Creation of the Fontan circulation allows for prolonged survival of patients with univentricular anatomy. Twenty-five-year survival for Fontan patients has been reported at 76%,Reference d’Udekem, Iyengar and Galati 1 but is estimated to be greater now with more recent surgical techniques, for which 25-year estimates have not yet been described. Despite this relative success, the Fontan circulation does not offer a normal life expectancy with many patients failing in their adult years or before. Fontan failure has been defined as death, transplantation, Fontan takedown or conversion, protein losing enteropathy, plastic bronchitis, or progression to New York Heart Association functional class III or IV.Reference d’Udekem, Iyengar and Galati 1
By its very nature, the Fontan circulation results in elevated systemic venous pressure. The loss of pulmonary artery pulsatile flow from the Glenn stage to Fontan completion leads to a lack of shear stress and subsequent endothelial dysfunction.Reference Gewillig and Goldberg 2 – Reference Zongtao, Huishan and Zengwei 4 This endothelial dysfunction, including altered release of nitric oxide, may induce further vascular remodelling and is believed to be a primary mechanism for the increase that can be seen in pulmonary vascular resistance (PVR).Reference Gewillig and Goldberg 2 – Reference Zongtao, Huishan and Zengwei 4 The resultant impedance to blood flow through the pulmonary vascular bed causes chronic preload deprivation leading to low cardiac output and ventricular remodelling, with reduced compliance and elevated ventricular end diastolic pressures.Reference Gewillig and Goldberg 2 Systolic dysfunction is also frequently seen in Fontan patients which may be a result of the original malformation but also damage from previous volume or pressure overloaded states. These all result in an “overgrown pump” which then cannot reach a filling point for optimal contractility in the pre-load deprived Fontan circulation.Reference Gewillig and Goldberg 2 , Reference Gewillig, Lundstrom and Deanfield 5 It is thought that this combination of elevated systemic venous pressure, elevated PVR, and chronic low cardiac output (with or without appreciable ventricular dysfunction) within the context of a patient who inherently has a narrow hemodynamic window of stability results in the clinical picture of Fontan failure.
We hypothesised that pulmonary artery structural changes at a microvascular level are likely present in Fontan patients due to this endothelial dysfunction and gradual elevation in PVR. However, pulmonary artery morphology is difficult to study in vivo beyond angiography. Optical coherence tomography (OCT) is a high-resolution (10 microns) intravascular imaging tool that has already proven its superior ability to conventional angiography in detecting early vessel wall changes in coronary arteriesReference Harris, Manouzi and Fung 6 , Reference Ulrich, Lehner and Birnbaum 7 and also at guiding coronary interventions.Reference Karanasos, Ligthart, Witberg, van Soest, Bruining and Regar 8 We employed OCT to assess for any structural changes within the pulmonary vasculature of Fontan patients compared to those with a normal pulmonary circulation.
Materials and methods
Patients were recruited between May 2016 and September 2017, in a single tertiary paediatric centre. Ethics approval was granted by The University of British Columbia Children’s and Women’s Health Centre’s Clinical Research Ethics Board and written informed consent and assent was obtained. All Fontan patients undergoing cardiac catheterisation during this time were approached for consent to perform pulmonary artery OCT at the end of their planned procedure. Patients with biventricular anatomy and without pulmonary arterial disease (e.g. electrophysiology patients) who were also undergoing cardiac catheterisation were approached to act as controls. Demographics (age, sex, and weight) and clinical data (systemic left or right ventricle, age at Fontan surgery, and pulmonary artery pressure pre-Fontan surgery) were collected for each Fontan patient. Pulmonary artery pressure, ventricular end diastolic pressure, cardiac index, and PVR were calculated for each Fontan patient’s current cardiac catheterisation.
