Brazilian high-altitude rocky complexes are ecosystems that occur at altitudes above 800 m above sea-level (asl) and are associated with a discontinuous mountain chain known as the Espinhaço Range that stretches in a north–south direction through north-eastern and south-eastern Brazil (Daly and Mitchell, Reference Daly, Mitchell and Lentz2000). These environments have characteristic soils and their vegetation is distinct from neighbouring areas, demonstrating high levels of diversity and endemism (Benites et al., Reference Benites, Caiafa, Mendonça, Schaeffer and Ker2003). The vegetation, known as campos rupestres, grows directly on the rocks themselves (quartzites) or in shallow soils with low water retention capacities that are poor in nutrients and extremely acidic (Giulietti et al., Reference Giulietti, Pirani, Harley, Davis, Heywood, Herrera-MacBryde, Villa-Lobos and Hamilton1997; Benites et al., Reference Benites, Schaefer, Simas and Santos2007). These conditions restrict the possibilities of colonization by many plants, but create opportunities for the establishment of species with xeromorphic characteristics, such as leaves that are coriaceous or reduced to thorns, with waxy coverings or abundant trichomes, succulence, and crassulacean acid metabolism (CAM) and C4 photosynthetic metabolism (Giulietti et al., Reference Giulietti, Pirani, Harley, Davis, Heywood, Herrera-MacBryde, Villa-Lobos and Hamilton1997).
The cactus family is well represented in campos rupestres vegetation, and has high species richness and endemism (Taylor and Zappi, Reference Taylor and Zappi2004). Among the many genera occurring in this environment, the genus Arthrocereus A. Berger comprises four species (one of which comprises three subspecies) that are endemic to Brazil and have columnar habits, highly restricted distributions and occupy very specific habitats. Three of these species are exclusive to the southern region of the Cadeia do Espinhaço Range (Taylor and Zappi, Reference Taylor and Zappi2004) and are classified as threatened to extinction (COPAM, 2008) due to their small population sizes and to the intense impact of human activities in occurrence areas (Giulietti et al., Reference Giulietti, Pirani, Harley, Davis, Heywood, Herrera-MacBryde, Villa-Lobos and Hamilton1997; Drummond et al., Reference Drummond, Martins, Machado, Sebaio and Antonini2005).
Studies concerning the germination behaviour of Arthrocereus seeds and their capacity to form persistent banks in the soil can aid in the management and conservation of these species. Germination processes involve the interaction of several abiotic factors such as temperature, light and soil moisture availability (Baskin and Baskin, Reference Baskin and Baskin1988), and these environmental factors have a fundamental role in the natural selection of strategies that reduce mortality and increase the probability of successful establishment (Daws et al., Reference Daws, Burslem, Crabtree, Kirkman, Mullins and Dalling2002; Jurado and Flores, Reference Jurado and Flores2005). Seed-bank formation constitutes an important strategy for plants that experience long periods under severe climatic conditions that may be unfavourable for future seedling development and survival (Moles et al., Reference Moles, Warton and Westoby2003).
Several species belonging to the Cactaceae family have been studied to determine their seed characteristics, their germination behaviour and their potential for forming soil seed-banks (de Viana, Reference de Viana1999; Bowers, Reference Bowers2000, Reference Bowers2005; Rojas-Aréchiga and Batis, Reference Rojas-Aréchiga and Batis2001; Montiel and Montaña, Reference Montiel and Montaña2003), showing the existence of a wide diversity of habits, germination characteristics and seed longevity (Rojas-Aréchiga and Vázquez-Yanes, Reference Rojas-Aréchiga and Vázquez-Yanes2000; Flores et al., Reference Flores, Jurado and Arredondo2006). In light of our lack of knowledge about the reproductive biology of the genus Arthrocereus and the fact that its subordinate taxa have very restricted distributions, the present study investigated the germination ecology of Arthrocereus spp. and their potential to form seed banks, in order to answer the following questions: (1) due to their close phylogenetic relationships, do the seeds of Arthrocereus spp. demonstrate similar germination patterns; (2) are these germination responses associated with the light and temperature conditions to which the seeds are exposed in their natural habitats; and (3) can the seeds of the taxa studied form persistent seed-banks in the soil?
Materials and methods
Areas and taxa studied
The fruits of four species of Arthrocereus were collected in 2007 and 2008 from natural populations growing within four different Conservation Reserves in the southern region of the Cadeia do Espinhaço Range in Minas Gerais State, south-eastern Brazil (Table 1). The climate in this region of the Espinhaço Range is classified as tropical–altitudinal (Cwb in the classification system of Köppen, Reference Köppen1923) and is characterized by dry winters and rainy summers, with an average annual temperature between 17 and 23°C. The average annual rainfall is near 1500 mm, with more than 80% being concentrated in the summer/rainy season (Galvão and Nimer, Reference Galvão and Nimer1965; INMET, 2008).
Table 1 Taxa of Arthrocereus studied, their collection site, geographical position, altitude (metres asl), date of collection (month/year) and conservation status (sensu COPAM, 2008)

EPA, Environmental Protection Area; SP, State Park; NP, National Park; CR, critically endangered; EN, endangered; VU, vulnerable.
Four taxa of Arthrocereus were studied, consisting of two species, one of which consists of three subspecies (Table 1). Reference specimens were deposited in the Herbarium of the Departamento de Botânica, Universidade Federal de Minas Gerais, Brazil (BHCB Herbarium). Another species that occurs in this same region was not found with fruits during the study period, so it was not included in the study.
