Depression is one of the most burdensome mental illnesses (Whiteford, Ferrari, Degenhardt, Feigin, & Vos, Reference Whiteford, Ferrari, Degenhardt, Feigin and Vos2015) and is a robust predictor of suicide (Avenevoli, Swendsen, He, Burstein, & Merikangas, Reference Avenevoli, Swendsen, He, Burstein and Merikangas2015). The onset of major depressive disorder often occurs during adolescence (Steinberg & Morris, Reference Steinberg and Morris2001), partly due to the development of neural circuitry underlying self-processing which results in heightened self-consciousness and susceptibility to peer influence (Sebastian, Burnett, & Blakemore, Reference Sebastian, Burnett and Blakemore2008). Self-processing involves the perceptions, memories, and representations of one's self. Research has shown that depressed individuals show excessive self-focus and negatively biased self-processing (Beck, Reference Beck2008; Bradley et al., Reference Bradley, Colcombe, Henderson, Alonso, Milham and Gabbay2016). In particular, excessive self-focus is thought to lead to self-regulatory cycles that attempt to reduce the perceived discrepancy between the current self and a salient ‘ideal’ standard (Carver & Scheier, Reference Carver and Scheier1998), and failure to exit such cycles might cause onset or endurance of depression (Beck, Reference Beck2008; Pyszczynski & Greenberg, Reference Pyszczynski and Greenberg1987). Because adolescence is a key window for self-identity development characterized by heightened self-focus and introspection (Sebastian et al., Reference Sebastian, Burnett and Blakemore2008), to inform future treatment strategies for adolescent depression, we need to identify psychological dimensions that modify depressed adolescents’ maladaptive self-processing, especially the neural mechanisms underlying such dimensions.
Self-compassion is an emotional and cognitive regulatory strategy which involves self-kindness, recognition of our common humanity, mindfulness and reduced self-judgment, isolation and over-identification in the face of negative life events (Neff, Reference Neff2003b; Terry & Leary, Reference Terry and Leary2011). Self-compassion is inversely associated with depression among adults (MacBeth & Gumley, Reference MacBeth and Gumley2012; Neff, Reference Neff2003a; Trompetter, de Kleine, & Bohlmeijer, Reference Trompetter, de Kleine and Bohlmeijer2017; Van Dam, Sheppard, Forsyth, & Earleywine, Reference Van Dam, Sheppard, Forsyth and Earleywine2011) and adolescents (Marsh, Chan, & MacBeth, Reference Marsh, Chan and MacBeth2018; Neff & McGehee, Reference Neff and McGehee2010; Tanaka, Wekerle, Schmuck, Paglia-Boak, & Team, Reference Tanaka, Wekerle, Schmuck, Paglia-Boak and Team2011), and less rumination, avoidance and worry mediate that inverse relationship (Krieger, Altenstein, Baettig, Doerig, & Holtforth, Reference Krieger, Altenstein, Baettig, Doerig and Holtforth2013; Raes, Reference Raes2010). Self-compassion interventions have demonstrated promising effects on reducing depression and/or distress among patients with diabetes or cancer (Campo et al., Reference Campo, Bluth, Santacroce, Knapik, Tan, Gold and Asher2017; Friis, Johnson, Cutfield, & Consedine, Reference Friis, Johnson, Cutfield and Consedine2016) and adolescents (Bluth, Gaylord, Campo, Mullarkey, & Hobbs, Reference Bluth, Gaylord, Campo, Mullarkey and Hobbs2016). Considering that self-compassion involves coping processes ‘to alleviate one's suffering and to heal or soothe oneself with kindness’ through a ‘recognition of one's common humanity’ (Neff, Reference Neff2003a), self-compassion may be a protective dimension that modulates maladaptive negative self-processing found in depressed youth.
So far, few studies have examined the neural basis of self-compassion. It has been found that state-level self-reassurance engaged the temporal pole and insula in response to negative v. neutral events (Longe et al., Reference Longe, Maratos, Gilbert, Evans, Volker, Rockliff and Rippon2010), while the traditionally studied trait-level self-compassion was negatively associated with the functional connectivity between ventromedial prefrontal cortex (VMPFC) and the right amygdala during negative v. neutral social feedback (Parrish et al., Reference Parrish, Inagaki, Muscatell, Haltom, Leary and Eisenberger2018). To our knowledge, no study has investigated how self-compassion relates to neural activity during self-processing, let alone the neural basis underlying the relationship between self-compassion and depression. Nevertheless, studies on the neural basis of self-processing in depressed patients have begun to shed light on this topic.
Lemogne et al. (Reference Lemogne, le Bastard, Mayberg, Volle, Bergouignan, Lehéricy and Fossati2009) found that both depressed patients and healthy controls (HCs) activated medial prefrontal cortex (MPFC) in self-judgments v. general judgments, but depressed patients showed greater activity in dorsomedial prefrontal cortex (DMPFC) and dorsolateral prefrontal cortex (DLPFC), as well as increased functional connectivity between them and dorsal anterior cingulate cortex (dACC). Such findings may represent the neural correlates of excessive self-focus (MPFC), its subsequent conflict monitoring (dACC), and a secondary compensatory mechanism attempting to use cognitive control (DLPFC) to exit the self-critical regulatory cycles in depressed patients. On the other hand, Bradley et al. (Reference Bradley, Colcombe, Henderson, Alonso, Milham and Gabbay2016) found that both depressed adolescents and HCs activated cortical midline structures (CMS) in response to self-judgments compared to general judgments, but depressed youth recruited the posterior cingulate cortex (PCC) and precuneus more for positive self-judgments. These findings suggest that CMS and DLPFC hyperactivity may underlie maladaptive self-processing in depressed patients.