OCT image acquisition and analysis
OCT (ILUMIEN OPTIS™ or OPTIS™ Integrated System, LightLab Imaging, St Jude Medical, St. Paul, Minnesota, United States of America (now Abbott)) was performed on one or more distal pulmonary artery branches. Which pulmonary artery branch or branches were imaged was dependent on ease of access, patient’s anatomy, and the discretion of the operator. Distal pulmonary artery branches were accessed with a 5 or 6 Fr wedge catheter, which was then exchanged out for a 5 or 6 Fr JR coronary catheter, 3.5 curve, 0.042 internal lumen (Vista britetip, Cordis). A 0.014″ wire was passed through the catheter with its floppy tip distal and over this; the OCT catheter was placed in a monorail fashion. The length of the imaging apparatus on the OCT catheter is denoted by two radio-opaque dots on fluoroscopy. We aimed to have the whole length of imaging apparatus outside of the JR coronary catheter, but without pulling the JR catheter back into a larger vessel, which would result in less effective occlusion of forward blood flow. A small hand injection of contrast was used to assess position of the wire and catheters and the relative size of the blood vessel. When desired catheter position was achieved, a contrast power injection at 300 PSI was delivered with cine image acquisition, which triggered pullback of the OCT catheter. Twenty millilitre of contrast at 5 ml/second was used for teenage patients >50 kg and smaller volumes for smaller patients depending on weight (∼0.5–1 ml/kg).
Image analysis
Quantitative analyses were performed on pullbacks with a series of ⩾5 cross-sectional frames that were ⩾1 mm apart. Cross-sectional frames containing artefacts or side branches comprising >25% of the image were excluded.Reference Tearney, Regar and Akasaka 9 Using digital planimetry, measurements of mean vessel wall thickness (mm) and the cross-sectional areas (CSA) of the lumen and the whole vessel were made. Wall CSA was derived as vessel CSA minus lumen CSA, and wall:vessel CSA ratio was calculated as a measure to allow comparisons between vessels in patients of different sizes. For each case, a summary value for wall thickness (mm) and normalised wall thickness (wall:vessel CSA) was derived as the median across all analysed cross-sectional frames from the case.
Statistical analyses
Descriptive sample statistics were calculated as frequencies (%) or median and interquartile range (IQR). Differences between groups were assessed by Mann Whitney U tests and associations between continuous variables were assessed by Spearman’s rank correlation (ρ). Analyses were carried out using Stata v. 14.1 (Stata Corp LP, College Station, Texas, United States of America) and significance was set at p < 0.05.
Results
Pulmonary artery OCT was successfully carried out on 12 Fontan patients and 11 controls with no complications. All Fontan patients had extracardiac Fontan conduits. All Fontan patients were clinically well and without evidence of Fontan failure. During the time of recruitment, there were no patients with failing Fontan physiology presenting for cardiac catheterisation. Our control patients were healthy patients with normal cardiac anatomy undergoing an electrophysiology study. Table 1 describes patient characteristics.
Table 1. Patient characteristics. Data are median (IQR) or n (%)

EP = Electrophysiology; LV = Left Ventricle; RV = Right Ventricle.
Pulmonary arteries are often visualised as a single-layered wallReference Jorge, Baptista and Calisto 10 , Reference Li, Zhang, Hou, Jang and Yu 11 in contrast to a coronary artery, where the three-layer structure is often more clearly delineated. Although difficult to measure and assign a quantitative value, we identified a vessel media in all control patients and, with greater difficulty, in the Fontan patients. The media was visualised with similar ease as controls, in only 4 of 12 Fontan patients. Figure 1 shows OCT of pulmonary arteries, with and without a visible media, compared to a coronary artery.

Figure 1. OCT imaging of PAs (( a ) with an indistinct media and ( b ) with media visualised denoted by arrow) and a coronary artery ( c ).
Median wall thickness for Fontan patients was 0.12 mm (IQR, 0.10–0.14) and for controls was 0.11 mm (IQR, 0.10–0.12; p = 0.23). Normalised wall thickness for Fontan patients was 0.13 (IQR, 0.12–0.16) and for control patients was 0.13 (IQR, 0.11–0.15; p = 0.32) (see Fig 2). There was no significant difference between groups for either parameter of wall thickness.