Fruit and seed measurements, germination and longevity
Seeds from the collected fruits were washed in running water to remove the fibrous pulp and subsequently left to dry for 3 d in the shade at room temperature (25 ± 2°C). Twenty fruits from at least 20 individuals of each taxon were collected to evaluate the average fruit size and the average number of seeds per fruit. The average seed size was obtained with a digital caliper using a sample of 200 seeds per species. To calculate the average dry mass of the seeds, four samples of 50 seeds for each species were weighed in an analytical balance (after drying to a constant weight at 105°C).
The germination of freshly collected seeds was evaluated at six different constant temperatures (10, 15, 20, 25, 30 and 35°C) under a 12-h photoperiod (30 μmol m− 2 s− 1), as well as in constant darkness, using samples of 100 seeds per treatment (four replicates of 25 seeds each). The seeds were germinated in Petri dishes lined with double layers of filter paper moistened with a solution of Nistatina fungicide (Medley, Brazil) (Oliveira and Garcia, Reference Oliveira and Garcia2011). Petri dishes submitted to the dark treatment were stored in opaque acrylic containers and were examined under green safe light. Germination (emergence of the radicle) was assessed daily until the germination percentage remained constant for ten consecutive days (or for 60 d in experiments showing no germination).
Longevity of A. glaziovii, A. melanurus subsp. magnus and A. melanurus subsp. odorus seeds was evaluated, but we did not collect sufficient amounts of A. melanurus subsp. melanurus seeds to perform this experiment. The potential for soil seed-bank formation was evaluated by burying the seeds and subsequently examining their germinability at regular intervals (Lunt, Reference Lunt1995; van Mourik et al., Reference van Mourik, Stomph and Murdoch2005; Buhk and Hensen, Reference Buhk and Hensen2008). The seeds were buried (up to 3 months after being collected) in nylon bags (0.5 mm weave) containing 25 seeds each at depths of approximately 5 cm in the same localities in which they naturally occur (Table 1). Four bags per taxon were removed to perform the germination tests every 2 months for the first 6 months of soil storage; after this time, the bags were removed at intervals of 4 months until reaching 14 months of storage. The seeds were tested in the laboratory for germinability under a 12-h photoperiod (30 μmol m− 2 s− 1) at their respective optimal germination temperatures (which had been pre-determined for each taxon in earlier germination tests). Seeds of A. glaziovii, A. melanurus subsp. magnus and A. melanurus subsp. odorus were also stored dry in amber-coloured flasks at room temperature for 12 months. At the end of this 12-month laboratory storage period their germinability was tested at their respective optimal temperature with a 12-h photoperiod (30 μmol m− 2 s− 1).
Data analysis
For each studied species we obtained final germination percentage and mean germination time ![]() , where n is the number of germinated seeds at day D and D is the number of days since the beginning of the experiment. The average time was calculated only when the average percentages of germination were greater than 10%. The optimal germination temperature was considered the one that gave the highest germination rate associated with the least mean germination time. Resulting data related to germination percentage and mean germination time were submitted to non-parametric statistical tests as they did not demonstrate normality and variance homogeneity. The Kruskal–Wallis test followed by pair comparisons using the Conover test was used to compare the temperatures and soil storage times. Resulting data related to the germination of recently collected seeds and those stored in the laboratory were compared using the Mann–Whitney test. The statistical analyses were performed separately for each species using BrightStat Software (Stricker, Reference Stricker2008) at a significance level of P < 0.05.
, where n is the number of germinated seeds at day D and D is the number of days since the beginning of the experiment. The average time was calculated only when the average percentages of germination were greater than 10%. The optimal germination temperature was considered the one that gave the highest germination rate associated with the least mean germination time. Resulting data related to germination percentage and mean germination time were submitted to non-parametric statistical tests as they did not demonstrate normality and variance homogeneity. The Kruskal–Wallis test followed by pair comparisons using the Conover test was used to compare the temperatures and soil storage times. Resulting data related to the germination of recently collected seeds and those stored in the laboratory were compared using the Mann–Whitney test. The statistical analyses were performed separately for each species using BrightStat Software (Stricker, Reference Stricker2008) at a significance level of P < 0.05.
Results
Fruit and seed measurements
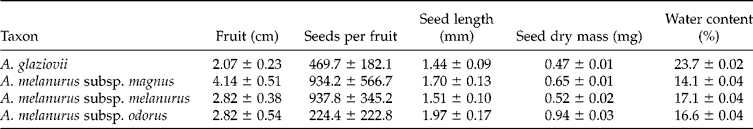
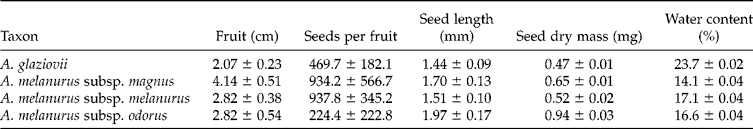
The four Arthrocereus taxa showed fleshy fruits with average sizes varying from 2.07 to 4.14 cm. Seeds were ovoid and compact, showing values between 1.44 and 1.97 mm long, and a dry mass ranging from 0.47 to 0.94 mg (Table 2). Number of seeds per fruit varied widely, both intra- and inter-specifically, resulting in high standard deviations. Seed water contents varied from 14.1 to 23.7%.