Compared to self-descriptive psychological attributes that elicit primarily verbally mediated self-processing, the self-face is an automatic and more direct cue for investigating self-processing (Kircher et al., Reference Kircher, Senior, Phillips, Rabe-Hesketh, Benson, Bullmore and David2001). Empirical studies and meta-analyses have shown that among healthy adults, while both verbal and facial self-processing engage the dACC and precuneus/PCC activity (Hu et al., Reference Hu, Di, Eickhoff, Zhang, Peng, Guo and Sui2016; Platek, Wathne, Tierney, & Thomson, Reference Platek, Wathne, Tierney and Thomson2008; Sugiura et al., Reference Sugiura, Watanabe, Maeda, Matsue, Fukuda and Kawashima2005), verbal self-processing additionally activates the MPFC (D'Argembeau et al., Reference D'Argembeau, Ruby, Collette, Degueldre, Balteau, Luxen and Salmon2007; Hu et al., Reference Hu, Di, Eickhoff, Zhang, Peng, Guo and Sui2016; Northoff et al., Reference Northoff, Heinzel, De Greck, Bermpohl, Dobrowolny and Panksepp2006), and recognizing our own face particularly activates a right lateral cortical network, including the right DLFPC (Hu et al., Reference Hu, Di, Eickhoff, Zhang, Peng, Guo and Sui2016; Morita et al., Reference Morita, Itakura, Saito, Nakashita, Harada, Kochiyama and Sadato2008; Platek et al., Reference Platek, Wathne, Tierney and Thomson2008). These networks are altered in depressed adolescents during emotional self-face recognition. Compared to HCs, depressed youth displayed increased right DLPFC activity during self-other face recognition, decreased MPFC and limbic (amygdala/hippocampus) activity during happy self v. other face recognition (Quevedo et al., Reference Quevedo, Ng, Scott, Martin, Smyda, Keener and Oppenheimer2016), and decreased limbic (amygdala/hippocampus) and fusiform activity during happy v. neutral self v. other face recognition (Quevedo et al., Reference Quevedo, Harms, Sauder, Scott, Mohamed, Thomas and Smyda2018). Furthermore, depressed adolescents displayed greater amygdala functional connectivity with DLPFC, DMPFC, and precuneus during self-other face recognition compared to HCs (Alarcón, Sauder, Teoh, Forbes, & Quevedo, Reference Alarcón, Sauder, Teoh, Forbes and Quevedo2019). Abnormal brain function has also been noted during the processing of unfamiliar faces, for example Henderson et al. (Reference Henderson, Vallejo, Ely, Kang, Roy, Pine and Gabbay2014) found that depression severity correlated positively with activity in VMPFC, insula, and limbic regions (e.g. amygdala and putamen) during sad face-processing among depressed adolescents. Collectively, these studies yielded cortical neural loci of maladaptive self-processing in depressed youth, perhaps especially for self-face recognition. These studies converge on altered (often heightened) DLPFC activity and higher limbic to DLPFC connectivity in depressed youth during self-other face recognition compared to healthy youth, again suggesting higher cognitive effort and/or compensatory cognitive control to counteract (or engage on) harsh self-judgments or ruminative thinking during self-processing.
The current study
The goal of this paper is to investigate whether neural activity during self-face recognition in loci previously found to be associated with maladaptive self-processing among depressed adolescents and adults (e.g. CMS and DLPFC) relates to self-compassion and might further explain the inverse relationship between self-compassion and depression. Due to a lack of evidence on this topic, this study is both novel and exploratory in its aims.
Because self-compassion is regarded as a protective psychological dimension against depression and sadness, our study focused on neural activity elicited by sad self-face recognition. Given that self-compassion entails less negative self-processing in response to suffering and negative events (Neff et al., Reference Neff, Long, Knox, Davidson, Kuchar, Costigan and Breines2018), we hypothesized: that higher self-compassion would be associated with reduced neural activity elicited by sad v. neutral self-face recognition in regions undergirding abnormal self-processing in depressed youth, such as CMS (e.g. MPFC, ACC and PCC/precuneus) and DLPFC, which would in turn relate to reduced depression severity. If the hypotheses above were supported, we would further explore whether the data fitted one of the three models that described the relationship between self-compassion, its neural activity correlates during sad v. neutral self-face recognition, and depression severity (Fig. 1). We only set up models with self-compassion as the antecedent variable of depression severity because previous research showed that self-compassion interventions reduced depression (Bluth et al., Reference Bluth, Gaylord, Campo, Mullarkey and Hobbs2016; Campo et al., Reference Campo, Bluth, Santacroce, Knapik, Tan, Gold and Asher2017; Friis et al., Reference Friis, Johnson, Cutfield and Consedine2016), implying a causal effect of self-compassion upon depression severity. These hypotheses were tested separately (1) in the total sample comprised of both HCs and depressed adolescents, as well as (2) in the HC and (3) in the depressed sub-sample.
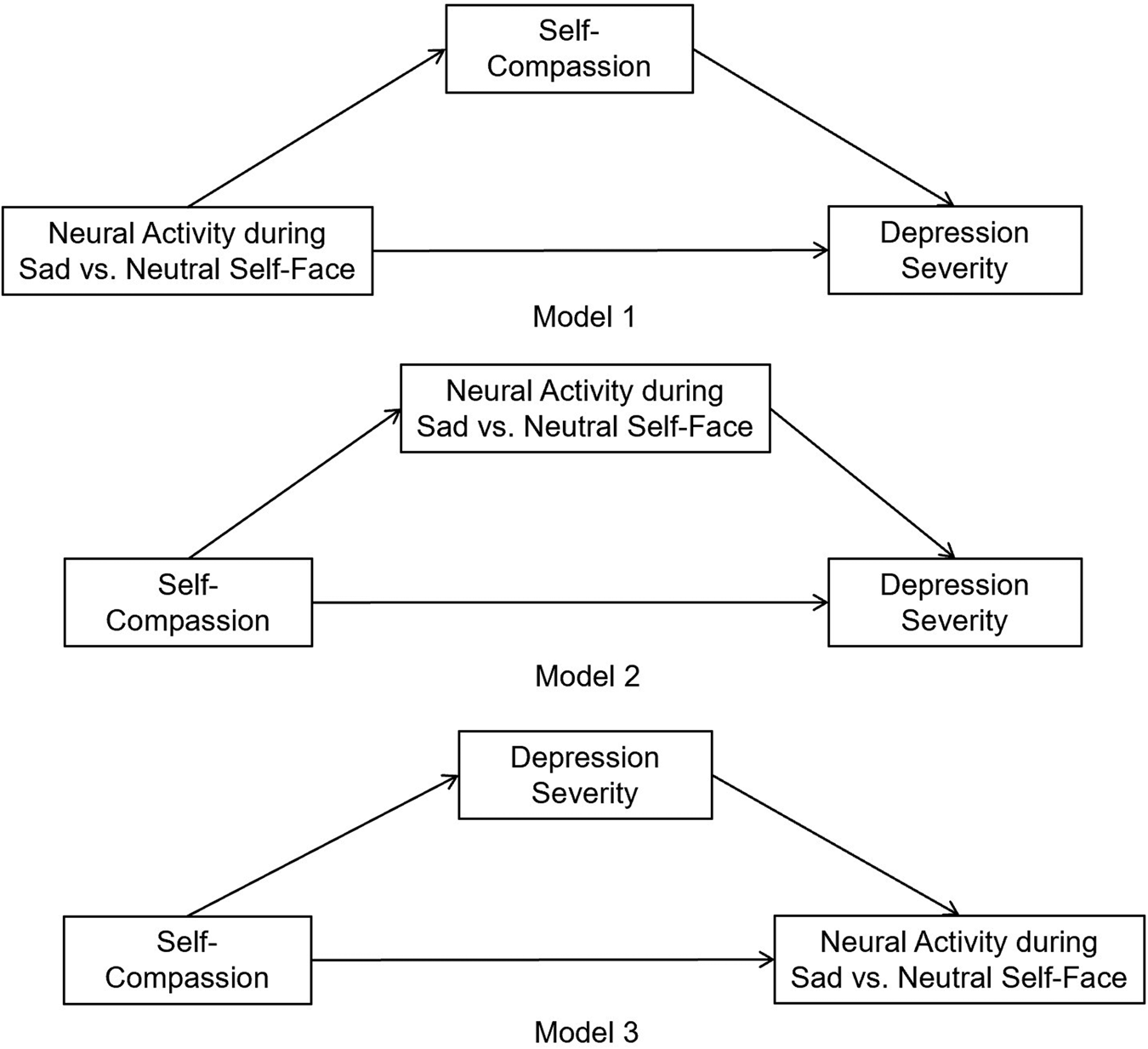
Fig. 1. Three mediation models were set up to explore the relationship between self-compassion, its neural activity correlates during sad v. neutral self-face recognition, and depression severity.
Methods
Participants
