Introduction
Research has yielded evidence for a substantial genetic impact on the development of schizophrenia. A recent Danish nation-wide twin study estimated the heritability of schizophrenia and the broader category of schizophrenia spectrum disorders to be 79% and 73%, respectively (Hilker et al., Reference Hilker, Helenius, Fagerlund, Skytthe, Christensen, Werge and Glenthøj2017). One of the core features of schizophrenia is widespread cognitive deficits (Fioravanti, Bianchi, & Cinti, Reference Fioravanti, Bianchi and Cinti2012) that often precede the onset of psychosis by several years (Meier et al., Reference Meier, Caspi, Reichenberg, Keefe, Fisher, Harrington and Moffitt2014). Cognitive deficits are also present in first-degree family members of patients with schizophrenia, although to a lesser extent (Sitskoorn, Aleman, Ebisch, Appels, & Kahn, Reference Sitskoorn, Aleman, Ebisch, Appels and Kahn2004). Cognitive deficits have thus been proposed to constitute endophenotypes for schizophrenia (Gottesman & Gould, Reference Gottesman and Gould2003; Gur et al., Reference Gur, Calkins, Gur, Horan, Nuechterlein, Seidman and Stone2007; Snitz, MacDonald, & Carter, Reference Snitz, MacDonald and Carter2006). A recent meta-analysis demonstrated that most cognitive phenotypes are under strong genetic control. Heritability of intelligence (IQ) was reported to be 64%, with no considerable difference between verbal IQ (67%) and performance IQ (65%). Besides IQ, the highest heritability estimates were observed for verbal ability (43–72%), visuospatial ability (20–80%), and attention/processing speed (28–74%), while the lowest heritability estimates were observed for executive functions (20–40%) (Blokland et al., Reference Blokland, Mesholam-Gately, Toulopoulou, Del Re, Lam, Delisi and Petryshen2017). Given that cognition is an important predictor of functional outcome (Bowie, Reichenberg, Patterson, Heaton, & Harvey, Reference Bowie, Reichenberg, Patterson, Heaton and Harvey2006; Green, Kern, Braff, & Mintz, Reference Green, Kern, Braff and Mintz2000) and relatively unaffected by antipsychotic treatment (Andersen et al., Reference Andersen, Fagerlund, Rasmussen, Ebdrup, Aggernaes, Gade and Glenthoj2011; Fagerlund, Mackeprang, Gade, Hemmingsen, & Glenthøj, Reference Fagerlund, Mackeprang, Gade, Hemmingsen and Glenthøj2004; Goldberg et al., Reference Goldberg, Goldman, Burdick, Malhotra, Lencz, Patel and Robinson2007), cognitive deficits are recognized as an important target for treatment (Pantelis, Wannan, Bartholomeusz, Allott, & McGorry, Reference Pantelis, Wannan, Bartholomeusz, Allott and McGorry2015; Wykes, Huddy, Cellard, Mcgurk, & Czobor, Reference Wykes, Huddy, Cellard, Mcgurk and Czobor2011).
In twin studies, multivariate analyses can be used to separate the covariance between two traits into genetic and environmental components (Rijsdijk & Sham, Reference Rijsdijk and Sham2002). Several twin studies have demonstrated genetic overlap between IQ and schizophrenia liability (Bohlken, Brouwer, Mandl, Kahn, & Hulshoff Pol, Reference Bohlken, Brouwer, Mandl, Kahn and Hulshoff Pol2016; Fowler, Zammit, Owen, & Rasmussen, Reference Fowler, Zammit, Owen and Rasmussen2012; Toulopoulou et al., Reference Toulopoulou, Picchioni, Rijsdijk, Hua-Hall, Ettinger, Sham and Murray2007, Reference Toulopoulou, Van Haren, Zhang, Sham, Cherny, Campbell and Kahn2015). Moreover, genetic variants implicating schizophrenia liability identified through genome-wide association studies have been found to overlap with genetic variants influencing IQ (Sniekers et al., Reference Sniekers, Stringer, Watanabe, Jansen, Coleman, Krapohl and Posthuma2017). Genetic overlap has also been reported between schizophrenia liability and specific cognitive functions such as memory (Owens et al., Reference Owens, Picchioni, Rijsdijk, Stahl, Vassos, Rodger and Toulopoulou2011a; Toulopoulou et al., Reference Toulopoulou, Goldberg, Mesa, Picchioni, Rijsdijk, Stahl and Murray2010) and some aspects of executive function (Owens et al., Reference Owens, Rijsdijk, Picchioni, Stahl, Nenadic, Murray and Toulopoulou2011b). One important consideration when examining the heritability of cognition and associations with schizophrenia is whether IQ may account for the findings. Most cognitive functions are highly correlated with IQ in the general population (Deary, Penke, & Johnson, Reference Deary, Penke and Johnson2010), and perhaps even stronger in schizophrenia patients (Dickinson, Goldberg, Gold, Elvevg, & Weinberger, Reference Dickinson, Goldberg, Gold, Elvevg and Weinberger2011). However, although IQ typically accounts for approximately 40% of the variance in tests of specific cognitive functions, it does not explain the entire variance (Deary et al., Reference Deary, Penke and Johnson2010). Moreover, even though some specific cognitive deficits observed in schizophrenia patients can be fully explained by impairments in IQ, other deficits remain after controlling for IQ (Joyce, Hutton, Mutsatsa, & Barnes, Reference Joyce, Hutton, Mutsatsa and Barnes2005; Martin, Mowry, Reutens, & Robinson, Reference Martin, Mowry, Reutens and Robinson2015; Roca et al., Reference Roca, Manes, Cetkovich, Bruno, Ibanez, Torralva and Duncan2014). This indicates that impairments in specific cognitive functions may constitute distinct risk factors for schizophrenia separate from the influence of IQ. From a developmental perspective, different cognitive functions show a differential pattern of maturation depending on the developmental stage of the underlying neural systems. These developmental trajectories may interact with the onset of the illness (Pantelis et al., Reference Pantelis, Wannan, Bartholomeusz, Allott and McGorry2015), which may impact the overlap between specific cognitive functions and schizophrenia liability.