Table 2 Fruit and seed length, number of seeds per fruit (mean±standard deviation), dry mass and seed water content (mean±standard error) of Arthrocereus taxa
Germination
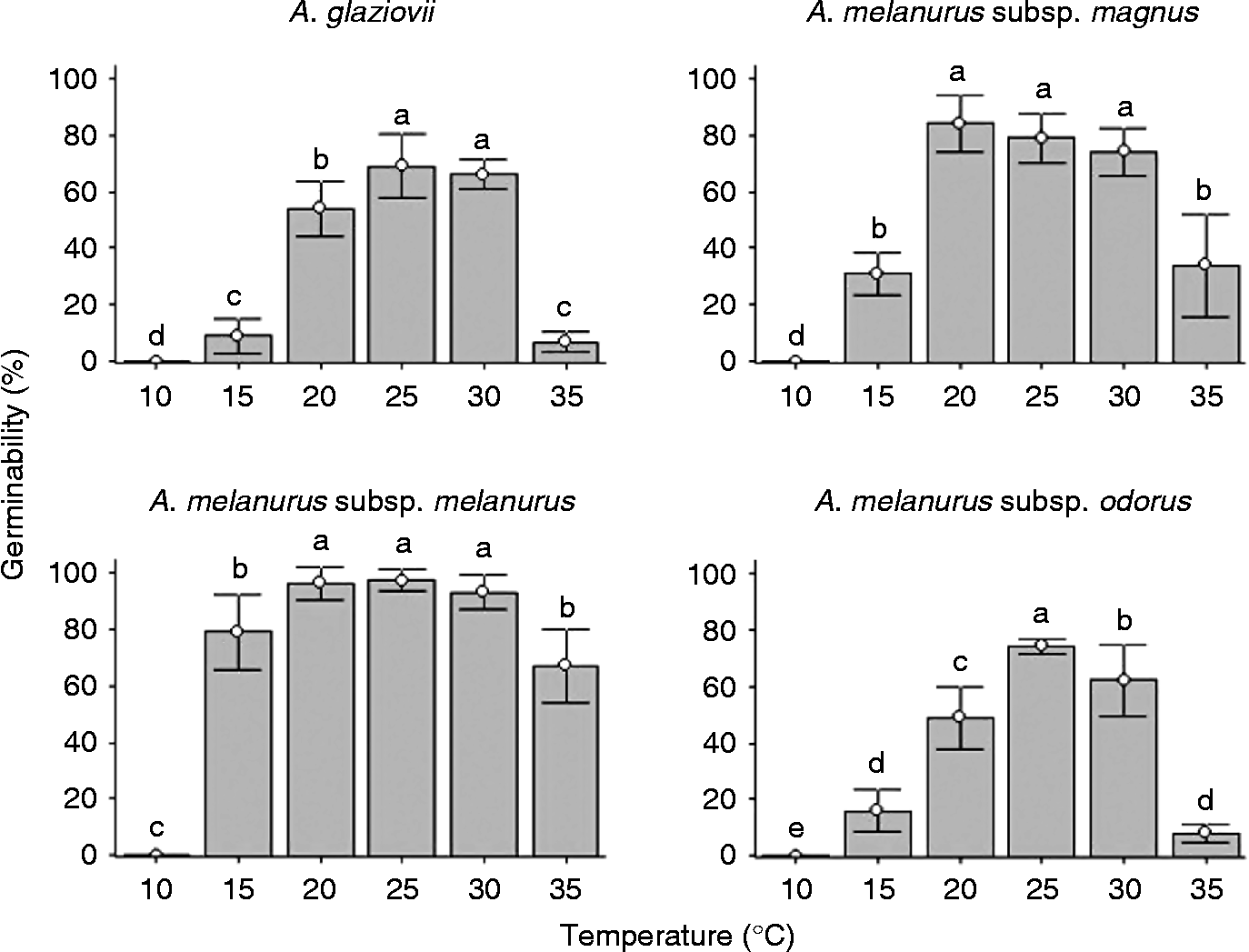
Seeds of A. glaziovii demonstrated germinability near 70% at temperatures of 25 and 30°C, but much lower percentages ( < 10%) at higher or lower temperatures (Fig. 1). There were significant variations in the germination percentage among A. melanurus subspecies, although they all demonstrated the same pattern (and similar percentages) between 20 and 30°C, but with a lower germination percentage at more extreme temperatures (15 and 35°C). The subspecies A. melanurus subsp. melanurus was distinct from the other taxa by having higher germinability percentages (Fig. 1). No taxa showed germination at 10°C in the presence of light, or at any temperature when maintained in the dark.