Recruitment and screening procedures were fully described in previous publications (Alarcón et al., Reference Alarcón, Sauder, Teoh, Forbes and Quevedo2019; Quevedo et al., Reference Quevedo, Ng, Scott, Martin, Smyda, Keener and Oppenheimer2016) and are only briefly presented here. Adolescents and their caregiver(s) were recruited from psychiatric clinics at the Universities of Minnesota (in Minneapolis) and Pittsburgh. Exclusion criteria included: IQ < 70, primary diagnosis other than depression, and left-handedness. Depression and presence of other psychiatric disorders were diagnosed by clinical evaluations with the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL) interview (Kaufman et al., Reference Kaufman, Birmaher, Brent, Rao, Flynn, Moreci and Ryan1997). All clinical interviews were videotaped and scored to determine diagnostic agreement. Three Ph.D.-level experts in child development reviewed and scored the videotapes with 98% agreement in symptoms severity and diagnosis. Given high comorbidity of anxiety and depression, a common comorbidity in both adolescent and adult patients, depressed adolescents with a comorbid diagnosis of anxiety disorder were not excluded. In the current study, 72.84% (N = 59) of the depressed sample also qualified for an anxiety disorder. Diagnostic discrepancies (i.e. disagreements between the coders) were resolved by the senior author, a licensed clinical psychologist. A total of 82 depressed adolescents (DEPs) and 37 HCs consented to participate in the study. This is a rather large total sample size for an imaging study. One participant whose brain images showed a large area of signal dropout in the right visual cortex was excluded from analyses. The final sample consisted of 81 DEPs (62 scanned at the Minneapolis site and 19 at the Pittsburgh site) and 37 HCs (21 at the Minneapolis site and 16 at the Pittsburgh site). Sample characteristics are presented in online Supplementary Table S1. Eight out of the 118 participants were excluded from behavioral analyses because their behavioral responses during the functional magnetic resonance imaging (fMRI) task were missing. This study was approved by the Institutional Review Boards at both the Universities of Minnesota and Pittsburgh.
Measures
Self-compassion was measured using the Self-Compassionate Reaction Inventory (SCRI) (Terry, Leary, Mehta, & Henderson, Reference Terry, Leary, Mehta and Henderson2013), which lists eight common negative events, and for each event, participants are asked to endorse two responses from four given reactions – two of which are self-compassionate and two of which are not self-compassionate. A total score is calculated by summing up the number of self-compassionate responses endorsed (ranging from 0 to 16). Scores in this questionnaire have been shown to highly correlate with those of Neff's (Reference Neff2003a) Self-Compassion Scale, and the two scales have comparable patterns of convergent and discriminant validity (Terry et al., Reference Terry, Leary, Mehta and Henderson2013). A continuous measure of depression severity was measured using the Children's Depression Rating Scale-Revised (CDRS-R) (Poznanski & Mokros, Reference Poznanski and Mokros1996), which was widely used and showed good reliability among adolescents (α = 0.74–0.92) (Mayes, Bernstein, Haley, Kennard, & Emslie, Reference Mayes, Bernstein, Haley, Kennard and Emslie2010).
fMRI task
Participants completed the Emotional Self-Other Morph-Query (ESOM-Q) task within 1–2 weeks after their clinical assessment. This task was described in previous publications (Alarcón et al., Reference Alarcón, Sauder, Teoh, Forbes and Quevedo2019; Quevedo et al., Reference Quevedo, Ng, Scott, Martin, Smyda, Keener and Oppenheimer2016; Quevedo et al., Reference Quevedo, Harms, Sauder, Scott, Mohamed, Thomas and Smyda2018). See online Supplementary material for details about stimuli generation of this task. In the scanner, participants were exposed to photographs of 150 faces displaying happy, sad, or neutral expressions with the task of identifying whether the picture looked like them or not, by pressing one of two buttons with fingers on the right hand (Fig. 2). The task consisted of one run and lasted 10 min 54 s. It was comprised of six different blocks of faces (self-happy, other-happy, self-sad, other-sad, self-neutral, and other-neutral). Instructions were presented at the beginning of each block and lasted 6 s each. At the start, the halfway and the end of each 70 s block an 18 s rest period (12 rest periods in total) with a presented fixation cross was announced by the phrase ‘rest now’. Each face was displayed for 2 s followed by a 0.5 s fixation cross. Blocks contained faces with high (i.e. self-blocks) or low degrees (i.e. other blocks) of facial morphing between the self and the other face. This resulted in blocks comprised of self faces or unfamiliar faces within happy, sad or neutral categories. Blocks were presented in five counterbalanced task orders and each contained 28 photos of faces presented randomly with regard to morphing percentages within any given self by emotion block, with the following means and standard deviation of morphs: Self blocks: M self = 83%, s.d. = 12%, Minself = 65%, Maxself = 100%; Other blocks: M self = 18%, s.d. = 12%, Minself = 0%, Maxself = 35%. Ambiguous stimuli (i.e. with 40–60% of self-features) were not used in any block. Within each of the six blocks, four faces of high opposite percentage in self level were shown to avoid response sets and keep the participants attentive to the actual identity of the faces (Other block: four faces = 90% or 80% self; Self block: four faces = 90% or 80% other). These incongruent faces were excluded from all present analyses. E-Prime software was used to present stimuli and record reaction time and accuracy.
