INTRODUCTION
While habitat loss and poaching are directly related to wild cat (felid) population declines, other contributing factors (e.g. indirect effects) could exacerbate such declines. For example, human–felid conflict (H-FC) has increased substantially (Inskip & Zimmerman Reference INSKIP and ZIMMERMANN2009) resulting in harassment and retaliatory killing of felids, particularly jaguar and puma. Potential indirect consequences of H-FC could be an increase in adrenal activity that negatively impacts reproductive rates and animal health, and heightens animal aggression resulting in more H-FC (Dobson & Smith Reference DOBSON and SMITH2000, Koolhaas et al. Reference KOOLHAAS, KORTE, DE BOER, VAN DER VEGT, VAN REENEN, HOPSTER, DE JONGA, RUIS and BLOKHUIS1999, Kruk et al. Reference KRUK, HALÁSZ, MEELIS and HALLER2004, Sgoifo et al. Reference SGOIFO, DE BOER, HALLER and KOOLHAAS1996). The detrimental effects of chronic increased adrenal activity in mammals are well documented and include suppression of reproductive, immune and neurological functions (Maccari & Morley-Fletcher Reference MACCARI and MORLEY-FLETCHER2007, Sapolsky et al. Reference SAPOLSKY, ROMERO and MUNCK2000, Zhao et al. Reference ZHAO, XU, XU and YOUNG2007). Adrenal activity has been measured in the form of faecal glucocorticoid metabolites (FGM) in captive Neotropical felids (Mesa-Cruz et al. Reference MESA-CRUZ, BROWN and KELLY2014, Morato et al. Reference MORATO, BUENO, MALMHEISTER, VERRESCHI and BARNABE2004, Moreira et al. Reference MOREIRA, BROWN, MORAES, SWANSON and ROYAL2007, Romano et al. Reference ROMANO, RODAS, VALDEZ, HERNÁNDEZ, GALINDO, CANALES and BROUSSET2010), and two species of temperate wild felid (Fanson et al. Reference FANSON, WIELEBNOWSKI, SHENK and LUCAS2012, Piñeiro et al. Reference PIÑEIRO, BARJA, OTERO, SILVÁN and ILLERA2015), but to our knowledge, there are no reports of FGM assessments in any free-living Neotropical felid in its native habitat.
In addition to FGM assessments, coprological (scat) studies provide an opportunity to monitor parasitic infection dynamics (Lafferty Reference LAFFERTY1997). Currently, however, there is little information on parasite loads in wild felids. While there has been a single endoparasite study from a scat felid survey in Cockscomb Basin, Belize (Patton & Rabinowitz Reference PATTON and RABINOWITZ1986), that study occurred only within a protected area, and used less sensitive parasite detection techniques than are currently available.
Scats can also be examined for diet content. A decrease in native prey availability outside protected areas could result in diet shifts that increase livestock predation on cattle for larger cats, and poultry predation for smaller cats, further increasing conflict. The diets of jaguar and puma in human-modified environments have been studied in only a few areas of their range (Cascelli de Azevedo Reference CASCELLI DE AZEVEDO2008, Novack et al. Reference NOVACK, MAIN, SUNQUIST and LABISKY2005, Polisar et al. Reference POLISAR, MAXIT, SCOGNAMILLO, FARRELL, SUNQUIST and EISENBERG2003). In Belize, Rabinowitz (Reference RABINOWITZ1986a) reported that most jaguars roaming in human-modified landscapes around Cockscomb Basin did not prey on livestock, but more recently, in the same region, one out of 10 jaguar scats contained evidence of cattle in the diet, while those of pumas did not contain livestock (Foster et al. Reference FOSTER, HARMSEN and DONCASTER2010). This area, however, is surrounded mostly by small family farms rather than large-scale industrial agriculture, as in our study. Additionally, there is little information on the diet of smaller felids such as ocelot, margay and jaguarundi.
We aimed to compare FGM concentrations, endoparasite species richness (ESR) and prey items in protected and non-protected areas. Obtaining biological data from wild Neotropical felids is challenging due to their secretive nature, thick habitat and the perceived or real risks of aggression towards humans. However, recent advances in non-invasive hormone and DNA analyses have improved the feasibility of scat sampling (Kelly et al. Reference KELLY, BETSCH, WULTSCH, MESA, MILLS, Boitani and Powell2012) and the use of detector dogs to find scat has greatly improved efficiency of sample collection (Long et al. Reference LONG, DONOVAN, MACKAY, ZIELINSKI and BUZAS2007, Wasser et al. Reference WASSER, DAVENPORT, RAMAGE, HUNT, PARKER, CLARKE and STENHOUSE2004, Wultsch et al. Reference WULTSCH, WAITS, HALLERMAN and KELLY2015). We hypothesized that H-FC would result in increased levels of adrenal activity (stress), increased ESR, and shifts in diet towards livestock and/or poultry in felids found outside versus inside protected areas in Belize, Central America.
METHODS
Study site
Belize, Central America, hosts over 150 species of mammal, including five species of wild felid: jaguar (Panthera onca Linnaeus, 1758), puma (Puma concolor Linnaeus, 1771), ocelot (Leopardus pardalis Linnaeus, 1758), jaguarundi (Puma yagouaroundi Geoffroy Saint-Hilaire, 1803) and margay (Leopardus wiedii Schinz, 1821) (Sunquist & Sunquist Reference SUNQUIST and SUNQUIST2002). Within protected areas, Belize has relatively large jaguar, puma and ocelot populations (Dillon & Kelly Reference DILLON and KELLY2007, Kelly et al. Reference KELLY, NOSS, DI BITETTI, MAFFEI, ARISPE, PAVIOLO, DE AGUDELO and DI BLANCO2008, Silver et al. Reference SILVER, OSTRO, MARSH, MAFFEI, NOSS, KELLY, WALLACE, GÓMEZ and AYALA2004), yet little is known about the ecology or factors that impact the well-being of these secretive species outside of protected areas (Foster et al. Reference FOSTER, HARMSEN and DONCASTER2010, Laundré & Hernández Reference LAUNDRÉ, HERNÁNDEZ, Hornocker and Negri2010). Additionally, there is scarce biological information about margay and jaguarundi throughout their entire range.