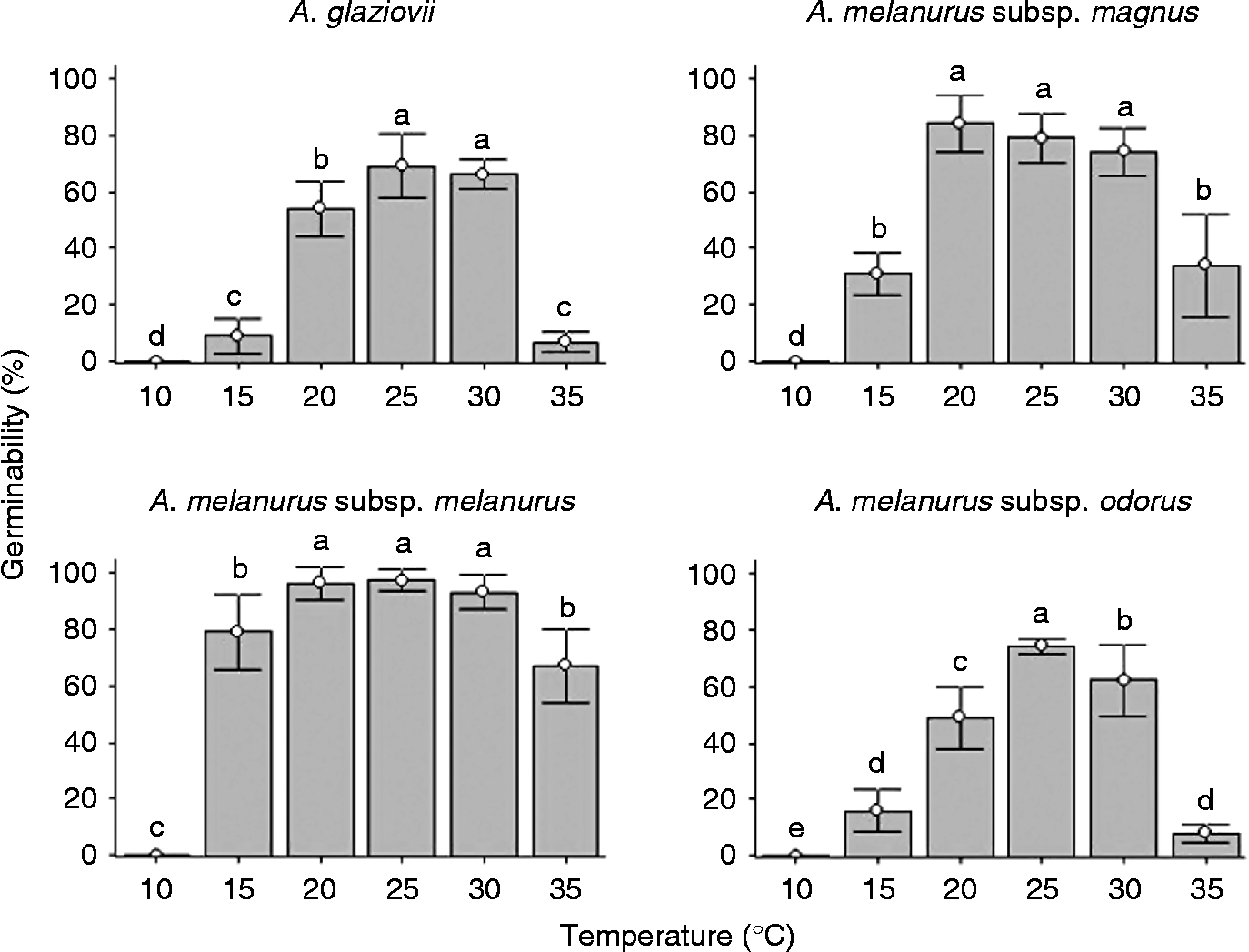
Figure 1 Germinability of fresh seeds of Arthrocereus taxa under constant temperatures and 12-h photoperiod. Bars represent standard deviation. Different letters show significant differences with Conover test (5%).
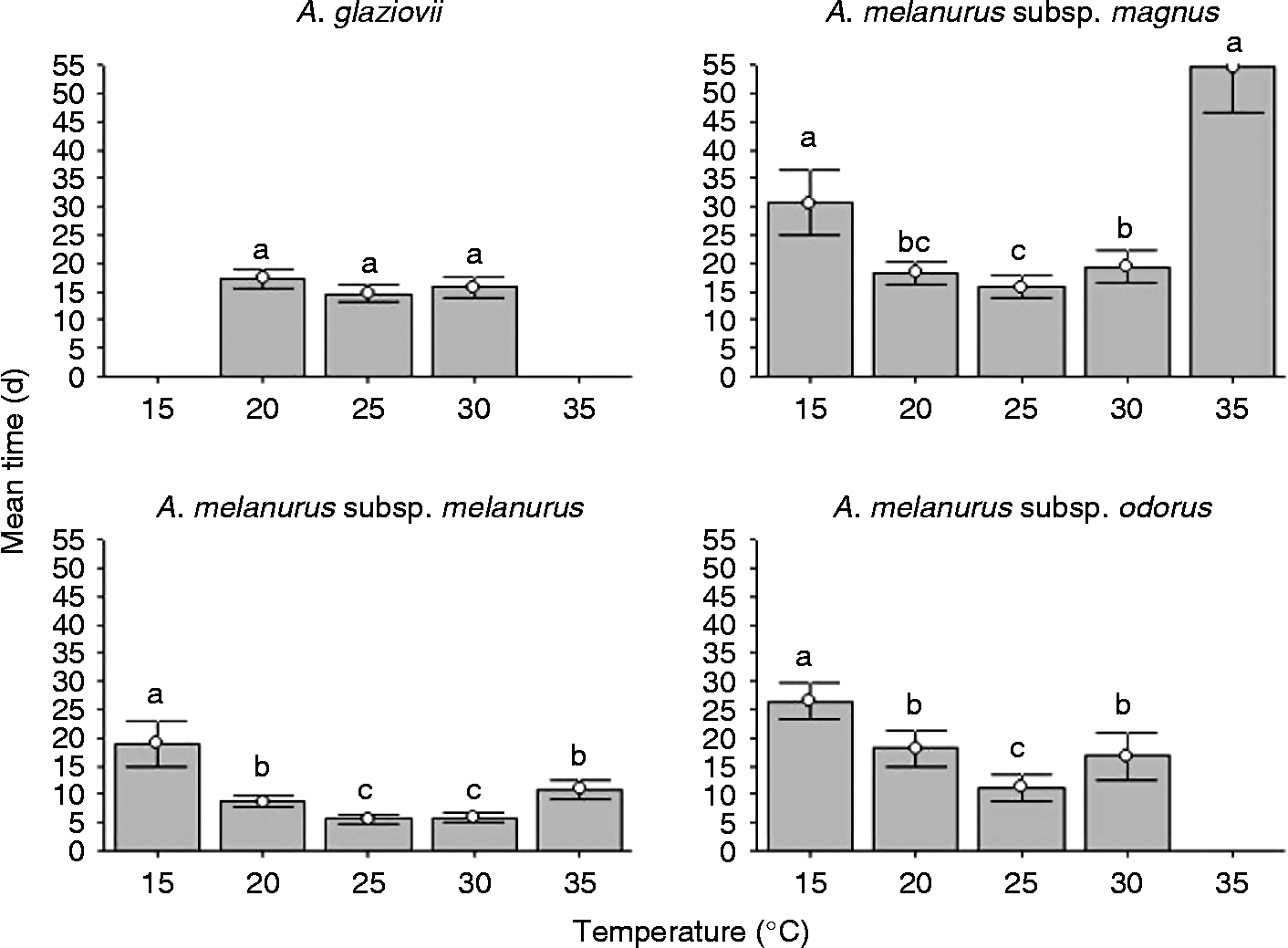
Although mean germination time differed among the different taxa, all of them demonstrated a significant increase in germination time at extreme temperatures (15 and 35°C) (Fig. 2). Seeds of A. glaziovii had a mean germination time of approximately 16 d at 20–30°C. Arthrocereus melanurus subsp. magnus had a mean germination time of approximately 17 d at 20 and 25°C, and the highest mean germination times (55 and 31 d) at 35 and 15°C, respectively. A. melanurus subsp. melanurus had the lowest mean germination time (approximately 6 d at 25 and 30°C, and 19 d at 15°C). A. melanurus subsp. odorus seeds had a mean germination time that varied from 11 days at 25°C to 26 d at 15°C.