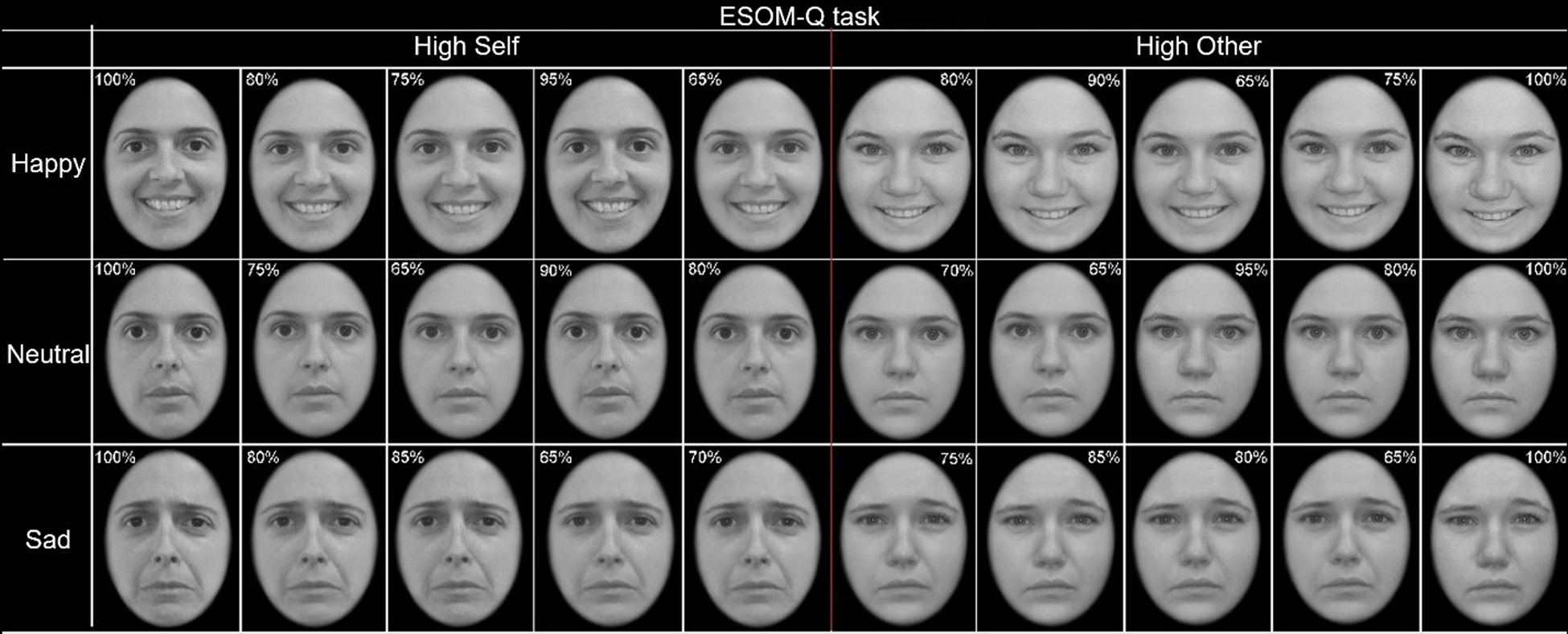
Fig. 2. The emotional self-other morph query (ESOM-Q) task entails recognizing the self v. an unfamiliar face via button press. The faces were displayed in random order within six blocks across happy, sad, and neutral faces.
fMRI data acquisition
Neuroimaging data were collected using 3T Siemens Trio MRI scanners at the two data collection sites. Structural 3D axial MPRAGE images were acquired in the same session (TR/TE: 2100 ms/3.31 ms; TI: 1050; flip angle = 8°; FOV: 256 × 200 mm; matrix = 256 × 200; 176 slices, slice thickness = 1 mm). Mean blood oxygenated level depended (BOLD) activity images were obtained using a gradient echo EPI sequence (TR/TE = 3340/30 ms, flip angle = 90°, FOV = 200 × 200 mm; matrix 80 × 80; 60 slices, slice thickness = 2 mm).
Image pre-processing
Scans were preprocessed with SPM12. Data for each participant was realigned to the first volume in the time series to correct for head motion. Realigned images were co-registered with subject's anatomical image, segmented, normalized to the MNI template, and spatially smoothed with a Gaussian kernel of 7 mm FWHM. Volumes with movement >2 mm or rotations >0.587 or that had global signal intensities greater than 9 were removed from first-level analysis using Artifact Detection Tools (ART) software (http://web.mit.edu/swg/software.htm).
Self-report analyses
Independent-sample t tests were conducted to determine whether self-compassion and depression severity differed between diagnostic groups (DEP/HC). Moderation analyses were performed with Preacher & Hayes's (Reference Preacher and Hayes2008) PROCESS package to test whether self-compassion had a main effect on depression severity and whether diagnostic group moderated the effect. Bias-corrected 95% confidence intervals (CIs) of the effects were estimated with bootstrap simulations of 5000 iterations and were evaluated for statistical significance using the criteria of excluding zero in the CIs.
fMRI analyses
Imaging data were processed and analyzed with SPM12. Block design first level analyses were conducted with the rest period and four incongruent faces in each block (condition) excluded from modeling. First-level general linear models with predictors including six conditions (sad self, neutral self, happy self, sad other, neutral other, and happy other) and nuisance regressors (including six movement parameters) were estimated for each participant at each voxel, resulting in t-statistic images for each of the six conditions. Contrasted images of sad self > neutral self were created for second-level analyses. Because our main hypotheses were only about sad self-face processing, fMRI analyses about happy self-face and sad other-face processing are described in the online Supplementary material.
