Introduction
The genus Desmodium is comprised of several species with a variety of uses including cover cropping, pasture (Morris, Reference Morris1997), green manure, insect or weed suppression (Kifuko-Koech et al., Reference Kifuko-Koech, Pypers, Okalebo, Othieno, Khan, Pickett, Kipkoech and Vanlauwe2012) and other phytochemical uses (Morris et al., Reference Morris, Hellier, Connett and Singh Ram2012). Some of the Desmodium species such as Desmodium intortum (Mill.) Urb. have been used as supplemental livestock feed in drought conditions (Boukila et al., Reference Boukila, Tendonkeng, Tendonkeng Pamo and Betfiang2009). Feeding goats with D. intortum has been shown to reduce the populations of the worm parasite Haemonchus contortus in them (Debela et al., Reference Debela, Tolera, Eik and Salte2012). Desmodium sandwicense E. Mey. plants have greater cold tolerance than D. intortum plants (Whiteman, Reference Whiteman1970). Natural pastures in the western frontier of the state of Rio Grande do Sul, Brazil, have mainly Desmodium incanum (G. Mey.) DC. plants along with many other species (Tanure et al., Reference Tanure, Potter and Lobato2011). Desmodium discolor (Vogel) plants produce hay and silage with good palatability (Boultwood, Reference Boultwood1964). Desmodium tortuosum (Sw.) DC. seeds are commonly sold as wild bird feed.
Since various flavonoids, oils and fatty acids are present in other legume species such as Macrotyloma uniflorum Lam. Verdc., Lablab purpureus (L.) Sweet, Neonotonia wightii (Wight & Arn) J.A. Lackey (Morris et al., Reference Morris, Wang, Grusak and Tonnis2013a, Reference Morris, Grusak, Wang, Tonnis and Kuangb, Reference Morris, Wang, Tonnis and El-Shemy Hanyc), some of these phytochemicals may also be present in Desmodium species. Legume flavonoids can be found in animal or human diets as quercetin, kaempferol, luteolin and apigenin have been reported to be present in the milk of cows consuming various grass forages (Besle et al., Reference Besle, Viala, Martin, Pradel, Meunier, Berague and Fraisse2010). Flavonoids have been reported to provide many health benefits to humans. Quercetin can effectively inhibit mast cell production in allergic and inflammatory diseases (Weng et al., Reference Weng, Zhang, Asadi, Sismanopoulos, Butcher, Fu, Katsarou-Katsari, Antoniou and Theoharides2012). Kaempferol induces apoptosis in ovarian cancer cells through the regulation of pro-apoptotic and anti-apoptotic protein expressions in apoptotic pathways (Luo et al., Reference Luo, Rankin, Li, Depriest and Chen2011). Isorhamnetin has been reported to have an antiproliferative effect and a greater cytotoxic effect in gastric cancer in combination with chemotherapeutic drugs (Ramachandran et al., Reference Ramachandran, Manu, Shanmugam, Li, Siveen, Vali, Kapoor, Abbasi, Surana, Smoot, Ashktorab, Tan, Ahn, Yap, Kumar and Sethi2012). Luteolin in combination with ( − )-epigallocatechin-3-gallate has been reported to induce apoptosis in both lung cancer and squamous cell carcinoma cells of the head and neck cancer cell lines (Amin et al., Reference Amin, Wang, Zhang, Peng, Shin, Brandes, Tighiouart, Khuri, Chen and Shin2010) in humans. Apigenin has been shown to block the progestin-dependent induction of the vascular endothelial growth factor in breast cancer cells (Mafuvadze et al., Reference Mafuvadze, Benakanakere and Hyder2010). Feeding livestock with oil crops or supplements that are high in unsaturated fats has been shown to increase their polyunsaturated fatty acid concentrations (Karsten and Baer, Reference Karsten, Baer, Wedin Walter and Fales Steven2009). Since there is little knowledge on important phytochemicals of Desmodium species, our objective was to determine the flavonoid concentrations, oil content and fatty acid compositions of 25 Desmodium accessions from five species including D. discolor (three accessions), D. incanum (four accessions), D. intortum (two accessions), D. sandwicense (ten accessions) and D. tortuosum (six accessions).