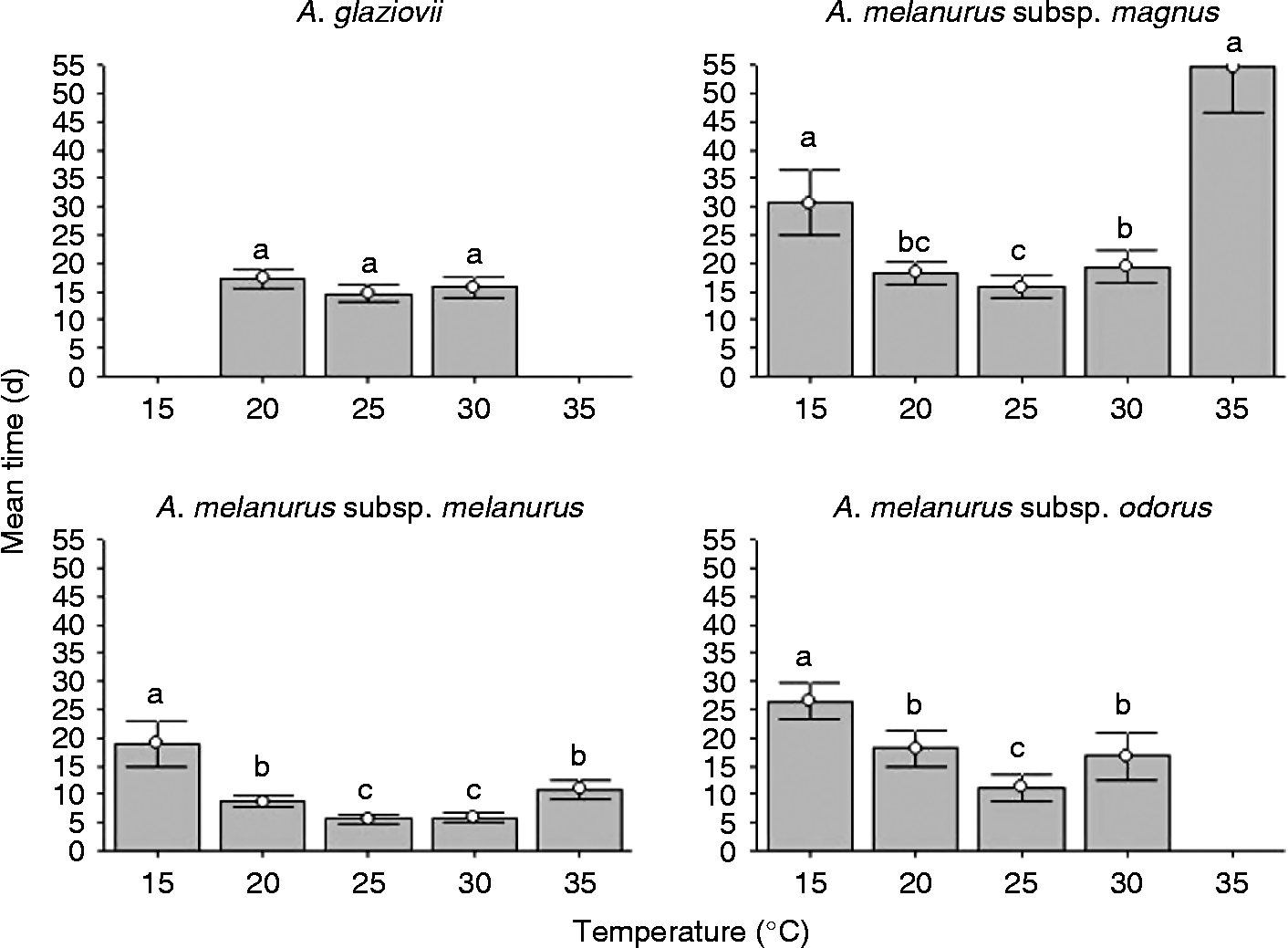
Figure 2 Mean germination time (d) of fresh seeds of Arthrocereus taxa exposed to five different constant temperatures and a 12-h photoperiod. Bars represent standard deviation. Different letters show significant differences with the Conover test (5%).