Identification of neural correlates of self-compassion during sad v. neutral self-face recognition in the total sample
Neural correlates of self-compassion were examined in the total sample. We also explored whether the correlates of self-compassion would differ by diagnostic group. Specifically, a second-level whole-brain regression model with self-compassion, diagnostic group (DEP/HC) and a self-compassion by diagnostic group interaction as covariates of interest, and with scanning site (Minneapolis or Pittsburgh) as confounding covariate, was estimated. Whole-brain cluster-extent thresholds of p FWE < 0.05 were calculated using Monte Carlo and 3dClustSim in AFNI 18 with a voxel-wise threshold of p uncorr < 0.001. Average BOLD activations of each cluster were extracted for visualization and correlation analyses with depression severity.
Identification of neural correlates of self-compassion during sad v. neutral self-face recognition in the HC and in the DEP sub-sample separately
Analyses with the same details as in the total sample were conducted in the HC and in the DEP sub-sample separately, the only difference being that there were no diagnostic group or group by self-compassion interaction regressor in the models.
Exploratory mediation analysis
To examine whether the following three variables, (1) self-compassion, (2) the neural substrates of self-compassion during sad v. neutral self-face recognition, and (3) depression severity, fitted one of the three mediation models as depicted in Fig. 1, we first tested whether the neural substrates of self-compassion correlated with depression severity in the total sample as well as in the HC and in the DEP sub-sample separately. If the correlation in any (sub-) sample was significant, mediation analysis was further performed with Preacher & Hayes's (Reference Preacher and Hayes2008) PROCESS package.
Results
Self-report results
Independent-sample t tests showed that DEPs reported lower levels of self-compassion than HCs, t(116) = −7.30, p < 0.001 (DEP: 7.00 ± 4.51, HC: 13.11 ± 3.48), and higher depression severity than HCs, t(116) = 23.06, p < 0.001 (DEP: 63.32 ± 14.49, HC: 20.27 ± 5.76). Moderation analysis showed that both the main effect of self-compassion (95% CI −0.35 to −0.14) and the diagnostic group by self-compassion interaction effect (95% CI −0.25 to −0.01) were significant. Further analyses showed that the conditional effect of self-compassion on depression severity was significant in DEPs (95% CI −0.45 to −0.22), but not in HCs (95% CI −0.27 to 0.18). Behavioral results on response time and accuracy can be found in the online Supplementary material.
fMRI activity results
Neural correlates of self-compassion during sad v. neutral self-face recognition in the total sample
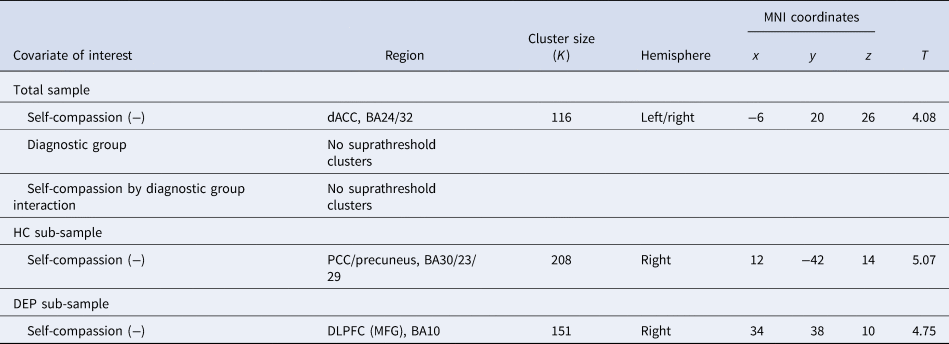
Whole-brain analysis showed that self-compassion related negatively to activity in the dACC (r = −0.17, p = 0.059) during sad v. neutral self-face recognition in the total sample (Table 1; Fig. 3a), yet no suprathreshold clusters were found related to either diagnostic group or its interaction with self-compassion. On the other hand, no suprathreshold clusters related to self-compassion, diagnostic group or their interaction during happy v. neutral self-face recognition in the total sample (see online Supplementary material). While no suprathreshold clusters related to self-compassion or diagnostic group during sad v. neutral other-face recognition in the total sample, self-compassion and diagnostic group showed an interaction effect in the left inferior parietal lobule (IPL) extending to postcentral gyrus and superior parietal lobule (online Supplementary Table S2). Specifically, the correlation between self-compassion and IPL activity was stronger in HCs than in depressed youth (see online Supplementary material).