Materials and methods
Planting
D. incanum was used as a control as it has better tolerance to continuous heavy grazing than D. intortum (Cook et al., Reference Cook, Pengelly, Brown, Donnelly, Eagles, Franco, Hanson, Mullen, Partridge, Peters and Schultze-Kraft2005). D. incanum plants have been used as medicinal diuretics, stomachics, fever reducers and haemostatics in Central America (Setyowati-Indarto and Brink, Reference Setyowati-Indarto, Brink, de Padua, Bunyapraphatsara and Lemmens1999). D. incanum plants are also resistant to root knot nematodes (Quesenberry et al., Reference Quesenberry, Dampier, Crow and Dickson2008). Seeds obtained from 25 Desmodium accessions (Table 1) were planted in 6.4 × 7.0 cm jiffy pots (Hummert International, Earth City, MO, USA) containing Promix HP potting soil (Griffin Greenhouse, Ball Ground, GA, USA) in 2010 and 2011 in the first week of April. The seedlings were grown in a greenhouse for 4 weeks without supplemental lighting at 21 to 26°C. Since Desmodium species are highly outcrossing, the same pool of seeds from each accession was used during both the years. Twenty-five to fifty seedlings representing each accession per plot were transplanted in a field in Griffin, Georgia, USA, in the first week of May in one 6 m row plot with an interval of 6 m between rows in an augmented randomized complete block design with two replications. The soil was a clayey, kaolinitic, thermic Typic Kanhapludult series. A supplemental fertilizer containing 10–10–10 NPK was applied to the field at a rate of 112 kg/ha. The plots were irrigated for proper plant growth and maximum seed production. Pods were harvested from each Desmodium accession on the maturation of seeds 3 to 6 months after transplantation. The pods were dried at 21°C and 25% relative humidity for 1 week and then threshed. This long sampling period was required to obtain enough seeds per accession for all the phytochemical analyses. Further studies should be carried out to evaluate the impact of harvest date and environment on flavonoid concentrations, oil content and fatty acid compositions in Desmodium species.
Table 1 Origin of Desmodium accessions used in the study

Determination of flavanoid concentrations
Desmodium seeds were ground to a fine powder in a coffee grinder and stored at − 20° C until the extraction of flavonoids. Approximately 0.1 g of seed tissue was placed into screw cap tubes. To each tube containing the seed tissue, 6 ml of extraction solvent containing 60% high-performance liquid chromatography (HPLC)-grade methanol with 1.2 M HCl were added. The samples were then mixed thoroughly and incubated at 80°C for 2 h with occasional mixing. A portion of the supernatant was filtered using a 0.45 μM membrane prior to injection into the chromatographic column. The flavonoids were separated by reversed-phase HPLC with a Kinetex solid-core, 4.6 × 100 mm, 2.6 μm, phenyl-hexyl column (Phenomenex, Inc., Torrance, CA, USA) at 40°C using an Agilent 1100 HPLC system with a binary pump and an autosampler. The mobile phase consisted of HPLC-grade methanol (B) and 0.1% formic acid in filtered, sterile water (A). The flow rate was 0.75 ml/min at the following gradient: 15% B initially followed by 60% B for 30 min. The column was washed with 95% B for 6 min and equilibrated at 15% B for 7 min between injections. The sample injection volume was 10 μl, and the flavonoids and flavonols were monitored using a diode-array detector at 340 and 370 nm, respectively. Flavonoid standards purchased from Indofine Chemical Co. (Hillsborough, NJ, USA) were dissolved in a 5:3:2 mixture of dimethyl sulphoxide–methanol–water. This mixture was diluted with 60% methanol to generate standard curves (ranging from 0.1 to 20.0 ng/μl) for the identification and quantification of peaks. All the samples were prepared and injected twice, and the results were averaged.