Considering the highest germination rate and the least mean germination time, the optimal germination temperature varied among the taxa, being 25 and 30°C for A. glaziovii and A. melanurus subsp. melanurus, 20 and 25°C for A. melanurus subsp. magnus, and 25°C for A. melanurus subsp. odorus.
Longevity
All buried seeds of all taxa were found to be intact and with no signs of deterioration or predation after 14 months of storage in the soil. Germinability of A. glaziovii seeds varied between 60 and 90% (Fig. 3), and no significant differences were observed in their germination percentages after 14 months of the experiment in relation to recently collected seeds. The mean germination time of the seeds stored in the soil was observed to diminish in relation to recently collected seeds (15 d), with much lower values (approximately 8 d) in the fourth, sixth and fourteenth soil-storage months (Fig. 3). Dry storage for 12 months in the laboratory did not alter seed germinability (69 and 67% for recently collected seeds and seeds stored for 1 year, respectively; P = 1.0).
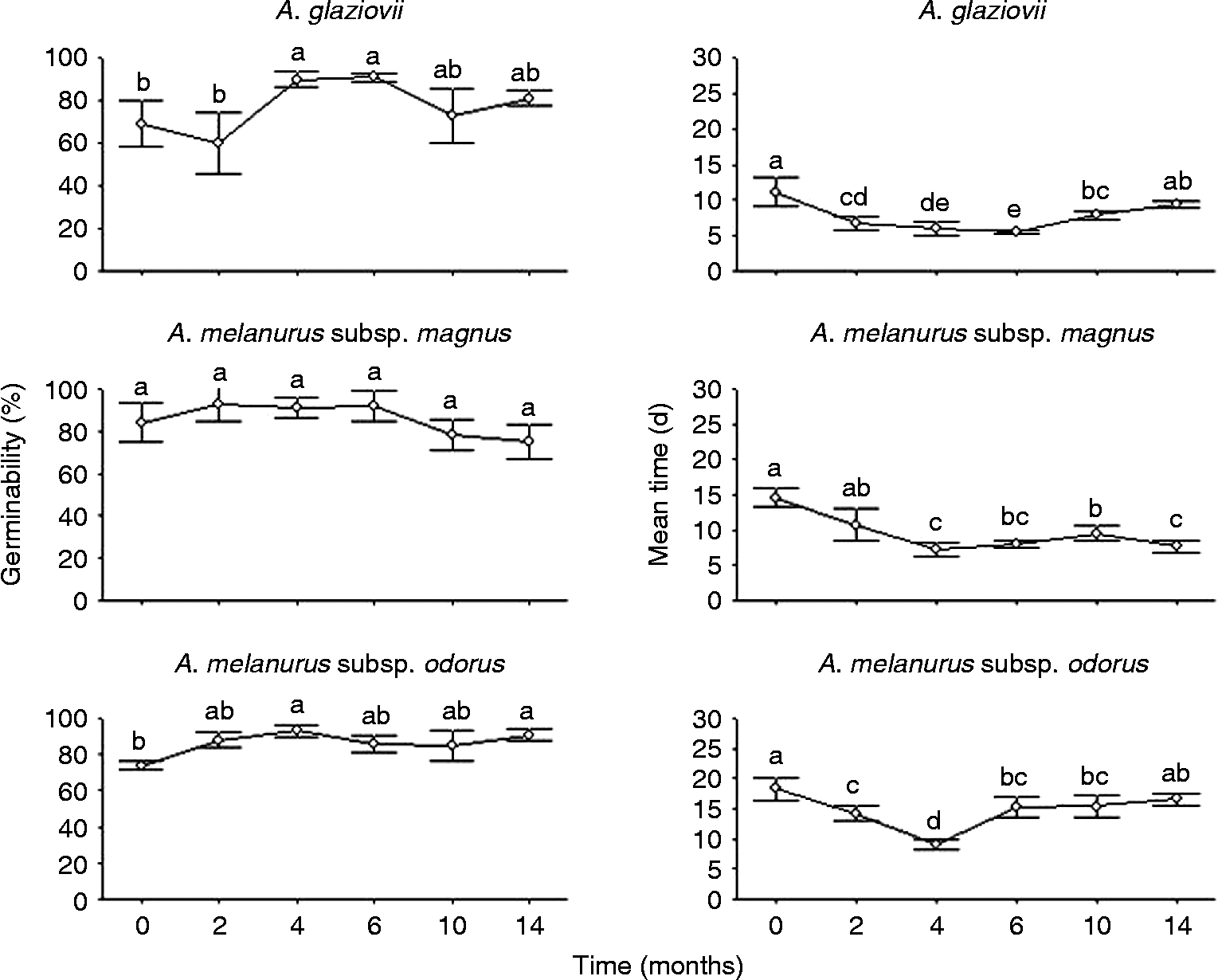
Figure 3 Germinability (%) and mean germination time (d), at 25°C and 12-h photoperiod, of three Arthocereus taxa seeds buried in the soil for 14 months under natural conditions. Data correspond to the mean ± SE of measurements made on seeds from four different bags with 25 seeds each. Different letters show significant differences with the Conover test (5%).
Germination percentages of A. melanurus subsp. magnus seeds remained high during the entire period of soil storage (Fig. 3), with 75% germination at the end of 14 months, with no significant differences from recently collected seeds (84%). The average germination time (9–17 d) for this species varied depending on the seed storage time, although no significant differences were observed between recently collected seeds and those stored for 14 months (Fig. 3). The germinability of the seeds stored for 12 months in the laboratory did not differ from the recently collected seeds (95% and 84%, respectively; P = 0.92).