We conducted scat surveys in north-western Belize in the Rio Bravo Conservation and Management Area (RBCMA), which is the largest private protected area in Belize and one of the largest protected areas in the country (1049 km2). The predominant ecotype is lowland moist forest. The jaguar is found at fairly high densities in the RBCMA (Kelly unpubl. data), while the density of other felids is unknown. The RBCMA is adjacent to non-protected highly modified land, dominated by cropland (e.g. corn, soy bean, sugarcane, onion and tropical fruits) and cattle ranching. In addition, there are several settlements with populations of fewer than 400 people, such as Indian Church, San Carlos and Blue Creek, and bigger settlements, with about 1000 or more inhabitants such as Indian Creek, Shipyard and San Felipe (Figure 1).

Figure 1. Map of the study area in Belize, Central America with location of collected faeces classified as felid species (jaguar (Panthera onca), puma (Puma concolor), ocelot (Leopardus pardalis), jaguarundi (Puma yagouaroundi) and domestic cat (Felis catus)) using molecular techniques. Samples classified as large-cat were identified by morphology and positive identification by detector dog, due to failure to amplify DNA via molecular techniques. Study sites included a protected area, A. Rio Bravo Conservation and Management Area – RBCMA (outlined in white); and human-modified non-protected areas, B. San Carlos Village; C. Indian Church Village; D. Indian Creek Village; E. Shipyard Village; F. San Felipe Village. Map layers reproduced with permission from Meerman & Clabaugh (2012), http://www.biodiversity.bz.
Field survey
From March to July 2011 a scat collection team, with a dog trained to detect scat of all five felid species, located samples across 45 transects (21 in human-modified areas and 24 in protected forests), in an opportunistic fashion. We adopted a systematic searching approach to ensure biological reliability of FGM concentrations in scats collected under Belizean conditions by visiting each transect (5–10 km) at a 4-d interval, as Mesa-Cruz et al. (Reference MESA-CRUZ, BROWN and KELLY2014) found FGM concentration to remain stable over this time period. Samples found in the first visit were cleared off the trails and were not included in the FGM analysis due to unknown scat age and hence possible degradation of FGM. We collected information on each scat including GPS location and surrounding habitat features such as: trail width (m), distance of the scat to main trail (m), habitat type, tree canopy cover (%), understorey vegetation type and ground cover type to test for habitat effects on DNA amplification.
Molecular species identification: nDNA and mtDNA
We used protocols for DNA preservation, extraction and genotyping previously developed and validated for Belizean felids (Wultsch et al. Reference WULTSCH, WAITS and KELLY2014, Reference WULTSCH, WAITS, HALLERMAN and KELLY2015). We used nuclear DNA (nDNA) to determine species, individual identity, and sex in all scat samples collected, while mitochondrial DNA (mtDNA) was amplified and sequenced in samples with low-quality nDNA and in two random samples in each defined genetic group for species confirmation classification obtained from nDNA allele frequencies.
For nDNA, we used a set of seven microsatellite loci (FCA043, FCA090, FCA096, F124, FCA126, FCA275, FCA391) and one sex marker (Zn-finger) specific to felids (Pilgrim et al. Reference PILGRIM, MCKELVEY, RIDDLE and SCHWARTZ2005, Wultsch et al. Reference WULTSCH, WAITS and KELLY2014). We performed PCR reactions and determined alleles, as described by Wultsch et al. (Reference WULTSCH, WAITS and KELLY2014). We quantified genotyping error by estimating both allelic dropout (ADO) and false allele (FA) frequencies following procedures by Broquet & Petit (Reference BROQUET and PETIT2004).
Carnivore-specific mitochondrial cytochrome b primers (146 bp) were employed in procedures slightly modified from Farrell et al. (Reference FARRELL, ROMAN and SUNQUIST2000). Our PCR reactions also included 0.6 mg mL BSA and 3 μL Amplitaq Gold® DNA polymerase (Applied Biosystems Foster City, CA). PCR amplifications included one denaturation cycle (10 min at 95°C), 55 cycles (30 s at 92°C, 45 s at 50°C, 40 s at 72°C), and two concluding steps, (2 min at 72°C, and 30 min at 4°C). After amplification, products were run on 2% agarose gels to test for positive amplification. We cleaned PCR products (10 μL) of samples presenting a positive band with SAM™ and XTerminator™ solutions (45 μL and 10 μL, respectively), by agitating for 30 min followed by centrifugation at 1000 g for 2 min. We performed a sequencing step by incubating 3 μL of cleaned PCR product, 2 μL Big Dye, 2 μL sequencing buffer, 2 μL of primer (2 μM), and 2 μL of distilled water, under the following conditions: an initial cycle (3 min at 96°C), 24 intermediate cycles (30 s at 95°C, 30 s at 50°C and 120 s at 60°C) and a final cycle (10 min at 4°C). We sequenced PCR products in an automated genetic analyser (3130xl Applied Biosystems, Foster City, CA). We used GeneMapper® (Applied Biosystems v3.7 2004) to edit the sequences and aligned those in the NCBI's basic local alignment search tool (BLAST®, accessed May 2013). Furthermore, we used mtDNA sequencing to confirm nDNA results for species assignment to the number of groups (i.e. species) detected by program STRUCTURE. We selected two samples at random from each distinct genetic group and assessed the following mtDNA regions: Carniv, 12Sv, 16S, 16Sco, and ATP6, described by Lopez et al. (Reference LOPEZ, CEVARIO and O'BRIEN1996).