Determination of seed oil content
Desmodium seed oil content was measured using a Minispec MQ10 nuclear magnetic resonance (NMR) analyzer (Bruker Optics, Inc., Billerica, MA, USA). The NMR analyzer was maintained at 40°C and operated at a resonance frequency of 9.95 MHz. For each signal acquisition, spin-echo parameters consisted of a 90° pulse of 10.44 μs, and the signal was recorded at 50 μs. Later, a 180° pulse of 21.38 μs (pulse spacing = variable) was used, and the signal was recorded at 7 ms. A recycle delay of 2 s was maintained between scans, and a total of 20 scans were collected for each sample. Soybean oil (Sigma-Aldrich, St. Louis, MO, USA) was used as a reference for establishing a standard curve as Desmodium seed oil is not available commercially. Shredded pieces of paper towels were added to a sample tube containing oil to serve as a matrix for each of nine standards. Moisture standards were prepared using soybean seeds having known moisture content. The mass of seed oil and water was converted into a percentage of the total weight of each sample. All the samples were measured thrice, and the results were averaged. Four Desmodium accessions including D. incanum (PI 322418, PI 322419 and PI 477072) and D. sandwicense (PI 316218) did not produce enough seeds for the adequate measurement of oil content. Therefore, seeds obtained from these accessions stored at − 18°C previously were used to determine oil content on a dry-weight basis.
Determination of fatty acid compositions
Fatty acid compositions were determined using an Agilent 7890A gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) with a split/splitless (S/Sl) inlet and a flame ionization detector. Desmodium seeds were ground to a fine powder in a coffee grinder. Oil from approximately 50 to 100 mg of these powdered Desmodium seeds was extracted into 3 ml of heptane and converted to fatty acid methyl esters (FAMEs) using a 0.5 N sodium methoxide catalyst in methanol. The organic layer containing these FAMEs was separated from the seed meal with the addition of water. An aliquot from this layer was transferred into a sample vial for injection. The peaks were separated using a DB-23 capillary column (15 m × 0.25 mm internal diameter) with a 0.25 μm film obtained from Agilent Technologies. Later, 1 μl of the prepared sample was injected at a 60:1 split ratio into the DB-23 capillary column using the following thermal gradient: 180° C for 1 min, 180 to 195°C at 5°C/min, and 195 to 240°C at 10°C/min. Helium was used as the carrier gas, and the inlet pressure was set to 12 psi (approximately 41 cm/s at 180° C). The peaks were identified by comparing the retention times with that of a FAME standard mix RM-3 (Sigma-Aldrich). The oven was equilibrated for 3.5 min between injections. All the samples were prepared and injected twice, and the results were averaged.
Statistical analyses
Analyses were carried out using Proc GLM of SAS (SAS 9.2; SAS Institute, Inc., Cary, NC, USA) (SAS Institute, 2009) to determine the significance of Desmodium accessions when compared with four D. incanum controls. Correlations were identified using Pearson's correlation analysis in SAS. Principal components were determined using PROC PRINCOMP (SAS 9.2; SAS Institute, Inc.), followed by a multivariate analysis of the data. Eigenvalues, the percentage of variances explained by each principal component, and eigenvectors were also determined. Clustering of the data was then carried out by entering the similarity matrix into PROC CLUSTER for cluster analysis with the unweighted paired group method using mathematical averages by specifying the AVERAGE option (SAS 9.2; SAS Institute, Inc.).