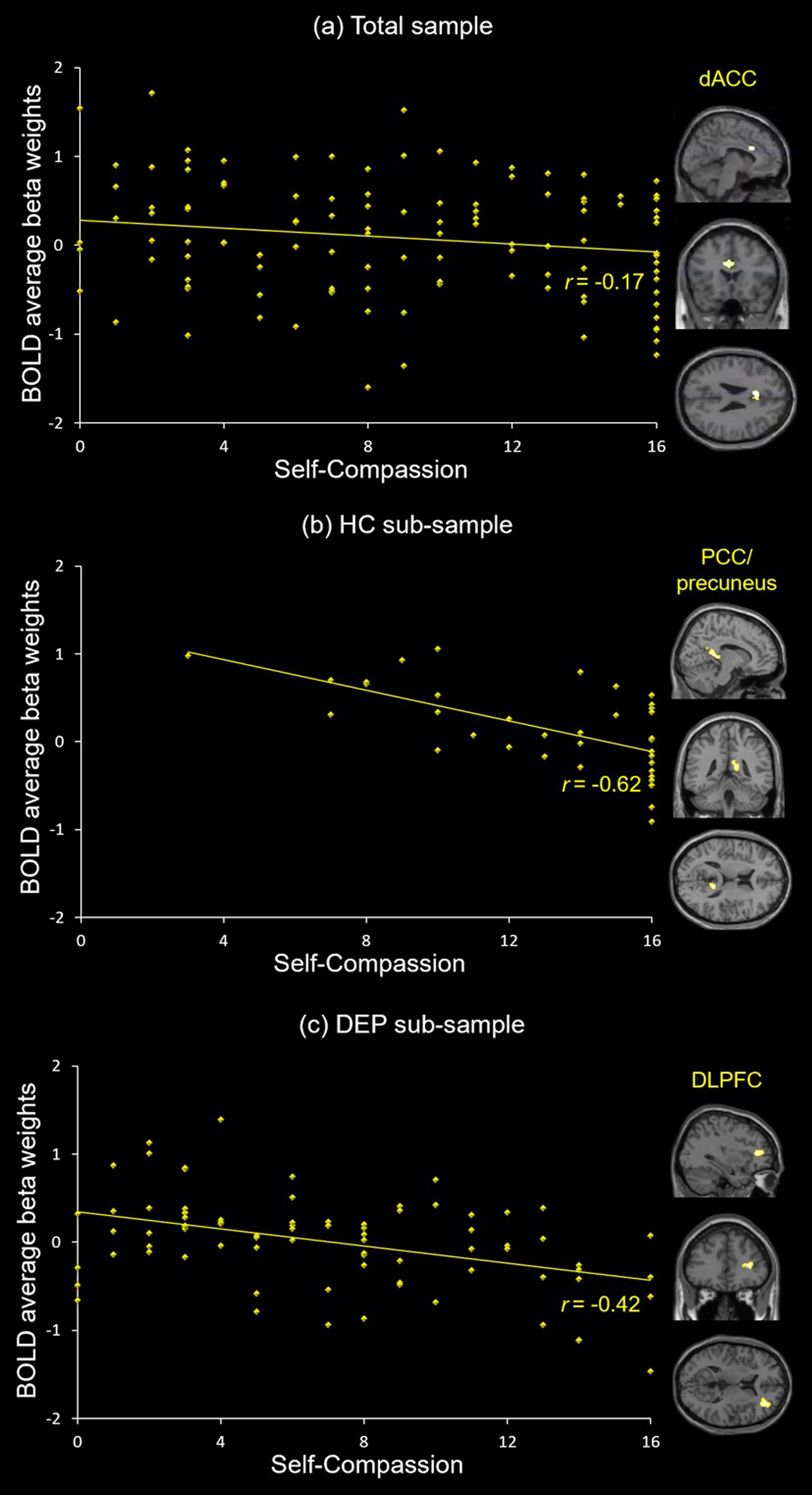
Fig. 3. Neural correlates of self-compassion. (a) Self-compassion is negatively associated with dACC activity during sad v. neutral self-face recognition in the total sample. (b) Self-compassion is negatively associated with right PCC/precuneus activity during sad v. neutral self-face recognition in the HC sub-sample. (c) Self-compassion is negatively associated with right DLPFC activity during sad v. neutral self-face recognition in the DEP sub-sample.
Table 1. Neural activity correlates during sad v. neutral self-face recognition (p uncorr < 0.001 at the voxel level, cluster-level p FWE < 0.05)

dACC, dorsal anterior cingulate cortex; BA, Brodmann area; PCC, posterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; MFG, middle frontal gyrus.
Neural correlates of self-compassion during sad v. neutral self-face recognition in the HC sub-sample
Whole-brain analysis showed that self-compassion related negatively to activity in the right PCC extending to precuneus (r = −0.62, p < 0.001) during sad v. neutral self-face recognition in the HC sub-sample (Table 1; Fig. 3b). On the other hand, no suprathreshold clusters were linked to self-compassion during happy v. neutral self-face recognition in the HC sub-sample (see online Supplementary material), and self-compassion related positively to activity in the left post-/precentral gyrus, the left insula and the right postcentral gyrus during sad v. neutral other-face recognition in the HC sub-sample (see online Supplementary Table S2 and material).
Neural correlates of self-compassion during sad v. neutral self-face recognition in the DEP sub-sample
Whole-brain analysis showed that self-compassion related negatively to activity in the right DLPFC (r = −0.42, p < 0.001) during sad v. neutral self-face recognition in the DEP sub-sample (Table 1; Fig. 3c). On the other hand, no suprathreshold clusters were linked to self-compassion during happy v. neutral self-face recognition or during sad v. neutral other-face recognition in the DEP sub-sample (see online Supplementary material).
Exploratory mediation analysis
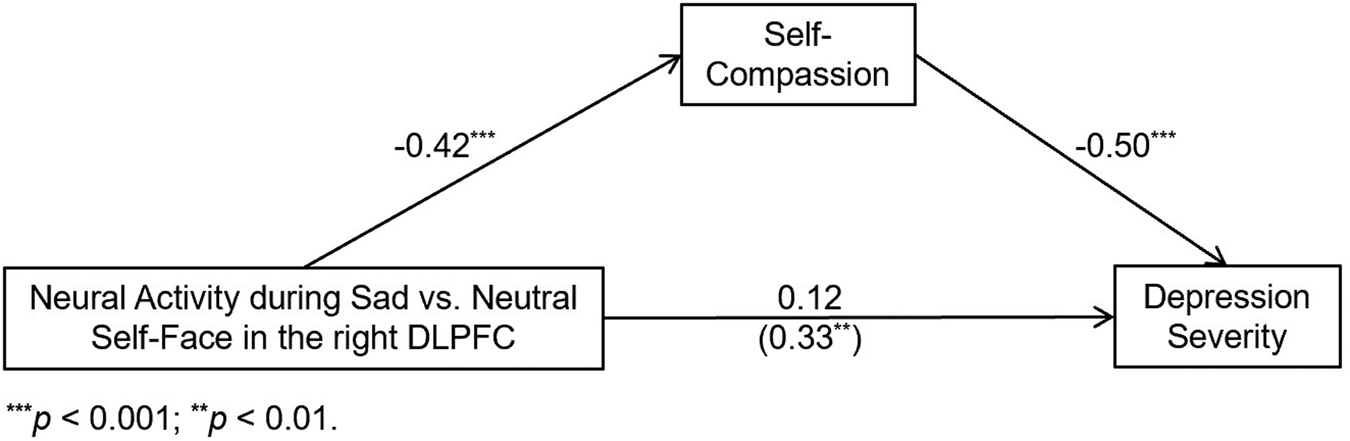
We first conducted correlation analyses between depression severity and activity in the neural substrates of self-compassion during sad v. neutral self-face recognition identified above in the various samples. Depression severity and neural activity were uncorrelated in both the total sample (dACC: r = −0.07, p = 0.48) and the HC sub-sample (PCC/precuneus: r = −0.11, p = 0.53). In the DEP sub-sample, however, right DLFPC activity during sad v. neutral self-face recognition positively correlated with depression severity (r = 0.33, p = 0.003). Given that right DLPFC activity during sad v. neutral self-face recognition related to both self-compassion and depression severity, mediation analyses were conducted with models depicted in Fig. 1. As shown in Fig. 4, these analyses showed that the direct effect of right DLPFC activity on depression severity became insignificant once self-compassion was added into the model (Model 1). The indirect effect via self-compassion (95% CI 3.16–9.05) was significant, indicating that higher self-compassion mediated the relationship between lower DLPFC activity during sad v. neutral self-face recognition and reduced depression severity. On the other hand, the indirect effect was not significant in either Model 2 or Model 3, and neural activity correlates of self-compassion during sad v. neutral other-face were uncorrelated with depression severity in any (sub-) sample (see online Supplementary material).