Storing A. melanurus subsp. odorus seeds in the soil favoured their germination and they maintained high germinability (greater than 80%) through the fourteenth month (Fig. 3). The mean germination time varied from 5 to 9 d during the storage periods tested, and no significant differences were observed between 14-month-old and recently collected seeds (Fig. 3). Seeds stored for 12 months in the laboratory maintained a high germinability and did not differ significantly from recently collected seeds (84% and 74%, respectively; P = 0.06).
Discussion
Arthrocereus species studied have fleshy fruits with fibrous pulp and sweet odour. These fruits constitute an important food resource for the native fauna of campos rupestres areas and are consumed by the wolf Chrysocyon brachyurus (Aragona and Setz, Reference Aragona and Setz2001), as well as by ants and rodents (as observed during this study). Large intra- and inter-specific variations in the number of seeds per fruit have been reported for other species of the Cactaceae (Rojas-Aréchiga and Vázquez-Yanes, Reference Rojas-Aréchiga and Vázquez-Yanes2000; Godínez-Álvarez et al., Reference Godínez-Álvarez, Valverde and Ortega-Baes2003) and seed number may depend on the age and size of the individual plants, as well as the number of fruits per plant (Rojas-Aréchiga and Vázquez-Yanes, Reference Rojas-Aréchiga and Vázquez-Yanes2000). Observed seed sizes on studied species were similar to those reported for other columnar cactus genera endemic to areas of campos rupestres vegetation (including Cipocereus, Micranthocereus and Pilosocereus) (Zappi and Taylor, Reference Zappi and Taylor2003).
The seeds of the taxa studied here demonstrated an absolute light requirement for germination, a phenomenon common in the Cactaceae (Rojas-Aréchiga and Vázquez-Yanes, Reference Rojas-Aréchiga and Vázquez-Yanes2000; de la Barrera and Nobel, Reference de la Barrera and Nobel2003; Benítez-Rodríguez et al., Reference Benítez-Rodríguez, Orozco-Segovia and Rojas-Aréchiga2004; Bowers, Reference Bowers2005; Flores et al., Reference Flores, Jurado and Arredondo2006, Reference Flores, Jurado, Chapa-Vargas, Ceroni-Stuva, Dávila-Aranda, Galíndez, Gurvich, León-Lobos, Ortega-Baes, Seal, Ulian and Pritchard2011; Ortega-Baes and Rojas-Aréchiga, Reference Ortega-Baes and Rojas-Aréchiga2007; Rojas-Aréchiga, Reference Rojas-Aréchiga2008; Rojas-Aréchiga et al., Reference Rojas-Aréchiga, Golubov, Romero and Mandujano2008; Meiado et al., Reference Meiado, Correa de Albuquerque, Rocha, Rojas-Aréchiga and Leal2010; Ortega-Baes et al., Reference Ortega-Baes, Aparício, Galíndez, del Fueyo, Sühring and Rojas-Aréchiga2010), as well as in many other campos rupestres plant families, such as Eriocaulaceae, Xyridaceae, Melastomataceae and Gesneriaceae (Silveira et al., Reference Silveira, Negreiros and Fernandes2004; Abreu and Garcia, Reference Abreu and Garcia2005; Oliveira and Garcia, Reference Oliveira and Garcia2005, Reference Oliveira and Garcia2011; Garcia et al., Reference Garcia, Barros and Lemos Filho2006; Ranieri et al., Reference Ranieri, Pezzini, Garcia, Chautems and França2011).
This light requirement for germination is probably related to the small size of these seeds ( < 2 mm and < 1 mg). Small seeds contain only limited nutrient reserves, and these are insufficient for deeply buried seedlings to reach the soil surface and initiate photosynthesis (Pons, Reference Pons and Fenner2000). As sunlight will only penetrate a few millimetres into the soil (Milberg et al., Reference Milberg, Andersson and Thompson2000; Pons, Reference Pons and Fenner2000), this light dependence represents an effective impediment to the germination of small seeds that are buried under more than just a very thin layer of soil.
In the presence of light, the seeds of Arthrocereus showed high germination percentages at intermediate temperatures (between 20 and 30°C), but low or nil germinability at 10, 15 and 35°C. Temperature is one of the principal environmental factors that can synchronize seed germination with environmental conditions favourable to seedling development (Probert, Reference Probert and Fenner1992), especially in habitats with marked seasonality such as the campos rupestres. Low seed germination under unfavourable conditions increases the chances of seedling survival (Venable and Lawlor, Reference Venable and Lawlor1980). The low germinability of Arthrocereus seeds at low temperatures (10 and 15°C) represents a mechanism that will constrain the germination of most seeds during the winter months when the climate is cold and dry in campos rupestres environments. Seed germination reduction was only partial, however, and may reflect the fact that winter months occasionally provide sufficient humidity for germination and growth at campos rupestres environments that tend to have high humidity levels and dew at night during that season (Giulietti et al., Reference Giulietti, Pirani, Harley, Davis, Heywood, Herrera-MacBryde, Villa-Lobos and Hamilton1997). At the highest temperature tested (35°C), seedlings may suffer deleterious effects from high transpiration rates. Some cacti can perform C3 photosynthesis during their first months of life after germination, which makes them more susceptible to dehydration at higher temperatures (de la Barrera and Nobel, Reference de la Barrera and Nobel2003).