Results
Flavonoid concentrations
Data obtained from the 2-year field experiments were analysed separately because of year effects. Desmodium accessions were compared with the D. incanum control accessions (including PI 322418, PI 322419, PI 477072 and PI 593057) using least mean squares. The best D. incanum control accession, PI 477072 from Uruguay, produced the highest concentrations of quercetin and kaempferol in 2010 (44.6 and 19.9 μg/g, respectively) as well as in 2011 (41.4 and 18.1 μg/g, respectively) (Supplementary Tables S1 and S2, available online). However, the second best control accession for the concentrations of quercetin (41.2 μg/g) and kaempferol (17.2 μg/g) was PI 593057 from Florida, USA, in 2010 (Supplementary Table S1, available online), while PI 322418 from Brazil was the second best control for the concentrations of quercetin (37.0 μg/g) and kaempferol (16.8 μg/g) in 2011 (Supplementary Table S2, available online). The best control accession for the concentration of apigenin (39.3 μg/g) was PI 593057, followed by PI 322419 from Brazil (37.7 μg/g) in 2010. In 2011, the best control accession for the concentration of apigenin (38.0 μg/g) was PI 322418, followed by PI 322419 (34.9 μg/g). None of the control accessions produced isorhamnetin or luteolin. During both the years, all the Desmodium accessions produced significantly greater concentrations of quercetin (135.3–876.3 μg/g) and kaempferol (76.5–200.6 μg/g) than the best control (D. incanum, PI 477072). All the Desmodium accessions produced significantly greater concentrations of isorhamnetin and luteolin (ranging from 14.2 to 925.4 μg/g) than all the four controls (0 μg/g) during both the years. All these Desmodium accessions produced significantly greater concentrations of apigenin (ranging from 57.4 to 150.8 μg/g) than the control PI 593057 (39.3 and 34.0 μg/g) in 2010 and 2011, respectively.
Oil content and fatty acid compositions
Oil content and fatty acid compositions are listed in Supplementary Tables S3 and S4 (available online). Oil content averaged 14.4% among the four controls during both the years, while oil content ranged from 8.5 to 11.2% among the additional Desmodium accessions. None of the accessions had significantly greater oil content when compared with the controls. Palmitic acid (16:0) and stearic acid (18:0) content ranged from 9.8 to 14.2 and from 2.6 to 5.4%, respectively, and was not significantly different from that of the control accessions during both the years. In 2010, only the D. discolor accessions (PI 271160 from India and PI 322442 from Brazil) and the D. tortuosum accessions (PI 275089 from India and PI 317054 from Trinidad and Tobago) did not have significantly greater oleic acid (18:1) content when compared with the second best controls (PI 322418 and PI 593057). In 2011, only the D. intortum accessions (PI 214107 from Spain and PI 317894 from Brazil) and the Australian D. sandwicense accessions (PI 316216, PI 316217, PI 316218, PI 316219, PI 316224 and PI 316225) had significantly greater oleic acid content than the second best control (D. incanum, PI 322418). All the Desmodium accessions ranging from 43.5 to 50.9% had significantly greater linoleic acid (18:2) content than the fourth best control (PI 477072) accession during both the years. Only the D. tortuosum accessions (PI 275089 and PI 317054) had significantly greater linolenic acid (18:3) content than the fourth best control (PI 593057) accession in 2010, while none of the accessions had significantly greater linolenic acid content when compared with the controls in 2011. Both PI 317894 (D. intortum) and PI 316218 (D. sandwicense) had significantly greater arachidic acid (20:0) content than the second best control (PI 477072) in 2010. There were no differences between any of the Desmodium and control accessions in 2011 for arachidic acid content. One D. intortum accession (PI 214107) and eight D. sandwicense accessions (PI 316216, PI 316217, PI 316219, PI 316220, PI 316221, PI 316222, PI 316224 and PI 316225) had significantly greater gadoleic acid (20:1) content than the fourth best control (PI 477072) in 2010; however, no differences were observed between the accessions and controls for gadoleic acid content in 2011. All the Desmodium accessions had significantly greater behenic acid (22:0) content than the best control (PI 593057) during both the years. Lignoceric acid content of all the Desmodium accessions and controls was similar in 2010 and 2011.