FGM analysis
We stored faecal material in plastic bags, transported it in a cooler to camp, and froze it at −20°C, within 6 h of collection, until processing at the Smithsonian Conservation Biology Institute (SCBI). Then, we freeze-dried faeces, and subsequently homogenized and pulverized the dried product. We separated the dry faecal powder from prey remains and extracted faecal steroids by boiling, as described by Mesa-Cruz et al. (Reference MESA-CRUZ, BROWN and KELLY2014). We assessed extraction efficiency recovery by adding radio-labelled cortisol (3H cortisol, 2500 dpm) to all samples prior to boiling extraction. We used the double-antibody 125I corticosterone radio-immuno-assay (RIA) (MP Biomedicals, LLC, Orangeburg, NY, USA) to estimate FGM concentrations, as described by Mesa-Cruz et al. (Reference MESA-CRUZ, BROWN and KELLY2014). This assay has been validated for jaguar, ocelot, margay and puma (Bonier et al. Reference BONIER, QUIGLEY and AUSTAD2004, Conforti et al. Reference CONFORTI, MORATO, AUGUSTO, DE OLIVEIRA SOUSA, DE AVILA, BROWN and REEVES2012, Dias et al. Reference DIAS, NICHI and GUIMARÃES2008, Young et al. Reference YOUNG, WALKER, LANTHIER, WADDELL, MONFORT and BROWN2004). We validated the 125I corticosterone RIA for the jaguarundi by testing for parallelism between the assay standards and a pool of jaguarundi faecal extracts.
Endoparasite analysis
In the field, we preserved a subsample of each scat (1–3 g) in buffered formalin (10%, pH 7) and stored subsamples at room temperature until analysis. We retrieved parasite eggs and larvae with a modified Wisconsin faecal flotation test as described by Zajac & Conboy (Reference ZAJAC and CONBOY2012). In the laboratory, we mixed preserved faeces with distilled water (1:5 v/v) and filtered them through gauze into a conical centrifuge tube. This solution was centrifuged for 10 min at 550 g. We removed the supernatant and resuspended the pellet in Sheather's sugar solution (1.27 specific gravity, Jorgensen Laboratories Inc., Loveland, CO, USA). Thereafter, we centrifuged samples for 10 min at 550 g. We observed and identified endoparasite eggs, oocysts and larvae present in the supernatant under the microscope; we recorded genera and number of propagules. We calculated prevalence and endoparasite species richness (ESR) as the total number of species observed in each individual and each felid species.
Prey remain analysis
In each scat, we analysed prey remains such as teeth, hair fibres, bones, claws, feathers and scales at the macroscopic and microscopic levels and identified diet items as previously described (Foster et al. Reference FOSTER, HARMSEN and DONCASTER2010). Our reference sample collection consisted of hair, claws, teeth and bone samples from over 30 potential prey species. We collected these reference samples at the Belize Zoo, from farms around the study sites, and through opportunistic sampling from road-killed animals observed during the scat surveys. We also used other reference materials to identify prey remains not accounted for in our collection (teeth and bone morphology – Engilis et al. Reference ENGILIS, COLE and CARO2012, García & Sánchez-González Reference GARCÍA and SÁNCHEZ-GONZÁLEZ2013, Goodwin Reference GOODWIN1969; hair morphology – Baca Ibarra & Sanchez-Cordero Reference BACA IBARRA and SANCHEZ-CORDERO2004, Lungu et al. Reference LUNGU, RECORDATI, FERRAZZI and GALLAZZI2007). We cleaned fragments of bone, teeth and claws and observed such remains under a dissecting microscope. We submerged hair and feathers in xylene for 2 h and then mounted hairs on microscope slides to observe medulla and cuticle casts. A similar procedure was performed with feathers, but only barbules and villi were characterized (Dove & Koch Reference DOVE and KOCH2010). We identified prey items in all samples with known felid genetic identity and also on those felid samples with unknown genetic identity. The latter were classified as felid samples by scat detector dog positive identification and we separated those samples into large felid (e.g. jaguar or puma) vs. small felid based on morphology. We conducted diet analysis only on these large-felid scats because small-felid scats are of ambiguous origin due to quick degradation rates in the field.
Statistical analysis
We used the software GeneAlEX 6.5 (Peakall & Smouse Reference PEAKALL and SMOUSE2012) to estimate Probability of Identity (P(ID)), (P(ID)sib), and to find matches in consensus genotypes to identify recaptured individuals. We used P(ID)sib < 0.010 as the criterion to determine the minimum number of loci required to identify individuals with high statistical significance. nDNA amplification success and individual ID was achieved if samples amplified at six or more loci. We used program STRUCTURE 2.3.4 (Pritchard et al. Reference PRITCHARD, STEPHENS and DONNELLY2000) to analyse the number of distinct genetic groups, k (i.e. species) including only one genotype per individual in the analysis and using 100000 burn-in period and 400000 McMC repetitions after burn-in, and the frequency for metropolis update (thinning rate) of 10. We used both the largest average probability of k given the simulated data (Ln P(D)) and the ad hoc statistic Δk (Evanno et al. Reference EVANNO, REGNAUT and GOUDET2005) to determine k. We used a contingency analysis and multiple linear regressions to compare DNA amplification success associated to habitat features in protected and non-protected areas.
We used a test for parallelism between the RIA and jaguarundi faecal extracts with multiple linear regression and linear contrasts of the least squares means of the regression to standard and serially diluted samples. We adjusted all FGM concentrations based on the extraction efficiency recovery. We combined and averaged FGM concentrations if multiple samples from the same individual were suitable for hormonal analysis.