The Cambridge Automated Neuropsychological Test Battery (CANTAB) is a series of computerized tests, specifically designed to identify different neural systems underlying specific cognitive functions (Robbins et al., Reference Robbins, James, Owen, Sahakian, McInnes and Rabbit1994; Sahakian & Owen, Reference Sahakian and Owen1992). Moreover, CANTAB is well validated (Barnett et al., Reference Barnett, Robbins, Leeson, Sahakian, Joyce and Blackwell2010; Evenden et al., Reference Evenden, Morris, Owen, Robbins, Roberts and Sahakian2013.; Robbins et al., Reference Robbins, James, Owen, Sahakian, Lawrence, Mcinnes and Rabbitt1998, Reference Robbins, James, Owen, Sahakian, McInnes and Rabbit1994), has a large normative dataset (Cambridge Cognition Ltd., 2013), and has been applied in a variety of clinical populations including schizophrenia (Fagerlund et al., Reference Fagerlund, Mackeprang, Gade, Hemmingsen and Glenthøj2004; Fagerlund, Pagsberg, & Hemmingsen, Reference Fagerlund, Pagsberg and Hemmingsen2006; Jepsen et al., Reference Jepsen, Fagerlund, Pagsberg, Christensen, Nordentoft and Mortensen2010; Levaux et al., Reference Levaux, Potvin, Sepehry, Sablier, Mendrek and Stip2007; Pantelis et al., Reference Pantelis, Barnes, Nelson, Tanner, Weatherley, Owen and Robbins1997, Reference Pantelis, Wood, Proffitt, Testa, Mahony, Brewer and McGorry2009), and those at high risk for psychosis (Wood et al., Reference Wood, Pantelis, Proffitt, Philips, Stuart, Buchanan and McGorry2003).
Previous twin studies in non-psychiatric samples have demonstrated a genetic component explaining variation in CANTAB measures (Singer, MacGregor, Cherkas, & Spector, Reference Singer, MacGregor, Cherkas and Spector2006; Steves, Jackson, & Spector, Reference Steves, Jackson and Spector2013; Zhou et al., Reference Zhou, Li, Xie, Xu, Cheung, Li and Chan2018). One study combined the applied CANTAB variables into four composite scores and reported significant heritability estimates for general memory, inspection time, and working memory. In contrast, reaction time was only explained by environmental effects (Singer et al., Reference Singer, MacGregor, Cherkas and Spector2006). Another study investigated the heritability of longitudinal changes in CANTAB performance over 10 years. At baseline, several of the reported outcome measures were found to be influenced by genetic factors and the heritability estimates increased in almost all measures at follow-up (Steves et al., Reference Steves, Jackson and Spector2013). Finally, a recent study reported moderate heritability for spatial working memory, whereas set-shifting was solely explained by unique environmental factors (Zhou et al., Reference Zhou, Li, Xie, Xu, Cheung, Li and Chan2018). How these cognitive functions relate genetically to schizophrenia is currently unknown.
The aims of the present study were (1) to identify genetic associations between specific cognitive functions measured by CANTAB and schizophrenia liability, (2) to quantify the genetic and environmental contributions to the variation in these specific cognitive functions, and (3) to investigate whether some functions are associated with schizophrenia liability independent of IQ. The goal was to identify cognitive endophenotypes and to disentangle the effects of specific cognitive functions and IQ in the development of schizophrenia.
To the best of our knowledge, this is the first twin study to examine genetic associations between specific cognitive functions measured by CANTAB and schizophrenia liability. Moreover, the study is novel in trying to disentangle the effects of IQ on these associations. A greater understanding of the heritability of cognition and genetic overlap with schizophrenia is important for increasing our understanding of the illness leading from genes to psychopathology.
Methods
The present study is part of the Vulnerability Indicators of Psychosis (VIP) study and has been approved by The Danish Health and Medicines Authority, The Danish Data Protection Agency (2010-41-5468), and The Danish National Committee on Health Research Ethics (H-2-2010-128). Informed consent was obtained from all participants. Data on cerebral blood flow and glutamate levels from this cohort have been previously published (Legind et al., Reference Legind, Broberg, Brouwer, Mandl, Ebdrup, Anhøj and Rostrup2019a, Reference Legind, Broberg, Mandl, Brouwer, Anhøj, Hilker and Glenthøj2019b).
Recruitment
To identify monozygotic (MZ) and dizygotic (DZ) twin pairs concordant or discordant for a diagnosis in the schizophrenia spectrum (main or secondary lifetime diagnosis in ICD-8 of 295, 297, 298.29, 298.39, 298.89, 298.99, 299.05, 299.09, 301.09, 301.29, or in ICD-10 of F2x.x), The Danish Twin Register (Skytthe, Ohm Kyvik, Vilstrup Holm, & Christensen, Reference Skytthe, Ohm Kyvik, Vilstrup Holm and Christensen2011) was linked with The Danish Psychiatric Central Research Register (Mors, Perto, & Mortensen, Reference Mors, Perto and Mortensen2011). The baseline population was restricted to twin pairs aged 18–60 years, where both twins were alive and resided in Denmark (MZ = 61, DZ = 143). All MZ pairs were invited to participate in clinical examinations. DZ proband pairs and healthy control (HC) pairs were subsequently recruited and matched on age and gender according to the included MZ proband pairs. Exclusion criteria included serious head trauma (recorded in the medical journal), drugs/alcohol addiction, serious physical illness, and pregnancy (due to MRI scans). HC pairs were excluded based on the presence of a diagnosis of major psychosis in any first-degree relatives (F2x.x, F30, F31, and F32.3). A total of 214 individuals participated in cognitive assessments: 32 complete MZ and 22 complete DZ proband pairs and 29 complete MZ and 20 complete DZ HC pairs. Five of the complete MZ proband pairs were concordant for a schizophrenia spectrum diagnosis. All the DZ proband pairs were discordant for the disease. Additionally, eight twins from proband pairs participated without their sibling (MZ patient = 1, MZ co twin = 1, DZ patient = 3, DZ co twin = 3).
Clinical and cognitive assessment
Zygosity was verified by blood samples (PsychCHIP v.1-1, Illumina, San Diego, California, USA). In cases where DNA was not available (N = 17) register-based information was used. Register diagnoses were verified according to ICD-10 criteria using the Schedules for Clinical Assessment in Neuropsychiatry (SCAN) interview (Wing et al., Reference Wing, Babor, Brugha, Burke, Cooper, Giel and Sartorius1990). In cases of discrepancy between the register and project diagnosis, the project diagnosis was used. Psychopathology was rated using the Positive and Negative Symptom Scale (PANSS) (Kay, Fiszbein, & Opler, Reference Kay, Fiszbein and Opler1987), Hamilton's Depression (HAM-D17) and Anxiety (HAM-A14) scale (Hamilton, Reference Hamilton1967, Reference Hamilton1969), and the Bech-Rafaelsen Mania Scale (MAS) (Bech, Reference Bech2002).
The neuropsychological battery applied in this study examined a broad range of cognitive functions, including IQ, attention, working memory, verbal and visual memory, executive functions, social cognition, and olfactory identification. The tests were administered by trained personnel in a fixed order. The battery took approximately 4 h to complete including breaks when needed. Here we only report on data from CANTAB and IQ tests. We included spatial span (SSP), spatial working memory (SWM), stockings of Cambridge (SOC), intra-extra dimensional set shift task (IED), rapid visual information processing (RVP), reaction time (RTI), and information sampling task (IST) from CANTAB. A brief description of the selected CANTAB tests and outcome measures can be found in the online Supplementary Material and detailed descriptions can be found elsewhere (Evenden et al., Reference Evenden, Morris, Owen, Robbins, Roberts and Sahakian2013). We included vocabulary, similarities, matrix reasoning, and block design from The Wechsler Adult Intelligence Scale (WAIS-III) (Wechsler, Reference Wechsler1997), because these four subtests have shown high correlations with Full-Scale IQ (Axelrod, Reference Axelrod2002) and the Danish version of the National Adult Reading Test (DART) (Nelson & O'Connell, Reference Nelson and O'Connell1978).