The capacity of seeds to respond to adequate environmental conditions for germination and growth is an adaptation that increases the probability for their successful establishment (Venable and Lawlor, Reference Venable and Lawlor1980; Meyer and Kitchen, Reference Meyer and Kitchen1994), and the time required for germination is critical to respond adequately to environmental changes. Seeds of the taxa studied here germinated fairly slowly, requiring more than 10 d after exposure to light and optimal temperatures to reach maximum germination capacity. Such slow germination is common in other campos rupestres species (Silveira et al., Reference Silveira, Negreiros and Fernandes2004; Abreu and Garcia, Reference Abreu and Garcia2005; Garcia et al., Reference Garcia, Barros and Lemos Filho2006, Reference Garcia, Jacobi and Ribeiro2007) as well as in species that occur in other areas with marked seasonal climates, such as Mediterranean-type climates (García-Fayos and Verdú, Reference García-Fayos and Verdú1998; Llorens et al., Reference Llorens, Pons, Gil and Boira2008) and deserts (Bowers, Reference Bowers2000; Flores et al., Reference Flores, Jurado and Arredondo2006). However, relatively slow germination can prevent the simultaneous germination of all of the seed pool in the seed bank during the brief winter rains that are generally insufficient for successful seedling establishment (Bowers, Reference Bowers2000). As such, the longer continuously humid periods required by Arthrocereus seeds favour the germination of most seeds during the rainy season, a period of reasonably regular rains that can better guarantee successive days of the abundant water resources needed for successful seedling establishment. This slow germination adaptive strategy is very important in inhospitable edapho-climatic environments such as campos rupestres, where soils are thin and have only limited capacities to retain humidity (Benites et al., Reference Benites, Schaefer, Simas and Santos2007) and dry periods last from five to seven consecutive months.
Burial did not alter the viability or germinability of Arthrocereus spp. seeds, and there was no evidence of their decomposition or physiological death. Seeds did not acquire dormancy, nor was there any loss of seeds due to precocious germination (due to their absolute light requirement). As a result, essentially all of the seeds of these species that become buried in the soil may be incorporated into the soil seed-bank. Burial and seed movement within the soil are influenced by both abiotic (water, soil characteristics) and biotic (fauna, seed characteristics) factors (Garwood, Reference Garwood, Leck, Parker and Simpson1989; Marone et al., Reference Marone, Rossi and Horno1998). The high frequency of rainfalls during the time when the seeds of the species studied here are dispersed, the high porosity of campos rupestres soils (Benites et al., Reference Benites, Schaefer, Simas and Santos2007) and the small size of the seeds of Arthrocereus spp. are all factors that may favour their burial. Small seeds are generally produced in large quantities (Moles et al., Reference Moles, Warton and Westoby2003) and are more easily buried (Thompson et al., Reference Thompson, Band and Hodgson1993; Bekker et al., Reference Bekker, Bakker, Grandin, Kalamees, Milberg, Poschlod, Thompson and Willems1998; Hölzel and Otte, Reference Hölzel and Otte2004), as they can penetrate into small fissures in the soil or be buried through the action of rainwater (Thompson et al., Reference Thompson, Band and Hodgson1993). In addition to these characteristics, the low germination velocity observed among the species studied here will increase their probability of being buried in the soil before they can germinate, thus increasing their potential to form seed banks.
Considering that the seeds of Arthrocereus spp. have an absolute light requirement for germination and can remain viable for more than a year when buried, it is possible to infer that they could be able to form a persistent seed-bank (sensu Thompson, Reference Thompson, Hendry and Grime1993). This fact is of significant importance for the conservation of these species as soil seed-banks aid in maintaining genetic diversity at the population level, reduce their vulnerability to local extinction (Stöcklin and Fischer, Reference Stöcklin and Fischer1999; Hill and Vander Kloet, Reference Hill and Vander Kloet2005) and foster natural regeneration after local disturbances (Parker et al., Reference Parker, Simpson, Leck, Leck, Parker and Simpson1989).
Additionally, the fact that Arthrocereus seeds retain their viability when stored under dry conditions makes it practical to maintain ex situ germplasm banks – a complementary strategy for their genetic preservation through restoration programmes (Vázquez-Yanes and Rojas-Aréchiga, Reference Vázquez-Yanes and Rojas-Aréchiga1996; Degreef et al., Reference Degreef, Rocha, Vanderborght and Baudoin2002). Due to the widespread destruction of the natural habitats of Arthrocereus species (which are now restricted to just a few small, preserved fragments) and reduced population size (IUCN, 2001), and the fact that all of the taxa studied here are threatened with extinction, their capacity to form soil seed-banks together with the possibility of maintaining the seeds ex situ offer viable options for their future conservation.
Our results have demonstrated germination behaviour patterns in Arthrocereus spp. that are adaptations to the seasonality of their natural environments. Seeds are small and sensitive to light, and are slow germinators even under a wide temperature range. Seed dispersal occurs during the summer and germination may occur immediately after dispersal if seeds are exposed to light and there is sufficient humidity and favourable environmental temperatures. Their slow germination may act, however, to limit the extent of their germination during the first rainy season, and their reduced germinability at extreme temperatures will impede or slow germination when environmental conditions are less than optimal for seedling establishment. Buried seeds will not be able to germinate but can maintain their viability for periods longer than a year, thus demonstrating the capacity to form persistent soil seed-banks.
Acknowledgements
The authors thank Coordenação de Aperfeiçoamento do Ensino Superior (CAPES) for the study grant awarded to the first author and Conselho Nacional de Pesquisa (CNPq) for the research productivity scholarship awarded to Q.S.G. We thank L. Arruda for his assistance during the execution of the field and laboratory work and we also greatly appreciate the comments and corrections suggested by M. Rojas-Aréchiga, L. Arruda and F. Vieira on an earlier draft of this paper.