We estimated prevalence of endoparasite genera for each felid species as the relative frequency of identification for each parasite (![]() $\frac{{Number\ of\ positive\ samples}}{{Total\ number\ of\ samples}} \times 100$). To avoid pseudoreplication, we combined results of parasite analysis and averaged them for individuals with more than one scat sample. ESR was compared across felid species with a one-way ANOVA.
$\frac{{Number\ of\ positive\ samples}}{{Total\ number\ of\ samples}} \times 100$). To avoid pseudoreplication, we combined results of parasite analysis and averaged them for individuals with more than one scat sample. ESR was compared across felid species with a one-way ANOVA.
We summarized the frequency of occurrence of prey items in scat of each felid species as: ![]() $\frac{{Number\ of\ scats\ containing\ prey\ item}}{{Total\ number\ of\ scats}} \times 100.\ $We analysed the prey results by examining the relationship between predator species (i.e. felids) and prey species consumed through correspondence analysis. Prey species were grouped into six different categories: reptiles, insects, birds and mammals – small (<1 kg body mass), medium (1–5 kg body mass) and large (>5 kg body mass). We examined the effects of habitat protection, species, FGM, ESR and prey items using an additive generalized linear mixed model. We assessed all data for normality using the Shapiro–Wilk goodness-of-fit test (α = 0.05) before applying a statistical test. Data distributed in a non-normal fashion were logarithmically transformed. We conducted all statistical analyses, except for the genetic assessments, in the statistical software JMP Pro 11 (Version 11.0.0; SAS Institute Inc., Cary, NC, USA).
$\frac{{Number\ of\ scats\ containing\ prey\ item}}{{Total\ number\ of\ scats}} \times 100.\ $We analysed the prey results by examining the relationship between predator species (i.e. felids) and prey species consumed through correspondence analysis. Prey species were grouped into six different categories: reptiles, insects, birds and mammals – small (<1 kg body mass), medium (1–5 kg body mass) and large (>5 kg body mass). We examined the effects of habitat protection, species, FGM, ESR and prey items using an additive generalized linear mixed model. We assessed all data for normality using the Shapiro–Wilk goodness-of-fit test (α = 0.05) before applying a statistical test. Data distributed in a non-normal fashion were logarithmically transformed. We conducted all statistical analyses, except for the genetic assessments, in the statistical software JMP Pro 11 (Version 11.0.0; SAS Institute Inc., Cary, NC, USA).
RESULTS
We surveyed 420 km in different habitat types with equivalent effort in protected and human-modified areas. We collected 336 scats samples (82 in RBCMA and 254 in human-modified areas) from wild felids across the study areas (Figure 1).
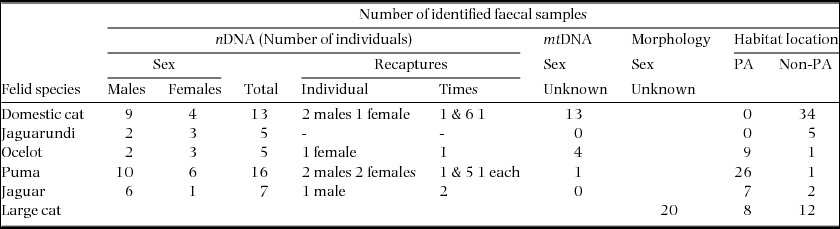
Molecular species identification
Results of P(ID)sib indicated that at least six loci were necessary to identify individuals in all felids. Consensus genotypes at six to seven loci were obtained for 71 samples (21.1%). These genotypes represented 46 individuals and five species (Table 1). Five different genetic groups were identified by (Ln P(D)) and Δk methods using program STRUCTURE and confirmed by mtDNA sequencing, corresponding to jaguar, puma, ocelot, jaguarundi and domestic cat (Felis catus Linnaeus, 1758). We did not find any margay samples. We found most jaguar, puma and ocelot samples in the protected area (RBCMA), whereas most jaguarundi and all domestic cat samples were found in human-modified areas (Table 1). We obtained overall ADO of 10.5% ± 3.1% and FA frequency of 1.1% ± 0.4%. Each locus exhibited the following genotyping error frequencies: FCA043: ADO = 2.3%, FA = 0.9%; FCA090: ADO = 5.0%, FA = 0.3%; FCA096: ADO = 17.4%, FA = 0.6%; F124: ADO = 12.1%, FA = 3.0%; FCA126: ADO = 9.1%, FA = 0.6%; FCA275: ADO = 3.4%, FA = 0.6%; and FCA391: ADO = 24.6%, FA = 1.8%.
Table 1. Total number of faecal samples from five felid species (jaguar (Panthera onca), puma (Puma concolor), ocelot (Leopardus pardalis), jaguarundi (Puma yagouaroundi) and domestic cat (Felis catus)) identified with nDNA, mtDNA and morphology through a non-invasive survey in a mosaic landscape in north-western Belize, Central America. Identification by morphology means that scat detector dog identified scat samples compatible by morphology with a large felid, but had low quality DNA and did not amplify for molecular species identification. Habitat location: PA = protected area, Non-PA = non-protected area.