Correlations
Highly significant correlations were observed among flavonoid concentrations (Supplementary Table S5, available online). The concentration of quercetin was significantly correlated with that of kaempferol (r 2= 0.69***) (Supplementary Table S5, available online); however, the concentration of quercetin exhibited a significantly negative correlation (r 2= − 0.41*) with that of isorhamnetin. Therefore, as the concentration of isorhamnetin decreased, that of quercetin increased. The concentration of isorhamnetin was significantly correlated with those of luteolin (r 2= 0.97***) and apigenin (r 2= 0.88***), while the concentration of luteolin was significantly correlated with that of apigenin (r 2= 0.92***). Highly significant correlations were also observed for oil content and fatty acid compositions (Supplementary Table S6, available online). Oil content was significantly correlated with palmitic acid (r 2= 0.61**), stearic acid (r 2= 0.81***), linolenic acid (r 2= 0.58**) and lignoceric acid (r 2= 0.80***) content. Several significantly negative correlations were also observed for oil content and linoleic acid (r 2= − 0.74***) and behenic acid (r 2= − 0.65**) content.
Principal component analysis
Large variations were observed for most of the traits tested except for oil, palmitic acid and stearic acid content (Table 2). The flavonoid concentration, oil content and fatty acid composition principal component analysis accounted for 47% of the total variation observed for the first principal component (Table 3). The amount of variation accounted for, cumulatively, by adding the second, third, fourth, fifth and sixth principal components was 88, 94, 98 and 99%, respectively. The first principal component was mostly correlated with quercetin, palmitic, oleic, gadoleic and behenic acid content (Table 4). The second principal component accounted for 41% of the variation and was mostly correlated with kaempferol and apigenin concentrations as well as with stearic acid, linoleic acid and oil content, while the third principal component explained 5% of the variation and was primarily correlated with stearic and linolenic acid content. The fourth principal component accounted for 4% of the variation and was mostly correlated with oleic and linoleic acid content. Both the fifth and sixth principal components accounted for 1% of the variation and were mainly correlated with apigenin, quercetin and kaempferol concentrations.
Table 2 Flavonoid, oil and fatty acid traits in diverse Desmodium genotypes based on data obtained in 2010–2011

Table 3 Eigenvalues and the proportion of total variability among diverse Desmodium genotypes (2010–2011) as explained by the principal components
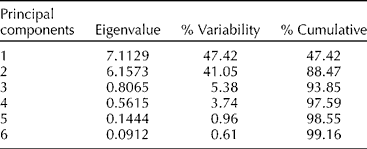
Table 4 Eigenvectors and principal components for 15 flavonoid, oil and fatty acid traits in diverse Desmodium genotypes (2010–2011)

Cluster analysis
The average distance cluster analysis grouped the original 25 Desmodium accessions into well-defined phenotypes with six distinct quercetin concentration accessions (Fig. 1). Clusters I and II represent four very-low- and five low-quercetin concentration accessions, respectively. Clusters III and IV consist of two very-high- and eight high-quercetin concentration accessions, respectively. However, clusters V and VI comprise two medium- and four zero-quercetin concentration accessions, respectively. These 25 Desmodium accessions were also clustered into well-defined phenotypes with four distinct isorhamnetin and luteolin concentration accessions (Fig. 2). Clusters I and II represent four medium- and five high-isorhamnetin and luteolin concentration accessions, respectively. However, clusters III and IV have 12 low- and 4 zero-isorhamnetin and luteolin concentration accessions, respectively. Using the distance values reported in Fig. 1, the clustering at any similarity level can be identified. For example, the D. discolor accessions PI 271160 and PI 322442 originating from India and Brazil, respectively, have a phenotypic distance index of 0.1385, which indicates close similarities among their quercetin, isorhamnetin and luteolin concentrations.