Statistical analyses
Preparing for model fitting
Z-scores were calculated for the applied CANTAB measures based on the mean and standard deviation (s.d.) of twin A from HC pairs. These z-scores were used in a principal component analysis (PCA) with varimax rotation to identify underlying latent cognitive components. Based on the PCA, factor scores were generated using variables loading more than 0.50 on a given component. Also based on initial PCA components, IQ was divided into verbal IQ (DART, vocabulary, and similarities) and performance IQ (block design and matrix reasoning). For completeness, we analyzed both cognitive components and individual CANTAB measures. Several variables of the latter were highly skewed and therefore log-transformed. Effects of age and gender were regressed out of CANTAB and IQ measures and outliers >3 s.d. from the mean were removed before model fitting (see online Supplementary Table S1 for number of subjects included for each measure). For some measures, data were only available from one member of a twin pair. These individuals were included in our analyses to increase power and to obtain a better estimate of group means and variances.
Genetic model fitting
The twin design allows for estimating contributions of latent variables A [additive genetic factors], C [common (shared) environmental factors], and E [unique environmental ( + measurement error) factors] that may influence a certain trait. We can separate the full variance V = (A + C + E) into the separate components making use of the fact that MZ twins share (almost) 100% of their genes, while DZ twins share (on average) 50% of their segregating genes. If MZ twins resemble each other more on a given trait compared to DZ twins, genetic factors are assumed to influence the trait. When MZ and DZ twins resemble each other to a similar extent, common environmental factors are thought to act on the trait. The variance that is not shared between twins is attributed to unique environmental effects. This intuition is quantifiable by modeling latent factors A, C, and E that act on a trait. Additive genetic factors A are assumed to correlate 1 in MZ pairs, and 0.5 in DZ pairs. Common environmental factors C are assumed to correlate 1 for both zygosities and unique environmental factors are independent by definition.
For schizophrenia, defined by a dichotomous diagnosis, a liability threshold model was assumed, where the risk of schizophrenia is normally distributed, and the disorder only occurs when a certain threshold is exceeded. Because this was not a population study, estimates for schizophrenia were based on a recent study from our group on the Danish population (Hilker et al., Reference Hilker, Helenius, Fagerlund, Skytthe, Christensen, Werge and Glenthøj2017). The liability threshold was fixed in correspondence with an overall population prevalence of 1.85%. Heritability of a schizophrenia spectrum disorder was fixed at 73% and unique environmental influences were assumed to be 27%. For brevity, schizophrenia liability is used in what follows as a reference to the entire psychosis spectrum. Heritability (h 2) of CANTAB measure in this model is defined as the variance attributed to genetic factors divided by the total variance ((a 12)2 + (a22)2)/((a 12)2 + (a 22)2 + (c 22)2 + (e 12)2 + (e 22)2). The proportion of the variance explained by common environmental effects (c 2) and unique environmental effects (e 2) is defined likewise (Fig. 1a).
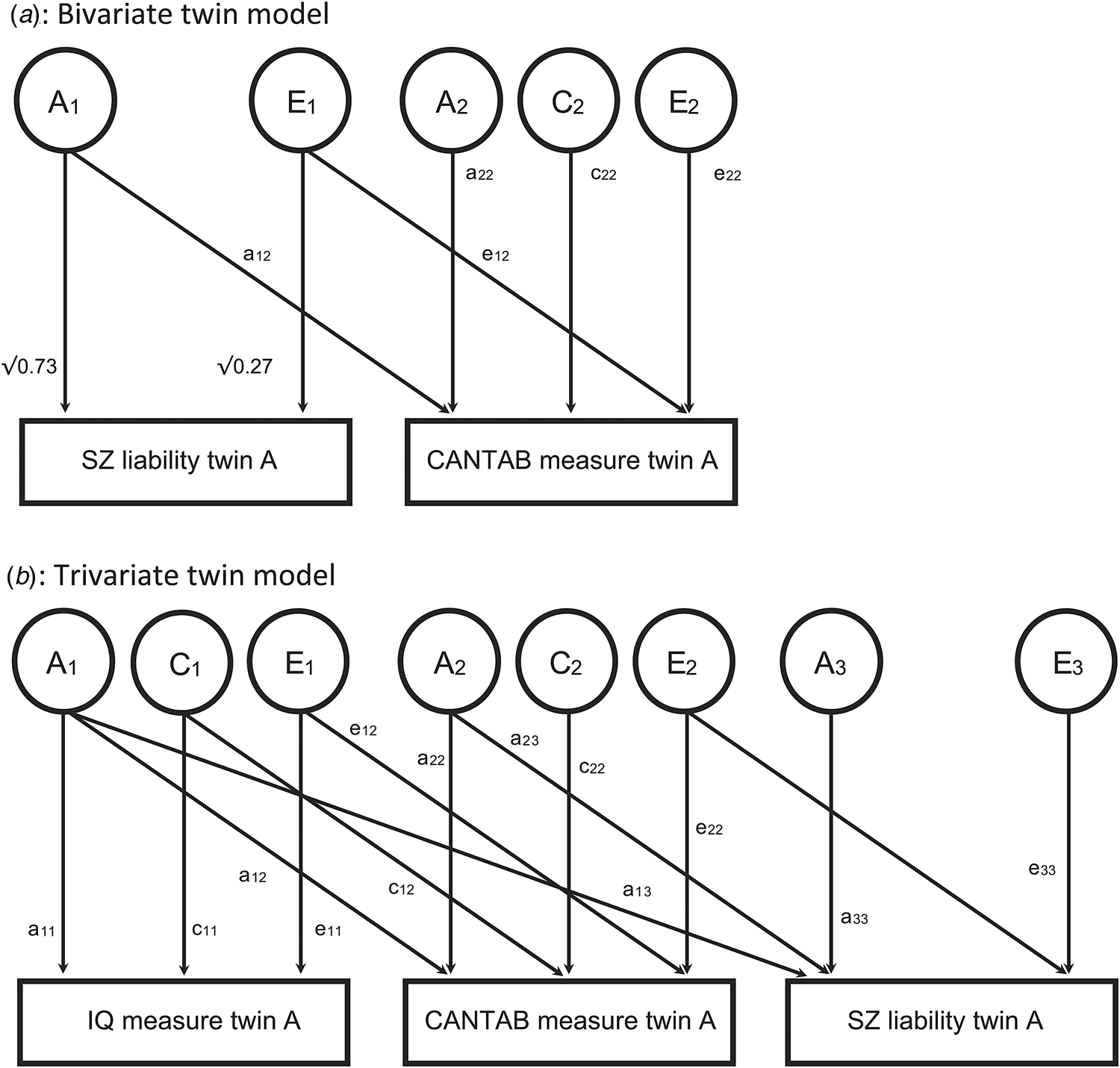
Fig. 1. Twin models. (a) Upper figure: Heritability (h 2) of CANTAB measure in this model is defined as ((a 12)2 + (a 22)2)/((a 12)2 + (a 22)2 + (c 22)2 + (e 12)2 + (e 22)2). The proportion of the variance explained by shared environmental effects (c 2) and unique environmental effects (e 2) is defined likewise. Heritability of schizophrenia spectrum disorder is fixed at 73% (Hilker et al., Reference Hilker, Helenius, Fagerlund, Skytthe, Christensen, Werge and Glenthøj2017). The covariance between liability for schizophrenia spectrum and the CANTAB is modeled as a 12 ×√0.73 + e 12√0.27. For simplicity, the model for only one of the twins is shown. Factor A1 (A2) of twin A is correlated to factor A1 (A2, respectively) of twin B with a correlation of 1 in monozygotic twins, and 0.5 in dizygotic twins. For both zygosities, C2 of twin A has correlation 1 with C2 twin B. Unique environmental factors are not correlated by definition. (b) Lower figure: Trivariate model to separate the covariance between CANTAB measures and schizophrenia liability (a 12 × a 13 + a 22 × a 23 + e 12 × e 23 + e 22 × e 23) into a genetic part not shared with IQ (a 22 × a 23), a genetic part shared with IQ (a 12 × a 13), an environmental part shared with IQ (e 12 × e 13) and an environmental part not shared with IQ (e 22 × e 23).