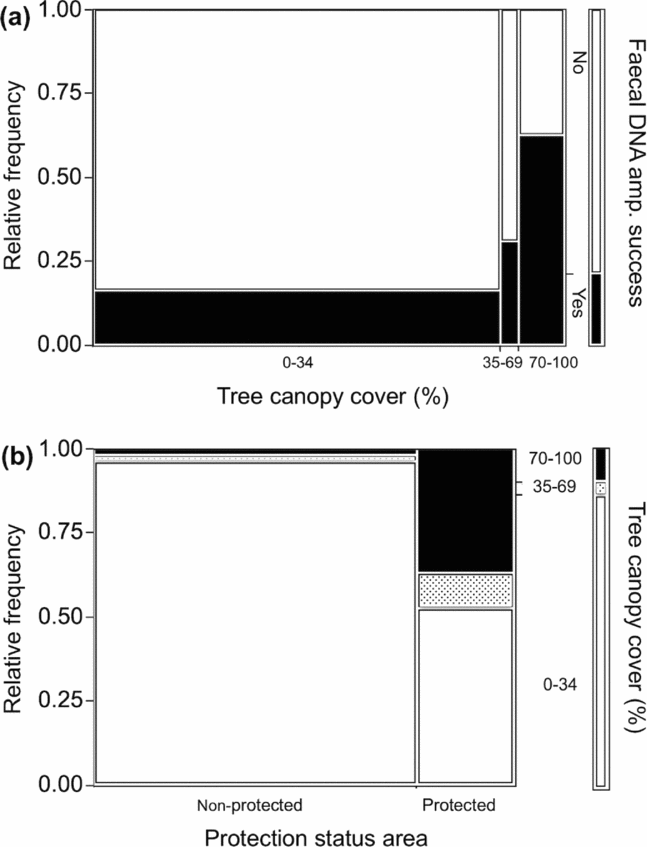
Marked differences in DNA amplification success were observed in scat samples found under different levels of canopy cover (R2(U) = 0.48, χ² = 18.3, P < 0.0001). DNA amplification success was highest (62.5%) in samples found under >70% canopy cover, while amplification success was lowest (16.3%) for those samples with very little (0–34%) canopy cover (Figure 2a). Canopy cover also varied greatly between the protected and non-protected areas. Scats found in RBCMA were five times more likely to successfully amplify for DNA than samples found in non-protected areas (R2(U) = 0.63, χ² = 98.9, P < 0.0001). Scats found in non-protected areas usually were located in areas with very little canopy cover (Figure 2b). Other habitat features were not significantly associated with DNA amplification.

Figure 2. Mosaic plots representing relationships between faecal DNA amplification success of five felid species (jaguar (Panthera onca), puma (Puma concolor), ocelot (Leopardus pardalis), jaguarundi (Puma yagouaroundi) and domestic cat (Felis catus)) (a), tree canopy cover (TCC), and protection status (b) of surveyed areas in Belize, Central America. Relative frequency of felid scats with successful DNA amplification at 6 ≥ or more loci (Yes = black area) or < 6 loci (No = white area), found under different TCC (0–34% (white area), 35–69% (dotted shade area) and 70–100% (black area)); faecal DNA amplification success increased significantly as TCC of scat location increased (R2(U) = 0.48, χ² = 18.3, P < 0.0001). TCC structure in protected and non-protected surveyed areas; non-protected areas, which were modified by humans, had significantly lower TCC than the protected area, RBCMA (R2(U) = 0.63, χ² = 98.9, P < 0.0001).
FGM
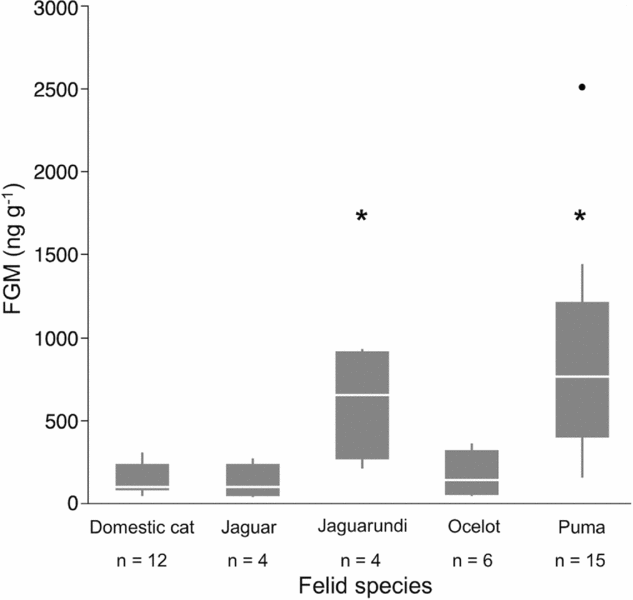
Immunoassay validation (e.g. test for parallelism) for jaguarundi faecal extracts showed that the curves were not significantly different from each other (t = −0.33, P = 0.748). Therefore, jaguarundi FGM can be measured with the corticosterone RIA. Extraction efficiency recoveries were, on average, above 84% for all species (jaguar: 84.5% ± 2.2%, puma: 85.8% ± 1.1%, ocelot: 84.4% ± 2.1%, jaguarundi: 84.5% ± 3.1%, domestic cat: 86.7% ± 0.7%). FGMs represented by more than one scat per individual were averaged in the analysis. Interestingly, puma and jaguarundi exhibited significantly higher concentrations of FGM than other felids (Figure 3; R2 = 0.66, puma: t = 2.39, P = 0.025; jaguarundi: t = 2.92; P = 0.007). We found no significant effects of habitat protection, ESR and diet on FGM concentrations across felids (R2 = 0.66, P > 0.1).

Figure 3. Faecal glucocorticoid metabolites (FGM) of five sympatric free-ranging felids (jaguar (Panthera onca), puma (Puma concolor), ocelot (Leopardus pardalis), jaguarundi (Puma yagouaroundi) and domestic cat (Felis catus)) surveyed non-invasively in north-western Belize, Central America. Multiple samples from individuals were averaged before statistical analysis; n corresponds to genetically unique individuals. Box plots designated with an asterisk are significantly different.