Fig. 4. Higher self-compassion fully mediates the relationship between lower right DLPFC activity during sad v. neutral self-face recognition and reduced depression severity in the depressed sub-sample.
Discussion
Our study provides the first evidence that higher right DLPFC activity during self-processing (operationalized as sad v. neutral self-face recognition) is associated with lower self-compassion and higher depression severity in depressed adolescents. Self-compassion was negatively associated with activity during sad v. neutral self-face recognition in the dACC in the total sample and in the right PCC/precuneus in HCs, respectively, but these regions’ activities were not significantly associated with depression severity. In depressed adolescents, however, lower right DLPFC activity during sad v. neutral self-face recognition was associated with both higher self-compassion and lower depression severity. Furthermore, higher self-compassion fully mediated the relationship between lower DLPFC activity and reduced depression severity among just the depressed youth (N = 81; Fig. 4).
CMS activity and self-compassion
We found that higher self-compassion was associated with lower dACC activity during sad v. neutral self-face recognition in the total sample, and with lower right PCC/precuneus activity in the HC sub-sample. These structures comprise CMS that have been broadly proposed to enable self-processing (Northoff & Bermpohl, Reference Northoff and Bermpohl2004; Uddin, Iacoboni, Lange, & Keenan, Reference Uddin, Iacoboni, Lange and Keenan2007), with the dACC involved in assigning salience to the physical self (Hu et al., Reference Hu, Di, Eickhoff, Zhang, Peng, Guo and Sui2016) and the PCC in getting ‘caught up’ in one's experience (Brewer, Garrison, & Whitfield-Gabrieli, Reference Brewer, Garrison and Whitfield-Gabrieli2013). Previous research has found that self-processing activates these regions in both healthy populations and depressed patients (Alarcón et al., Reference Alarcón, Sauder, Teoh, Forbes and Quevedo2019; Bradley et al., Reference Bradley, Colcombe, Henderson, Alonso, Milham and Gabbay2016; Lemogne et al., Reference Lemogne, le Bastard, Mayberg, Volle, Bergouignan, Lehéricy and Fossati2009; Quevedo et al., Reference Quevedo, Ng, Scott, Martin, Smyda, Keener and Oppenheimer2016). It has been proposed that self-compassion might promote hypo-egoic (i.e. psychological states characterized by relatively little involvement of the self) responses to negative events (Johnson & O'Brien, Reference Johnson and O'Brien2013; Leary & Terry, Reference Leary and Terry2012; Neff & Seppälä, Reference Neff, Seppälä, Brown and Leary2016) by involving the concept of common humanity rather than seeing oneself as separate from others (Neff, Reference Neff, Bauer and Wayments2008). Our findings on the inverse relationship between self-compassion and dACC as well as PCC/precuneus activity (loci within CMS) during sad self-face recognition lend some support to this speculation. In addition to self-processing, the dACC is also associated with conflict monitoring (MacDonald, Cohen, Stenger, & Carter, Reference MacDonald, Cohen, Stenger and Carter2000) and the PCC/precuneus with attentional focus (Leech & Sharp, Reference Leech and Sharp2014) and experiential immersion (Brewer et al., Reference Brewer, Garrison and Whitfield-Gabrieli2013). Our findings suggest that adolescents higher in self-compassion might show less vigilant attentional focus or self-involvement (Cavanna & Trimble, Reference Cavanna and Trimble2006; Fransson & Marrelec, Reference Fransson and Marrelec2008) when processing negative self-relevant stimuli (e.g. sad self-face) and/or experience less mental conflicts regarding negative self-relevant cues. However, we did not find significant correlations between dACC or PCC/precuneus activity and depression severity, which suggests that lower activity in these regions during sad v. neutral self-face recognition does not explain the inverse relationship between self-compassion and depression severity in our study.
DLPFC activity and self-compassion
Within the depressed sub-sample, we found that higher self-compassion was associated with lower right DLPFC activity during sad v. neutral self-face recognition. Moreover, we found no association between self-compassion and DLPFC activity, but rather positive associations between self-compassion and activity in empathy-related regions (e.g. insula, postcentral gyrus, and IPL) among HCs during sad v. neutral other-face recognition (see online Supplementary material). These findings appear to imply that the inverse association between self-compassion and DLPFC activity is specific to sad self-face recognition. Self-compassion involves less self-criticism during personal failures (Neff et al., Reference Neff, Long, Knox, Davidson, Kuchar, Costigan and Breines2018) and might promote soothing regulatory responses to negative events that reduce threat system activation and depressive symptoms (Johnson & O'Brien, Reference Johnson and O'Brien2013; Leary & Terry, Reference Leary and Terry2012; Neff & Seppälä, Reference Neff, Seppälä, Brown and Leary2016). Our findings provide further evidence to this line of research at the neural level. The higher DLPFC activity associated with lower self-compassion might represent engagement of higher cognitive control (Diamond, Reference Diamond2013; Miller & Cohen, Reference Miller and Cohen2001) to avoid dwelling on the sad self-faces or deployment of cognitive regulatory processes to dampen negative emotions elicited by them (Etkin, Büchel, & Gross, Reference Etkin, Büchel and Gross2015; Ochsner & Gross, Reference Ochsner and Gross2005). Alternatively, heightened DLPFC might underpin ruminative self-critical thoughts elicited by recognizing the sad self-face (Cooney, Joormann, Eugène, Dennis, & Gotlib, Reference Cooney, Joormann, Eugène, Dennis and Gotlib2010). This implies that self-compassionate depressed adolescents might be more able to swiftly process negative self-relevant stimuli without dwelling on self-recrimination or might be better at regulating the negative emotions induced by them without engaging cognitive control resources. Because the right DLPFC was consistently activated during self-face recognition in past research (Hu et al., Reference Hu, Di, Eickhoff, Zhang, Peng, Guo and Sui2016; Morita et al., Reference Morita, Itakura, Saito, Nakashita, Harada, Kochiyama and Sadato2008; Platek et al., Reference Platek, Wathne, Tierney and Thomson2008), its lower activation during sad self-faces in self-compassionate depressed adolescents might be due to reduced over-identification with the sad-face stimuli or less need of associative cognitive resources for a simple self-face recognition task.