To investigate potential associations between cognition and schizophrenia liability, we estimated the covariance between the two traits. Following path tracing rules, the covariance between liability for schizophrenia spectrum and CANTAB was then modeled as a 12 × √0.73 + e 12 × √0.27. We estimated the phenotypic correlation R ph as this covariance divided by the standard deviation of the traits: (a 12 × √0.73 + e 12 × √0.27)/(1 × √(a 12)2 + (a 22)2 + (c 22)2 + (e 12)2 + (e 22)2). The phenotypic correlation can be thought of as the correlation between a CANTAB measure, and the liability for schizophrenia (Fig. 1a).
Genetic correlations (R a) and unique environmental (R e) correlations can be estimated by comparing cross-twin cross-trait correlations in MZ and DZ twins. Intuitively, if cross-twin cross-trait correlations are higher in MZ twins than in DZ twins, then genetic factors can be assumed to explain the correlation between the two traits. Mathematically, R a is defined as (a 12 × √0.73)/(√0.73 × √((a 12)2 + (a 22)2)). Likewise, R e is defined as (e 12 × √0.27)/(√0.27 × (√((e 12)2 + (e 22)2))). A common environmental correlation was not modeled, as there was no latent variable C modeled for schizophrenia liability (Hilker et al., Reference Hilker, Helenius, Fagerlund, Skytthe, Christensen, Werge and Glenthøj2017). If common environmental factors are not contributing to variation in schizophrenia liability, they also cannot explain covariance between schizophrenia liability and another trait.
The R a and R e correlations do not consider the heritability of either trait and it is thus possible that a large genetic correlation explains very little of the observed covariation between these traits. Therefore, the correlations were combined with the heritability estimates of each trait to calculate the part of the phenotypic correlation (R ph) due to genetic effects (R ph-a; sometimes called bivariate heritability) by (√0.73 × R a × √(h 2CANTAB)), and unique environmental effects (R ph-e) by (√0.23 × R e × √(e 2CANTAB)) (Toulopoulou et al., Reference Toulopoulou, Picchioni, Rijsdijk, Hua-Hall, Ettinger, Sham and Murray2007), with h 2CANTAB the estimated heritability of the CANTAB measures as defined above. Similarly, e 2CANTAB is the estimated proportion due to unique environmental effects of the CANTAB measures. Please note that R ph-a + R ph-e equals R ph (see online Supplementary Fig. S1 for an example output of the bivariate model).
Finally, to investigate whether IQ influenced potential associations between CANTAB components and schizophrenia liability, we extended the model to a trivariate model (Fig. 1b). Here the covariance between CANTAB components and schizophrenia liability (a 12 × a 13 + a 22 × a 23 + e 12 × e 23 + e 22 × e 23) was separated into a genetic part not shared with IQ (a 22 × a 23), a genetic part shared with IQ (a 12 × a 13), an environmental part shared with IQ (e 12 × e 13) and an environmental part not shared with IQ (e 22 × e 23), separately for verbal and performance IQ. When these parts not shared with IQ were significantly different from zero, we conclude that the association between CANTAB and schizophrenia liability is not completely explained by IQ.
The models described above were implemented in the OpenMx software package (2.9.6) installed on the R platform (3.3.2) and were fitted using maximum likelihood. Significance of variance components was based on comparing the full model with the model in which the variance component was constrained at zero. Minus two times the difference in the log-likelihood of these models is distributed as a 50:50 mixture of a χ2 distribution with zero and one degree of freedom, respectively (Dominicus, Skrondal, Gjessing, Pedersen, & Palmgren, Reference Dominicus, Skrondal, Gjessing, Pedersen and Palmgren2006). Significances for R ph, R a, R e, R ph-a, and R ph-e were based on the 95% confidence intervals. Similarly, we computed the 95% confidence intervals of the parts of the covariance between CANTAB and schizophrenia shared and non-shared with IQ in the trivariate models to assess significance in the individual components.
Sensitivity analyses
We reran the analyses in the narrow schizophrenia group (excluding all proband pairs with a diagnosis other than schizophrenia). Secondly, to test for the effect of fixing the variance components to the estimates in the Danish population [which notably, did not show significant common environmental effects on schizophrenia or the psychosis spectrum (Hilker et al., Reference Hilker, Helenius, Fagerlund, Skytthe, Christensen, Werge and Glenthøj2017)] we reran analyses fixing the heritability at 81%, common environmental influences at 11% and unique environmental influences at 8% (Sullivan, Kendler, & Neale, Reference Sullivan, Kendler and Neale2003). Thirdly, to assess the influence of excluding outliers, we reran the analyses in the full dataset. Finally, we also reran the analyses without regressing out age and gender effects.
Results
Demographics
The demographic and clinical data are summarized in Table 1. Of the 214 individuals who participated in cognitive assessments, five patients did not complete any CANTAB tests, one patient did not complete any WAIS-III tests and nine (four patients, four co-twins, and one HC) did not complete DART.