Endoparasite richness (ESR)
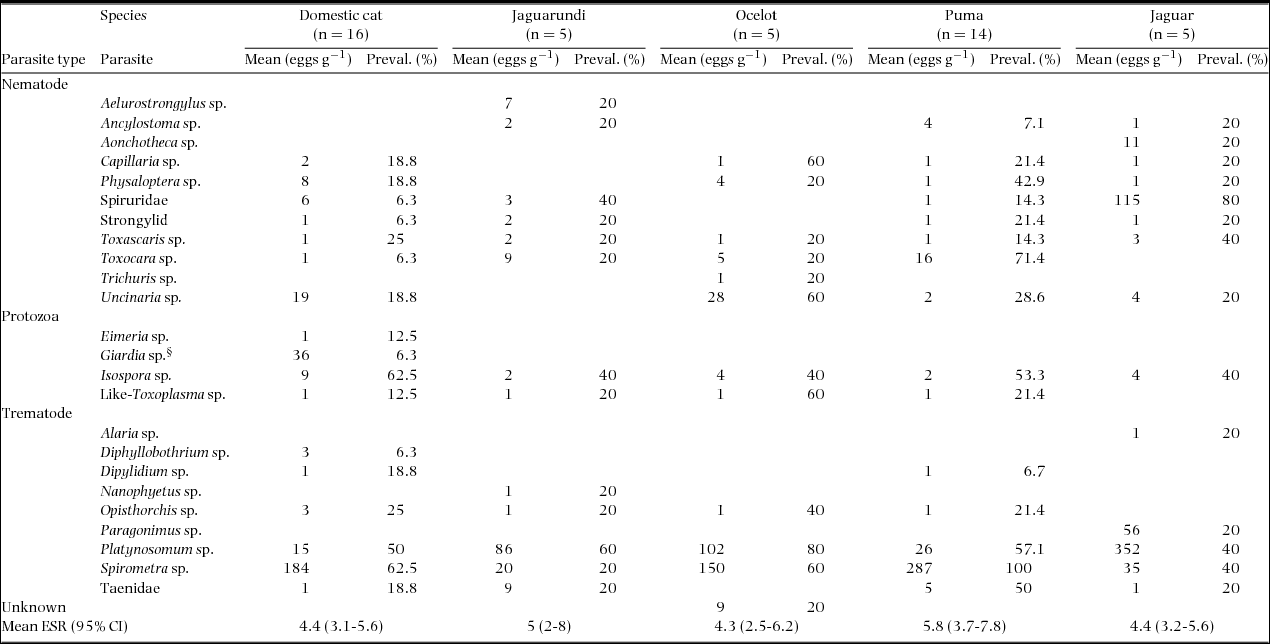
We identified a total of 24 genera of endoparasite and one unidentified species (Table 2). Most scat samples were positive for nematodes (60%), trematodes (70%) and protozoans (85%). Average ESR within species ranged from 3 to 6.6, but was not significantly different across felid species (R2 = 0.04, df = 40; F = 0.43; P = 0.782) (Table 2). Numbers of parasite eggs were highly variable within and among felid species (range = 1–352). Two trematode species, Spirometra sp. and Platynosomum sp., were found at the highest prevalence across the felid species. ESR was highest for the domestic cat (18), followed by the puma (15), the jaguar (15), the jaguarundi (13) and the ocelot (12).
Table 2. Summary of nematode, trematode, and protozoan eggs, larvae, and oocysts identified in faeces of five sympatric felid species (jaguar (Panthera onca), puma (Puma concolor), ocelot (Leopardus pardalis), jaguarundi (Puma yagouaroundi) and domestic cat (Felis catus)). Faeces were obtained using a scat-detector dog survey in north-western Belize, Central America. n corresponds to genetically unique individuals. Number of eggs was calculated per gram of faeces and averaged if more than one sample per individual was analysed (mean eggs g−1). Prevalence (Preval. %) was calculated as ![]() $\frac{{Number\ of\ positive\ samples}}{{Total\ number\ of\ samples}} \times 100$. § = Giardia sp. was identified in the form of trophozoite. Endoparasite species richness (ESR) is the total number of parasite species affecting a single individual. CI = Confidence interval. Parasite species with no values indicates that it was not observed for that felid species.
$\frac{{Number\ of\ positive\ samples}}{{Total\ number\ of\ samples}} \times 100$. § = Giardia sp. was identified in the form of trophozoite. Endoparasite species richness (ESR) is the total number of parasite species affecting a single individual. CI = Confidence interval. Parasite species with no values indicates that it was not observed for that felid species.

Prey remains analysis
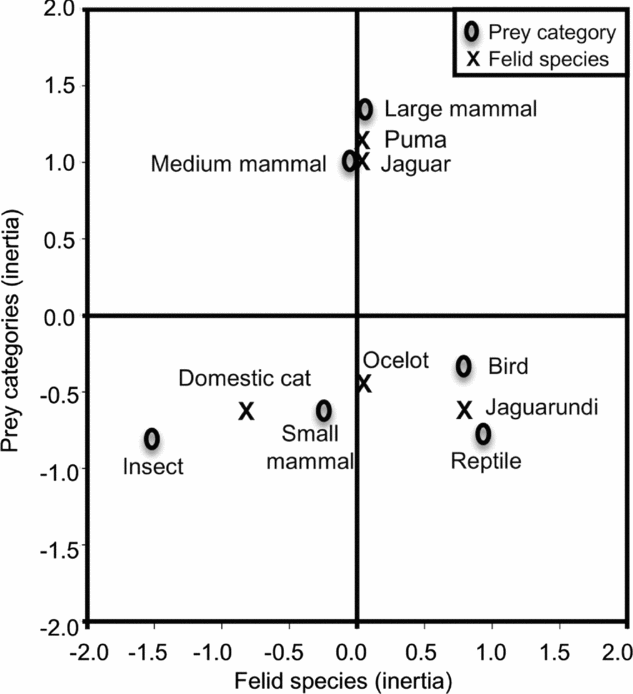
We identified a total of 35 animal prey species in felid scats in this study (Appendix 1). Jaguar more frequently consumed peccary spp. (Tayassuidae) and armadillo (Dasypus novemcinctus Linnaeus 1758), whereas puma consumed more cervids (e.g. red brocket – Mazama americana Erxleben 1777 and white-tailed deer – Odocoileus virginianus Zimmermann 1780). Cotton rats (Sigmodon spp.) were the most frequently consumed prey item for jaguarundi, ocelot and domestic cat. Correspondence analysis indicated that the puma and jaguar were more associated with medium and large prey, the ocelot was associated with small-mammal prey and to a lesser extent with birds, the jaguarundi was associated with birds and reptiles, and the domestic cat was associated with the consumption of small mammals and insects (93.8% cumulative explained variation, χ² = 828, P < 0.0001) (Figure 4).