Mediating role of self-compassion
Lower right DLPFC activity during sad v. neutral self-face recognition was associated with reduced depression severity among depressed youth, and higher self-compassion fully mediated their relationship. Moreover, neural activity correlates of self-compassion during sad v. neutral other-face recognition were uncorrelated with depression severity in any (sub-) sample (see online Supplementary material). These findings appear to suggest that the association between self-compassion, its neural substrates and depression severity is specific to sad self-face recognition. Previous findings with the same dataset found that compared to HCs, depressed adolescents showed higher activity in the right DLPFC during self-other face recognition (Quevedo et al., Reference Quevedo, Ng, Scott, Martin, Smyda, Keener and Oppenheimer2016), and higher DLPFC activity during self-referential processing has also been reported in depressed adults (Lemogne et al., Reference Lemogne, le Bastard, Mayberg, Volle, Bergouignan, Lehéricy and Fossati2009). These results suggest that lower self-compassion might be linked to greater need for cognitive control, self-focused rumination, and/or engagement of cognitive effort during sad v. neutral self-face recognition and more severe depressions among adolescents. The association between heightened DLPFC activity and lower self-compassion/higher depression severity might be due to the role of DLPFC as the structure enabling self-focused rumination. Rumination is a maladaptive form of self-processing and affect dysregulation that characterizes depression (Cooney et al., Reference Cooney, Joormann, Eugène, Dennis and Gotlib2010). Sad self-face recognition might have temporarily heightened depressed adolescents’ rumination concerning their distress and/or engaged the same cognitive processes for redirecting such distress. It is possible that perceived discrepancies between one's current state (or one's sad-self face) and a salient self-relevant standard might have led to regulatory attempts to decrease the discrepancy and/or to avoid self-focus (Lemogne et al., Reference Lemogne, le Bastard, Mayberg, Volle, Bergouignan, Lehéricy and Fossati2009; Ochsner & Gross, Reference Ochsner and Gross2005). On the other hand, our previous findings with the same dataset found that depressed youth showed greater amygdala connectivity with DLPFC during self-other face recognition compared to HCs (Alarcón et al., Reference Alarcón, Sauder, Teoh, Forbes and Quevedo2019). Thus, lower DLPFC activity linked to higher self-compassion might represent less need for cognitive control to regulate distress (including strong emotions borne by limbic areas such as the amygdala) elicited by negative self-relevant stimuli. If these speculations were true, lower DLPFC activity and/or amygdala connectivity with this area might represent (among a host of other processes) a biological marker for more acceptance of personal negative information or less need for top-down regulation of elicited emotional experiences and reduced depression.
Limitations
This study has several limitations. First, although depression was the primary diagnosis, some participants had co-morbid anxiety disorder, though it is quite common particularly among young patients. Second, our findings are correlational rather than causal. Studies with intervention validated for adolescents (Bluth et al., Reference Bluth, Gaylord, Campo, Mullarkey and Hobbs2016) are needed to provide direct evidence to the causal relationship between DLPFC activity during sad v. neutral self-face recognition, self-compassion, and depression severity. Third, we only presented sad faces among negative emotions in the current study, so it is not known whether the present findings are general to faces of all negative emotions (e.g. angry, disgusted, and fearful faces) or specific to sad faces. Similarly, it is not clear whether the present findings are general to all types of self-processing or specific to self-face processing. Future studies should examine the neural activity correlates of self-compassion in tasks with more negative emotions (e.g. fear and anger) as well as verbal self-processing. Finally, the scale (SCRI) by which we measured self-compassion is not widely used and does not assess mindfulness and over-identification. Though the SCRI correlates highly with the more widely-used measure of self-compassion (Terry et al., Reference Terry, Leary, Mehta and Henderson2013), i.e. Neff's (Reference Neff2003a) Self-Compassion Scale (SCS), future studies should test the replicability of our findings with the SCS. In addition, future researchers may examine to what degree our findings are generalizable across different age groups, given that age was found to moderate the associations between SCS scores and depression (Bluth, Campo, Futch, & Gaylord, Reference Bluth, Campo, Futch and Gaylord2017) or other psychopathological problems (e.g. social anxiety; Werner et al., Reference Werner, Jazaieri, Goldin, Ziv, Heimberg and Gross2012). It is also important for future research to supplement the self-report measure with a behavioral measure of self-compassion comprised of behaviors that are theoretically linked to self-compassion and reflect a stance of caring and respect for oneself.
Conclusions
Higher self-compassion relates to lower neural responses in the right DLPFC during sad v. neutral self-face recognition and mediates the relationship between lower DLPFC activity and reduced depression severity among depressed adolescents. These results advance our understanding of the neural mechanisms of self-compassion and suggest that DLPFC activity might be a biological marker of a successful self-compassion intervention as a potential treatment for adolescent depression.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291720002482
Acknowledgements
Funding provided by K01MH092601 and two NARSAD grants to the last author (KQ). The second author (NZ)'s work was funded by an NRSA (T32 DA039772) by NIDA through the Department of Psychology and the REACH Institude at Arizona State University. The authors would like to thank Drs. Mary Phillips, Kathleen Thomas, and Daniel Pine for facilitating this work and helping with conceptualizations of the research ideas in this study.
Conflict of interest
Dr Richard J. Davidson is the founder, president, and serves on the board of directors for the non-profit organization, Healthy Minds Innovations, Inc.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.