Table 1. Demographics
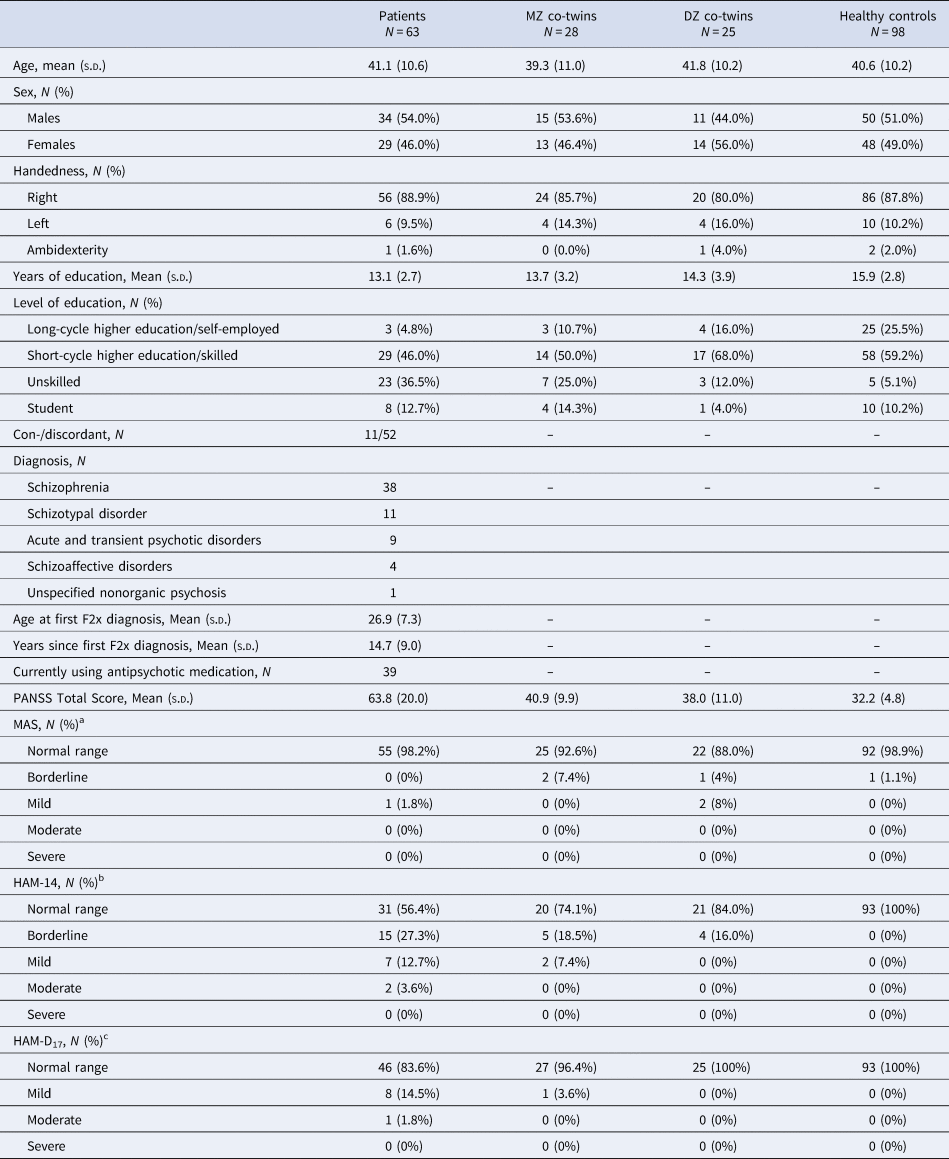
Note: Age at first F2x diagnosis and years since F2x diagnosis are based on the first contact with the secondary health care system under this diagnosis.
PANSS, Positive and Negative Symptom Scale; MAS, Bech-Rafaelsen Mania Scale; HAM-A14, Hamilton's Anxiety Scale; HAM-D17, Hamilton's Depression Scale.
a Missing data from seven patients, one MZ co-twin, and five healthy controls.
b Missing data from eight patients, one MZ co-twin, and five healthy controls.
c Missing data from eight patients and five healthy controls.
Cognitive components
Figure 2 illustrates performance on the selected CANTAB tests for patients, MZ co-twins, DZ co-twins, and HCs (mean raw scores are presented in online Supplementary Table S1). The PCA of the CANTAB measures resulted in seven components with an eigenvalue above 1.00, accounting for 78.05% of the variance. The components represented planning/spatial span, self-ordered spatial working memory, sustained attention, movement time, set-shifting, reflection impulsivity, and thinking time (detailed in Table 2).
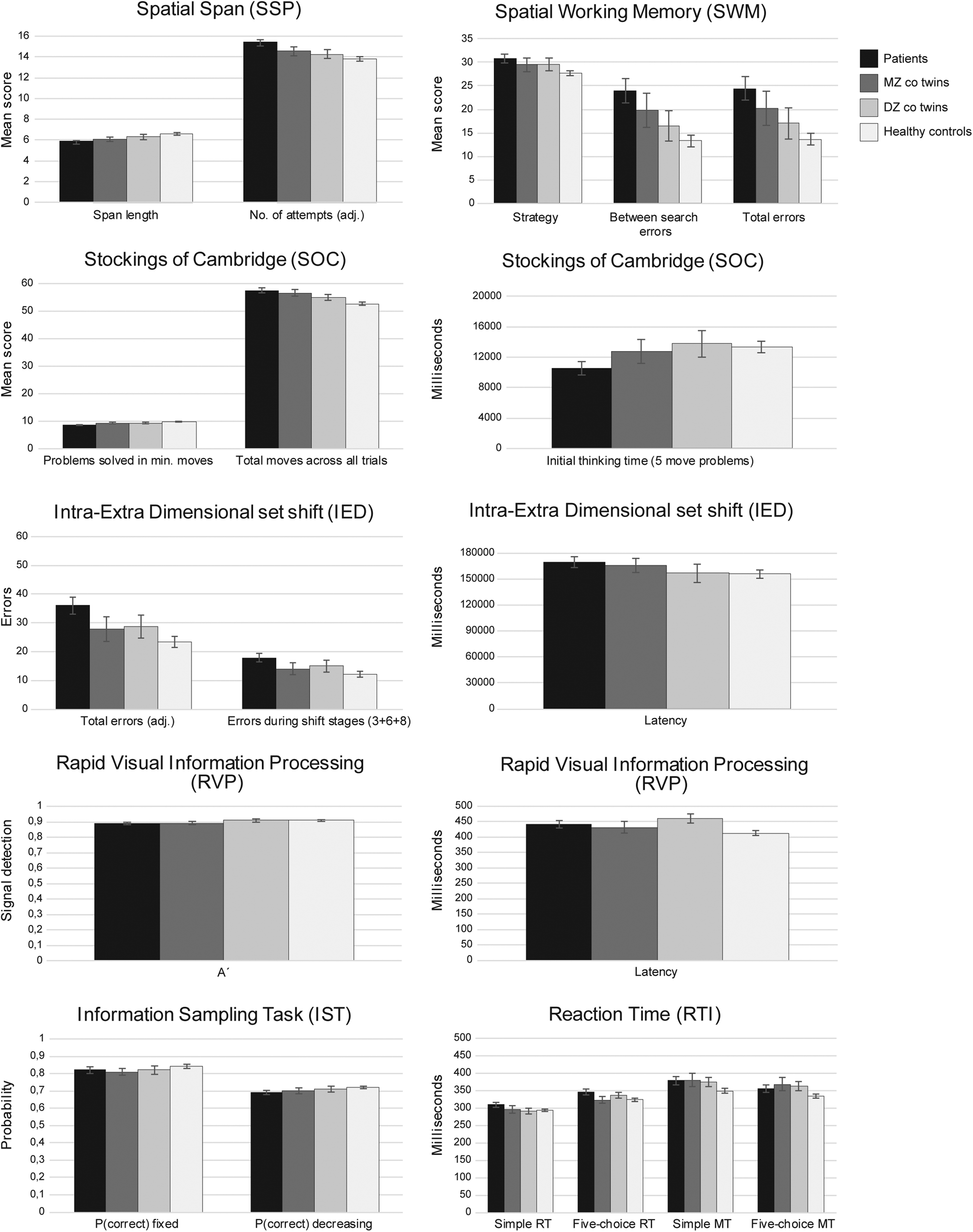
Fig. 2. Mean raw scores of individual outcome measures form the applied CANTAB tests for patients, MZ and DZ co-twins, and healthy controls. Error bars represent standard error of the mean (SEM). For the following measures, higher scores indicate better performance: SOC span length, SOC problems solved in min moves and initial thinking time, RVP A′, IST P(correct), DART, vocabulary, similarities, block design, and matrix reasoning. For the following measures, lower scores indicate better performance: SSP number of attempts to attain span, SWM strategy score, between search errors and total errors, SOC total moves, RVP latency, all IED measures, and all RTI measures.