Fig. 1 (colour online) Dendrogram of the distance between clusters based on flavonoid concentration, oil content and fatty acid composition differences. Accession numbers are given (Acc). Values on the baseline indicate average phytochemical distances between the accessions. Six distinct clusters for quercetin concentrations can be distinguished.

Fig. 2 (colour online) Dendrogram of the distance between clusters based on flavonoid concentration, oil content and fatty acid composition differences. Accession numbers are given (Acc). Values on the baseline indicate average phytochemical distances between the accessions. Four distinct clusters for isorhamnetin and luteolin concentrations can be distinguished.
Discussion
Biochemical analysis of the flavonoid concentrations, oil content and fatty acid compositions of Desmodium species can identify and quantify accessions useful for potential livestock nutrition. Several significant correlations among flavonoid concentrations, oil content and fatty acid compositions were observed in this study; therefore, potential Desmodium cultivars could be developed with improved phytochemical profiles. In addition, the bioavailability and assimilation of these seed phytochemicals for possible human consumption should be evaluated in future research studies. The Desmodium accessions could provide livestock with nutraceuticals or functional feed supplements containing flavonoid concentrations, oil content and fatty acid compositions for improved health.
There have been a limited number of studies reporting fatty acid compositions for these Desmodium species. Maestri et al. (Reference Maestri, Fortunato, Guzman, Torres and Lamarque2002) reported similar fatty acid compositions for D. tortuosum. Several Desmodium species having fatty acids including linoleate were temperature sensitive when grown in northern and southern areas of the United States (Cherry et al., Reference Cherry, Bishop, Hasegawa and Lefflert1985), which may explain the year effect differences observed in this study. Principal components and cluster analysis provide quality characterization information on the flavonoid concentration, oil content and fatty acid composition profiles of the Desmodium accessions. Principal component analysis is a useful method for determining as how much each flavonoid, oil and fatty acid trait contributes to the variation observed in the Desmodium species. It has successfully been used to characterize flavonoids in legume species such as Macrotyloma uniflorum (Lam.) Verdc., Lablab purpureus (L.) Sweet and Neonotonia wightii (Wight & Arn.) J.A. Lackey (Morris et al., Reference Morris, Wang, Grusak and Tonnis2013a, Reference Morris, Grusak, Wang, Tonnis and Kuangb, Reference Morris, Wang, Tonnis and El-Shemy Hanyc).
The average linkage cluster and multivariate analysis carried out on the data were able to separate accessions producing very low concentrations of quercetin, isorhamnetin and luteolin from those producing high concentrations of these flavonoids. All six clusters were represented by collected or donated Desmodium accessions from Australia, Brazil, India, Spain, Tanzania, Trinidad and Tobago, Uruguay, the United States and the Virgin Islands, USA (Table 1). These Desmodium accessions originate from a relatively small number of geographical locations. However, the clusters of accessions producing quercetin tended to define accession groups with similar geographical origins. For example, seven of the high-quercetin concentration accessions originated from Australia, while one originated from Brazil. The accessions producing the highest concentrations of isorhamnetin and luteolin consisted of two from Australia and one from Brazil and India each. Ten of the low-isorhamnetin and luteolin concentration accessions originated from Australia also. This will be useful when selecting accessions from Desmodium genetic resources for potential flavonoid enhancement. The analysis revealed tighter clustering among the high-quercetin, low-isorhamnetin, and luteolin concentration accessions. This indicates greater genetic variability in the accessions producing low-to-medium quercetin concentrations and the accessions producing medium-to-high isorhamnetin and luteolin concentrations.
More than 240 accessions representing 39 Desmodium spp. remain to be evaluated for flavonoid concentrations, oil content and fatty acid compositions in the USDA, ARS, PGRCU collection. Desmodium accessions evaluated for these traits will provide breeders with valuable germplasm for the development of future cultivars with superior flavonoid concentrations, oil content and fatty acid compositions.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1479262113000397
Acknowledgements
The authors thank Ken Manley for his excellent technical assistance during seed harvesting and preparation for chemical analysis.