Figure 4. Correspondence analysis among five sympatric felids and prey items found in faeces through a non-invasive survey in north-western Belize, Central America. There was a significant relationship between prey species (mammals (small < 1 kg, medium 1–5 kg and large > 5 kg body mass), birds, reptiles and insects) and felid species (jaguar (Panthera onca), puma (Puma concolor), ocelot (Leopardus pardalis), jaguarundi (Puma yagouaroundi) and domestic cat (Felis catus)) (χ² = 828, P < 0.0001). Correspondence analysis indicates that the x and y axes for felid and prey species explain 27.7% and 66.8% of the model variation, respectively.
We found a total of 20 large-cat samples (i.e. those samples where DNA did not amplify but were classified as large cat by morphology and scat-dog positive identification), eight in the RBCMA and 12 in human-modified areas. In these scats, small-sized prey were more commonly found in human-modified areas (small 50%; medium: 40%; large: 10% prey items), while medium-sized prey were more frequent in the protected area (small 25%; medium: 75%). However we were not able to account for pseudoreplication in the samples identified as large-cat. Nonetheless, livestock or other domestic animals were not found in any felid scats including those recognized by the scat dog and categorized by morphology as large cats.
DISCUSSION
To our knowledge, this is the first study using non-invasive scat surveys to assess simultaneously felid DNA, adrenal activity, endoparasites and diet in the Neotropics. The overall 21.1% nDNA amplification success was low compared with a previous study by Wultsch et al. (Reference WULTSCH, WAITS and KELLY2014), which was ~60%. However, that study collected scat only in protected areas and our results were similar at 58.5% success for samples collected in the RBCMA. Lack of canopy cover in human-modified areas most likely caused low DNA amplification success (at 12%) due to higher temperatures at ground level (e.g. > 28°C) and higher UV radiation. Other studies also have found high temperatures to affect viability of faecal DNA (DeMay et al. Reference DEMAY, BECKER, EIDSON, RACHLOW, JOHNSON and WAITS2013, Nsubuga et al. Reference NSUBUGA, ROBBINS, ROEDER, MORIN, BOESCH and VIGILANT2004) and that DNA in scat exposed directly to the sun degraded more rapidly than when sheltered (Santini et al. Reference SANTINI, LUCCHINI, FABBRI and RANDI2007). Despite poor amplification success outside protected areas, jaguar, puma and ocelot were present in both the RBCMA and in surrounding human-modified habitats, whereas the jaguarundi was detected only in the human-modified areas. This is consistent with previous findings of jaguarundi ranging in heterogeneous habitats with intermingled closed and open areas (Sunquist & Sunquist Reference SUNQUIST and SUNQUIST2002).
The jaguarundi and margay are known to be present in the RBCMA as they are photographed by remote cameras (Kelly unpubl. data), but both are rare. Additionally, the margay is known to be arboreal in nature (Sunquist & Sunquist Reference SUNQUIST and SUNQUIST2002), perhaps depositing faeces in trees out of the detector dog's scent reach. The margay, however, is thought to be very sensitive to human disturbance (Carvajal-Villarreal et al. Reference CARVAJAL-VILLARREAL, CASO, DOWNEY, MORENO, TEWES and GRASSMAN2012), potentially preventing them from inhabiting non-protected areas.
Domestic cats were found only in human-modified habitats despite the close proximity to the RBCMA. This is important for local conservation efforts at RBCMA, suggesting that domestic cats have not colonized this protected forest as they have in other areas across the world (Farris et al. Reference FARRIS, GERBER, KARPANTY, MURPHY, ANDRIANJAKARIVELO, RATELOLAHY and KELLY2015, Kays & DeWan Reference KAYS and DEWAN2004). The native carnivores may be out-competing domestic cats within the protected forest. This is encouraging since feral cats are known to consume native small mammals and birds worldwide (Loss et al. Reference LOSS, WILL and MARRA2013, Medina et al. Reference MEDINA, BONNAUD, VIDAL, TERSHY, ZAVALETA, DONLAN, KEITT, CORRE, HORWATH and NOGALES2011).
Contrary to our hypotheses, we did not find higher FGM concentrations, potentially indicative of stress, in felids in human-modified areas compared with protected areas for the three felids with samples found in both areas (jaguar, puma, ocelot). However, this may be attributed to low sample sizes in human-modified areas, hampering our ability to compare FGM between protected and non-protected areas for these species. We did, however, observe differences in FGM across species, with higher concentrations in puma and jaguarundi than in other felids. Romano et al. (Reference ROMANO, RODAS, VALDEZ, HERNÁNDEZ, GALINDO, CANALES and BROUSSET2010) and Bonier et al. (Reference BONIER, QUIGLEY and AUSTAD2004) also observed higher FGM concentrations in captive jaguarundi and puma than in other felids. Higher FGM in these two species, therefore, does not appear to be related to high adrenal activity. Instead, there could be a glucocorticoid metabolic particularity, either in the steroid conjugation step for excretion, or in the gut breakdown, that is shared by these two felids; especially since they are clustered in the same phylogenetic lineage (Pecon Slattery & O'Brien Reference PECON SLATTERY and O'BRIEN1998).