Table 2. Heritability estimates and associations with schizophrenia spectrum liability

Note: Results from the cognitive components are displayed in grey rows. Under each component score are the results from the individual measures included in the component. Significant effects displayed in bold, unique environmental effects are significant by default. Brackets include 95% confidence intervals. Effects of age and gender regressed out before model fitting.
a Log-transformed before model fitting. A: Additive genetic effects, C: Common environmental effects, E: Unique environmental effects, R ph: Phenotypic correlation with schizophrenia spectrum liability, R ph-a: Association due to genetic overlap: R ph-e: Association due to unique environmental overlap. R a: Genetic correlation, R e: unique environmental correlation. The estimates applied for schizophrenia spectrum disorders did not show a significant C-component and thus the phenotypic correlation cannot be due to common environmental factors.
SSP, spatial span; SWM, spatial working memory; SOC, stockings of Cambridge; IED, intra-extra dimensional set shift; RVP, rapid visual information processing; RTI, reaction time; IST, information sampling task;DART, Danish version of the National Adult Reading Test.
Associations between cognition and schizophrenia liability
As shown in Table 2, schizophrenia liability was negatively associated with planning/spatial span, self-ordered spatial working memory, sustained attention, and set-shifting. For the first two of the above components, genetic covariance accounted for the observed associations. For individual CANTAB variables, only RVP latency, RTI simple reaction time, RTI five-choice movement time, and IST P(correct) did not correlate with schizophrenia liability. Performance, but not verbal IQ was associated with schizophrenia liability and this association was due to a genetic overlap. For individual WAIS-III subtests, block design and matrix reasoning were associated with schizophrenia liability due to a genetic overlap, whereas DART, vocabulary, and similarities were not associated with the illness.
Genetic and environmental influences on cognition
Heritability estimates are also given in Table 2 for both cognitive components and individual cognitive measures. Genetic factors contributed significantly to planning/spatial span, self-ordered spatial working memory, sustained attention, and movement time, whereas only unique environmental factors ( + measurement error) contributed significantly to set-shifting, reflection impulsivity, and thinking time. IQ was found to be highly heritable, with genetic factors significantly contributing to the variance in both verbal and performance IQ.
Cognitive components, IQ, and schizophrenia liability
All CANTAB components correlated moderately with both verbal and performance IQ (except for thinking time and performance IQ) (Table 3). Several of these associations were due to a genetic overlap. However, the associations between planning/spatial span and performance IQ as well as between thinking time and verbal IQ were explained by overlapping environmental factors. The bivariate model was then extended to a trivariate model including CANTAB components, IQ and schizophrenia liability. Figure 3 illustrates the associations between the seven CANTAB components and schizophrenia liability, separated on overlap shared with verbal and performance IQ, respectively. In this trivariate model, again planning/spatial span, self-ordered spatial working memory, sustained attention, and set-shifting were significantly associated with schizophrenia liability. Moreover, due to an increase in power by adding IQ which correlated both with schizophrenia liability and movement time, the association between movement time and schizophrenia liability also reached significance. Verbal IQ did not significantly contribute to the observed associations between the CANTAB components and schizophrenia liability. On the other hand, performance IQ did contribute significantly to the covariance between schizophrenia and self-ordered spatial working memory, sustained attention, and movement time. However, for the association between planning/spatial span and schizophrenia, we observed a significant genetic contribution independent from performance IQ. For the association between schizophrenia and set-shifting, none of the components reached significance. In sum, planning/spatial span was genetically associated with schizophrenia liability independent of both performance and verbal IQ. For the remaining CANTAB components, the genetic associations with schizophrenia were shared with performance, but not verbal IQ.

Fig. 3. Covariance between CANTAB components and schizophrenia liability, separated on overlap with verbal IQ (top) and performance IQ (bottom). Stars next to bars indicate a significant association between the CANTAB component and schizophrenia liability. Stars on bars indicate a significant contribution to the covariance.
Table 3. Correlations between CANTAB components and intelligence

Note: Significant effects are displayed in bold. Here we have estimates of A, C, and E of both traits and therefore also report the part of the phenotypic correlation due to common environmental factors, R ph: Phenotypic correlation with schizophrenia spectrum liability, R ph-a: Association due to genetic overlap, R ph-e: Association due to unique environmental overlap. R ph-c: Association due to common environmental overlap.
Sensitivity analyses
Rerunning the analyses only including proband pairs with a diagnosis of schizophrenia showed very similar results (online Supplementary Table S2). By fixing the heritability estimate to 81% and allowing for common environmental influences on schizophrenia (11%) (Sullivan et al., Reference Sullivan, Kendler and Neale2003), we obtained very similar findings. Although planning/spatial span and movement time were no longer significantly heritable, the numerical values of the genetic influences were very similar to the original analyses (online Supplementary Table S3). When we included outliers, the heritability for set-shifting reached significance, although the numeric value of the estimate actually decreased. It did not influence the phenotypic correlations between cognition and schizophrenia, but the R ph-a for sustained attention and set-shifting became significant (online Supplementary Table S4). Finally, rerunning the analyses without regressing out age and sex did not change the conclusions (online Supplementary Table S5).