Figure 2. Mean wall thickness and normalised wall thickness (wall CSA:vessel CSA ratio) in Fontan patients versus controls.
In Fontan patients, there was no difference in normalised wall thickness between those with a systemic left ventricle (n = 7) and those with a systemic right ventricle (n = 5) (p = 0.68). Normalised wall thickness was not significantly related to age at time of catheterisation, current pulmonary artery pressure, current PVR, current ventricular end diastolic pressure, current cardiac index, age at Fontan completion, years since Fontan completion, and pulmonary artery pressure prior to Fontan completion (Table 2).
Table 2. Fontan patient characteristics, catheterisation data, and associations with normalised wall thickness
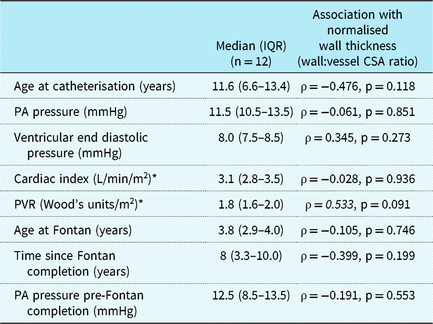
PA = Pulmonary Artery; PVR = Pulmonary vascular resistance.
* Available for n = 11 only (due to extensive aorto-pulmonary collateral burden in one patient).
Discussion
OCT of the coronary arteries in the setting of atherosclerotic heart disease or coronary allograft vasculopathy is becoming more commonplace, but imaging of the pulmonary arteries is less well described. OCT imaging of the pulmonary arteries in children has been reported twiceReference Hayabuchi, Sakata and Kagami 12 , Reference Homma, Hayabuchi, Ono and Kagami 13 and has never been described in Fontan patients. From this initial study, our OCT findings suggest that during childhood, pulmonary artery wall dimensions are normal in healthy children with a Fontan circulation and reassuring hemodynamics. Our ability to identify a media more readily in control patients versus Fontan patients may suggest that loss of the media is an early sign of vascular change, analogous to that seen in coronary arteries affected by Kawasaki disease.Reference Harris, Manouzi and Fung 6 Increased apoptosis of smooth muscle cells has also been described in an animal model of non-pulsatile pulmonary blood flow,Reference Zongtao, Huishan and Zengwei 4 which may explain this finding. However, further studies with a larger sample size, evaluation of Fontan patients with abnormal hemodynamics and serial evaluation into adulthood, are needed to conclude on the utility of OCT for identifying early pulmonary artery structural changes. This study serves to provide a baseline for the OCT appearance of clinically well paediatric Fontan patients, to which OCT images from adult Fontan patients or failing Fontan patients can be compared.
Our inability to identify a difference in wall thickness between Fontan patients and controls may be attributable to our small sample size. It is also possible that our assessment in young, healthy Fontan patients was simply premature, as many molecular and histological studies do support the presence of functional and structural changes in the pulmonary arteries of univentricular patients.Reference Zongtao, Huishan and Zengwei 4 , Reference Kurotobi, Sano and Kogaki 14 – Reference Ridderbos, Wolf and Timmer 18 An in vitro study of 10 healthy patients with a bidirectional Glenn supported endothelial functional attenuation by demonstrating reduced pulmonary flow velocity in response to acetylcholine.Reference Kurotobi, Sano and Kogaki 14 Decreased synthesis of endothelial nitric oxide synthase has also been described.Reference Zongtao, Huishan and Zengwei 4 Many histological studies have reported abnormal structural changes in the pulmonary arteries of univentricular patients with passive pulmonary blood flow, but these findings have often been conflicting. Increased wall thickness of intra-acinar vessels from abnormal smooth muscle extension,Reference Lévy, Danel, Tamisier, Vouhé and Leca 15 attenuation of the muscular component of the medial layer with disarray of elastic fibres,Reference Adachi, Ueno, Hori and Sawa 16 and increased medial thickness which correlated with mean pulmonary artery pressure and outcomesReference Maeda, Yanaki, Kado, Asou, Murakami and Takamoto 17 have all been described. Although most of these histological studies report abnormalities in unwell univentricular patients, Maeda et alReference Maeda, Yanaki, Kado, Asou, Murakami and Takamoto 17 also noted less