While parasite richness (ESR) can provide a coarse assessment of the ability of the host to control infections (Muehlenbein Reference MUEHLENBEIN2006), we did not find differences in ESR between protected and human-modified areas, nor did we find associations between ESR and adrenal activity based on FGM. Sample sizes, however, were very low outside of protected areas. We did, however, find almost twice that number of endoparasite genera in faeces of wild Belizean felids than a previous study (Patton & Rabinowitz Reference PATTON and RABINOWITZ1986). This is likely due to increased sensitivity of the modified (double centrifugation) Wisconsin flotation technique compared with the standard Wisconsin method, as previously documented (Zajac & Conboy Reference ZAJAC and CONBOY2012), rather than an increase in felid parasites in the region. Therefore, it is unclear if our range of ESR for Neotropical felids is normal since there are no previous reports of ESR in Belize, using similar techniques. We suggest that future studies adopt the modified Wisconsin technique to assess gastrointestinal parasites in felid scat to obtain higher sensitivity and comparable results.
It should be noted, however, that some propagules, such as protozoa, could originate from prey species and may not have any direct pathological effect on felid hosts. For instance, we found Eimeria sp. in two domestic cats, yet these protozoa are known to cause infections in New World rodents and birds, but not in felids (Berto et al. Reference BERTO, FLAUSINO, MCINTOSH, TEIXEIRA-FILHO and LOPES2011, Upton et al. Reference UPTON, MCALLISTER, BRILLHART, DUSZYNSKI and WASH1992). Nevertheless, we did not find evidence that the endoparasite loads and FGM concentrations we found are causing pathogenic effects on felids, but larger sample sizes are needed from human-dominated areas to more thoroughly address this issue.
Dietary resources appear well partitioned among wild felid species. Our results are congruent with previous reports that showed the puma preys more on deer and paca (Cuniculus paca Linnaeus, 1766), the jaguar preys more on peccaries (Tayassuidae) and armadillo, and smaller cats relied more on smaller mammals, birds and reptiles (Aranda et al. Reference ARANDA, SANCHEZ-CORDERO and SÁNCHEZ-CORDERO1996, Cascelli de Azevedo Reference CASCELLI DE AZEVEDO2008, Foster et al. Reference FOSTER, HARMSEN and DONCASTER2010, Rabinowitz Reference RABINOWITZ1986b). Dietary overlap among small felid species indicates that domestic cats are likely competing for smaller prey with ocelot and jaguarundi in the human-modified areas. Interestingly, scats from large cats in human-modified areas predominantly contained remains of small- and medium-sized mammals. Thus habitat modification outside of RBCMA may cause the diet shift to smaller prey by jaguar and puma, but habitat modification may not be strong enough to shift to livestock prey entirely. Native prey still exists in these modified areas along riparian corridors or overgrown fields, and it appears that large cats target them, presumably to avoid risk in using open areas to prey on livestock. However, reports of livestock kills do occur, and more research is needed to determine when predators might switch to livestock.
This study demonstrates that non-invasive scat surveys via detector dogs are feasible to assess adrenal activity, endoparasites and diet of free-ranging Neotropical felids, as long as experimental designs account for low amplification success outside protected areas. Future studies could increase sampling effort in human-modified landscapes to overcome degradation issues by using more than one dog detection team, revisiting transects at 2-d intervals instead of 4 d, and/or by extending the duration of field surveys. Nonetheless, we provide a baseline for FGM, endoparasites and diet in Belizean wild felids within and outside of protected areas providing a template for expanding this approach to future studies across Mesoamerica.
ACKNOWLEDGEMENTS
We thank our funding institutions: Conservation, Research, and Education Opportunities (CREO), Cleveland Zoo's Scott Neotropical Fund, Smithsonian Institution, the Philadelphia Zoo, Virginia Tech's Graduate School, and the Department of Fish and Wildlife Conservation at Virginia Tech. We give special thanks to the Lamanai Field Research Center and the Lamanai Outpost Lodge for hosting the team while performing the field survey. We also thank the Belize Zoo and the Tropical Education Center (TEC), the director, Sharon Matola and keeper staff for their collaboration on diet reference sample collection. We thank endocrine laboratory manager, Nicole Presley at SCBI, PackLeader LLC for their training support and detector dog, ‘C.J.’. Christine Proctor, Claudia Wultsch, Jennifer Adams and David Montague, provided field and technical support. Julie Golla, Christopher Satter and numerous other field technicians made scat collection surveys possible. We thank laboratory technicians Ankit Patel and Clark DeHart for their assistance in prey remains sorting and parasite processing. We also thank Dr William Hopkins and anonymous reviewers for comments on the manuscript.
Appendix 1. Summary of prey species found in faeces of five sympatric felid species (jaguar (Panthera onca), puma (Puma concolor), ocelot (Leopardus pardalis), jaguarundi (Puma yagouaroundi) and domestic cat (Felis catus)) organized by class, order and family. Faeces were obtained using a scat detector dog survey in north-western Belize. n corresponds to genetically unique individuals. Numbers in parentheses are the number of species observed as prey items in each class. Frequency of occurrence of prey species (%) = ![]() $\frac{{Number\ of\ scats\ containing\ prey\ item}}{{Total\ sample\ size\ of\ scats\ per\ species}} \times 100.$ Large cat category is represented by scat samples that were compatible by morphology with a large felid and had positive identification by the scat detector dog, but had low quality DNA and did not amplify for molecular species identification.
$\frac{{Number\ of\ scats\ containing\ prey\ item}}{{Total\ sample\ size\ of\ scats\ per\ species}} \times 100.$ Large cat category is represented by scat samples that were compatible by morphology with a large felid and had positive identification by the scat detector dog, but had low quality DNA and did not amplify for molecular species identification.