Discussion
The first aim of the current study was to investigate (genetic or environmental) associations between specific cognitive functions measured by CANTAB and schizophrenia liability. We identified seven cognitive components from the applied CANTAB tests. Planning/spatial span, self-ordered spatial working memory, sustained attention, and set-shifting were negatively associated with schizophrenia liability, indicating that a higher liability to schizophrenia is associated with a poorer performance in these measures. For planning/spatial span and self-ordered spatial working memory, genetic overlap accounted for the observed associations with schizophrenia, suggesting that shared genes may regulate the development of these cognitive functions and schizophrenia risk. This is in line with previous studies demonstrating overlap between cognition and schizophrenia liability (Owens et al., Reference Owens, Picchioni, Rijsdijk, Stahl, Vassos, Rodger and Toulopoulou2011a, Reference Owens, Rijsdijk, Picchioni, Stahl, Nenadic, Murray and Toulopoulou2011b; Toulopoulou et al., Reference Toulopoulou, Goldberg, Mesa, Picchioni, Rijsdijk, Stahl and Murray2010).
We also observed a genetic association between performance IQ and schizophrenia liability consistent with previous studies. However, verbal IQ was not associated with schizophrenia liability. This supports findings from a previous study demonstrating an association between the polygenic risk score for schizophrenia and lower performance, but not verbal IQ in children (Hubbard et al., Reference Hubbard, Tansey, Rai, Jones, Ripke, Chambert and Zammit2016). Together the evidence suggests a difference between verbal and performance IQ in relation to schizophrenia liability. One explanation may be that verbal IQ tests are less sensitive to various disease processes and insults during development (Rinaldi & Karmiloff-Smith, Reference Rinaldi and Karmiloff-Smith2017; Russell et al., Reference Russell, Munro, Jones, Hayward, Hemsley and Murray2000). Accordingly, premorbid IQ is estimated using verbal tasks, with overlapping construct validity with several of the verbal subtests from the WAIS scales (Bright & van der Linde, Reference Bright and van der Linde2018).
Our second aim was to examine genetic and environmental influences on specific cognitive functions. Here we demonstrate that several of the CANTAB measures are heritable, consistent with the recent meta-analysis showing strong genetic influences on many cognitive functions (Blokland et al., Reference Blokland, Mesholam-Gately, Toulopoulou, Del Re, Lam, Delisi and Petryshen2017). In addition, we found both verbal and performance IQ to be highly heritable, also consistent with existing literature (Blokland et al., Reference Blokland, Mesholam-Gately, Toulopoulou, Del Re, Lam, Delisi and Petryshen2017; Deary et al., Reference Deary, Penke and Johnson2010). We observed the highest heritability estimates for self-ordered spatial working memory and sustained attention, but planning/spatial span and movement time were also influenced by genetic factors. Singer et al. (Reference Singer, MacGregor, Cherkas and Spector2006) applied different composite scores, making the results difficult to compare directly. However, their working memory score was similar to our self-ordered spatial working memory and in line with our results, the authors found working memory to be heritable. Steves et al. (Reference Steves, Jackson and Spector2013) reported significant heritability for SSP and SWM consistent with our results. In contrast to our findings, they reported significant heritability for both five-choice and simple reaction times from RTI, whereas we only observed significant heritability for simple reaction time. The discrepant findings may be explained by different study populations. Finally, in agreement with our results, Zhou et al. (Reference Zhou, Li, Xie, Xu, Cheung, Li and Chan2018) reported significant heritability of SWM.
On the other hand, some of the cognitive components were only explained by unique environmental factors, i.e. set-shifting, reflection impulsivity, and thinking time. Previous studies support the finding that set-shifting is not heritable (Ceaser et al., Reference Ceaser, Goldberg, Egan, McMahon, Weinberger and Gold2008; Zhou et al., Reference Zhou, Li, Xie, Xu, Cheung, Li and Chan2018). Moreover, some individual outcome measures showed heritability estimates close to zero, e.g. IST P(correct) decreasing, RVP A′, and IED latency. This is surprising for cognition (Blokland et al., Reference Blokland, Mesholam-Gately, Toulopoulou, Del Re, Lam, Delisi and Petryshen2017), and could be due to low reliability of these measures. Reliability is generally high for the majority of the applied CANTAB tests (Gonçalves, Pinho, & Simões, Reference Gonçalves, Pinho and Simões2016; Lowe & Rabbitt, Reference Lowe and Rabbitt1998); however, low reliability has previously been reported for some IED measures, although latency was not included (Lowe & Rabbitt, Reference Lowe and Rabbitt1998). A simpler version of the RVP has been found to show good reliability (Gonçalves et al., Reference Gonçalves, Pinho and Simões2016), but we cannot rule out that a more complex task would change the reliability. For IST, we could not find any available reliability measures, and it is therefore entirely possible that low reliability may have influenced the obtained estimates.
Among the cognitive components in the current study is one shared between the tests of planning (SOC) and spatial span (SSP) that is somehow not dependent simply on spatial working memory, as performance on the SWM test did not load significantly with performance on SOC and SSP. The most obvious cognitive requirement shared by the SOC and SSP tests may be that of response sequencing, which is also not the predominant requirement of the SWM test. Whether this observation can be generalized to other tests of sequencing and whether it relates to a discrete neural network closely related, but not identical to that for spatial working memory, remains a topic of future investigation.
Finally, the third aim of the study was to separate the effects of IQ on the associations between specific cognitive functions and schizophrenia liability. All seven CANTAB components were associated with IQ and several of these associations were due to overlapping genetics, indicating that there are common genes involved in the development of IQ and specific cognitive abilities. Moreover, for self-ordered spatial working memory, sustained attention, and movement time, the observed association with schizophrenia was shared with performance, but not verbal IQ.
However, for planning/spatial span, we observed a significant genetic contribution to the covariance with schizophrenia liability independent of both verbal and performance IQ. This is supported by the finding that even though planning/spatial span is associated with performance IQ, this association is explained by overlapping environmental factors. This suggests different genetically-driven pathways for planning/spatial span and performance IQ involved in the development of schizophrenia, indicating that low performance on these two cognitive domains constitutes separate types of risk for schizophrenia.
Based on the current analyses, we cannot determine the direction of causality of the observed associations between cognition and schizophrenia. However, recent studies suggest that cognitive deficits contribute substantially to schizophrenia liability and not the other way around (Toulopoulou et al., Reference Toulopoulou, Van Haren, Zhang, Sham, Cherny, Campbell and Kahn2015, Reference Toulopoulou, Zhang, Cherny, Dickinson, Berman, Straub and Weinberger2018). This underscores the importance of cognitive deficits as a potential target for intervention (Pantelis et al., Reference Pantelis, Wannan, Bartholomeusz, Allott and McGorry2015). Here it is important to note that even though both traits are highly heritable, it does not mean that environmental interventions cannot have an effect: only an environment that varies across subjects can be measured in our model.