marked medial layer thickening in patient with good outcomes. Ridderbos et alReference Ridderbos, Wolf and Timmer 18 studied lung tissue samples from deceased Fontan patients and described increased medial thickness in patients who died peri-operatively but conversely decreased smooth muscle in the media and rather increased intimal thickening, due to acellular fibrosis and increased collagen, in late Fontan deaths. However, our OCT study cannot be directly compared to these histological studies. For example, although Adachi et alReference Adachi, Ueno, Hori and Sawa 16 did report a case of a markedly reduced medial layer in the main pulmonary artery of a 35-year-old Fontan patient, most of the histological studies describe pre- or intra-acinar vessel changes,Reference Lévy, Danel, Tamisier, Vouhé and Leca 15 , Reference Maeda, Yanaki, Kado, Asou, Murakami and Takamoto 17 – Reference Ishida, Kogaki and Ichimori 19 and they largely report abnormalities in patients with poor outcomes.Reference Lévy, Danel, Tamisier, Vouhé and Leca 15 , Reference Adachi, Ueno, Hori and Sawa 16 , Reference Ridderbos, Wolf and Timmer 18 As OCT cannot image the pre- or intra-acinar vessels due to the size limitations imposed by the catheter, it is possible that the arteries we imaged are too proximal to exhibit early pathological changes.
To date, OCT of the pulmonary arteries has been shown to successfully identify increased vessel wall thickness in the setting of pulmonary arterial hypertension.Reference Jorge, Baptista and Calisto 20 In some cases, OCT indices of increased wall thickness have correlated with pulmonary artery pressure and PVR.Reference Dai, Fukumoto and Tatebe 21 , Reference Jorge, Baptista and Calisto 10 Several studies have also verified the accuracy of OCT by demonstrating a strong correlation between histological and OCT measurements of pulmonary artery wall thickness in patients with pulmonary arterial hypertension.Reference Li, Zhang, Hou, Jang and Yu 11 , Reference Domingo, Grignola and Aguilar 22 , Reference Brunner, Zamanian and Ikeno 23 These studies support that OCT has the ability to identify true changes if present. If future studies conclude that OCT is able to identify early structural changes in the PAs of Fontan patients, it may be a useful tool for prognostication or an adjunct for surgical or medical decision-making. For example, should medial hypertrophy be observed, this could support the use of pulmonary vasodilators. However, Ridderbos et alReference Ridderbos, Wolf and Timmer 18 suggested aggressive anticoagulation may be a more useful strategy if intimal thickness is identified as the primary pathological process, which they reported as being largely due to in situ thrombosis based on histopathology. Although less invasive and more convenient to carry out than lung biopsy, OCT of the pulmonary arteries provides a more limited assessment than lung tissue histopathology. The inability to consistently identify the media and the difficulty in its quantification (as it is frequently not seen circumferentially) would limit our ability to report on whether wall thickening is due to intimal or medial layer thickening.
This study was the first to report on OCT imaging of pulmonary arteries in Fontan patients. We have shown that this technique is safe and feasible. Although we did not identify a significant difference in vessel wall thickness between Fontan patients and controls, this may be explained by our small sample size, the lesser likelihood of significant differences being present in the larger vessels accessible to OCT or most likely because we only studied young, healthy Fontan patients. A larger study evaluating Fontan patients with abnormal hemodynamics and serial evaluation into adulthood is needed to conclude on the utility of OCT for identifying early pulmonary artery structural changes and subsequently ascertain if OCT would be a useful tool to help risk stratify patients and determine best treatment approach.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
K.C. Harris is a consultant for St Jude Medical (now Abbott) and a proctor for Gore. All other authors have no conflicts of interest.