One possible limitation of this study is that we included patients with schizophrenia spectrum disorders when examining genetic influences on cognition. Patients were only ‘mildly’ ill according to the Total PANSS scores, whereas both MZ and DZ co-twins as well as healthy controls scored within the normal range (Leucht et al., Reference Leucht, Kane, Kissling, Hamann, Etschel and Engel2005). Still, the heritability of cognition may be lower in schizophrenia patients compared to the general population due to environmental/illness-related factors such as medication, clinical state during testing or higher rates of smoking and substance abuse. However, a previous comparison of heritability in non-psychiatric and schizophrenia families reported comparable estimates, suggesting that schizophrenia patients are equally informative for genetic studies of cognition (Blokland et al., Reference Blokland, Mesholam-Gately, Toulopoulou, Del Re, Lam, Delisi and Petryshen2017). Other forms of psychopathology, such as anxiety, mania, and depression, could also influence cognition. However, this is most likely not a major factor in the current study, given that the majority of patients, co-twins, and healthy controls scored within the normal range on the MAS and Hamilton Anxiety and Depression (Table 1). Moreover, twin pairs were specifically selected based on a schizophrenia spectrum diagnosis, and therefore model parameters for schizophrenia were not directly estimated. The estimate for common environmental influences for schizophrenia and the psychosis spectrum used in our analyses was fixed at zero. Since there was no common environmental influence explaining variance in schizophrenia liability, the correlation between schizophrenia liability and cognition could not be explained by common environmental factors, which is a limitation of our model. However, these estimates were based on the Danish twin registry and this nation-wide estimate is therefore the best estimate for our cohort (Hilker et al., Reference Hilker, Helenius, Fagerlund, Skytthe, Christensen, Werge and Glenthøj2017). Results were very similar using a model that included common environmental influences (Sullivan et al., Reference Sullivan, Kendler and Neale2003). Another potential limitation concerns the number of participants included in the study. Even though we were able to identify all eligible twin pairs nationwide through the registers, the scarcity of twins with schizophrenia in combination with the fact that this patient group is typically difficult to recruit, especially for a study with such a comprehensive examination program, raises concerns about power issues in the current analyses. We did observe quite large confidence intervals for many estimates, and a substantial proportion of the common environmental effects were estimated to 0. It is entirely possible that some of the common environmental effects may have been missed due to power issues, as common environmental factors are known to be harder to detect (Visscher, Gordon, & Neale, Reference Visscher, Gordon and Neale2008). Finally, the current sample size did not allow us to explore potential gene–environment correlations or interactions that likely exist.
In summary, this study provides further evidence that genetic factors influence cognition and overlap with the genetics of schizophrenia, suggesting a partially shared etiology. These results highlight cognition as a core feature of the disease and underscore the importance of cognitive deficits as a risk factor for schizophrenia. A better understanding of the genetic associations of these cognitive deficits helps elucidate the mechanisms by which genetic vulnerability impacts brain functioning in schizophrenia spectrum disorders. The heritable cognitive components associated with schizophrenia spectrum liability, i.e. planning/spatial span, self-ordered spatial working memory, and sustained attention, could represent endophenotypes for the disorder consistent with previously proposed cognitive endophenotypes (Gur et al., Reference Gur, Calkins, Gur, Horan, Nuechterlein, Seidman and Stone2007; O'Connor et al., Reference O'Connor, Harris, McIntosh, Owens, Lawrie and Johnstone2009; Saperstein et al., Reference Saperstein, Fuller, Avila, Adami, McMahon, Thaker and Gold2006). The observed heritability estimates for the potential cognitive endophenotypes are lower than the estimated heritability of 79% for schizophrenia and 73% for the schizophrenia spectrum (Hilker et al., Reference Hilker, Helenius, Fagerlund, Skytthe, Christensen, Werge and Glenthøj2017). However, by examining specific parts of the phenotypic presentation, e.g. cognitive deficits, we are attempting to reduce the complexity of the symptoms associated with schizophrenia and delineate the entire heritability of schizophrenia into components with specific underlying neurobiology. Therefore, it might not be possible to find a single endophenotype that is more heritable than schizophrenia itself, and the goal should rather be to find a collection of endophenotypes that additively resemble the heritability of schizophrenia (Braff, Freedman, Schork, & Gottesman, Reference Braff, Freedman, Schork and Gottesman2007; Greenwood et al., Reference Greenwood, Braff, Light, Cadenhead, Calkins, Dobie and Schork2014). Finally, this is the first study to demonstrate that some specific cognitive functions are genetically linked to schizophrenia independently of IQ. Planning/spatial span was associated with schizophrenia through a genetic pathway separate from both performance and verbal IQ, pointing to an endophenotype for schizophrenia that is not driven by IQ. Future studies should examine how cognitive performance is influenced by interactions among genetic and environmental factors.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291720002858
Acknowledgements
The authors would like to thank research assistants Helle Schæbel (CRN) and Mikkel Erlang Sørensen (MSc) for valuable contributions to the recruitment and data collection.
Financial support
This work was funded by Lundbeck Foundation grants (25-A2701, R155-2013-16337); a Ph.D. grant from The Mental Health Services, Capital Region of Denmark to CKL; and CP was supported by an NHMRC Senior Principal Research Fellowship (1105825) and by a grant from the Lundbeck Foundation (R246-2016-3237).
Conflict of interest
BJS consults for Cambridge Cognition. TWR consults for Cambridge Cognition, Unilever, Greenfields Inc. Research Grants, Shionogi, and Small Pharma; has received royalties from Cambridge Cognition (CANTAB); and is Editorial honoraria for Springer-Nature and Elsevier. RWH has received lecture fees from Lundbeck Pharma. CP has participated on Advisory Boards for Janssen-Cilag, Astra-Zeneca, Lundbeck, and Servier. He has received honoraria for talks presented at educational meetings organized by Astra-Zeneca, Janssen-Cilag, Eli-Lilly, Pfizer, Lundbeck, and Shire. BYG is the leader of a Lundbeck Foundation Centre of Excellence for Clinical Intervention and Neuropsychiatric Schizophrenia Research (CINS), which is partially financed by an independent grant from the Lundbeck Foundation, based on international review and partially financed by the Mental Health Services in the Capital Region of Denmark, the University of Copenhagen, and other foundations. Her group has also received a research grant from Lundbeck A/S for another independent investigator-initiated study. All grants are the property of the Mental Health Services in the Capital Region of Denmark and administrated by them. The remaining authors report no conflicts of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.









